A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Determining Soil-transmitted Helminth Infection Status and Physical Fitness of School-aged Children
In This Article
Summary
Chronic infection with soil-transmitted helminths (STHs) causes malabsorption, stunting, and wasting in the growing child. Hence, it is plausible that these infections also reduce the physical fitness of children. Here, we visualize two techniques for the diagnosis of STHs and the 20-meter shuttle run test for assessing children's physical fitness.
Abstract
Soil-transmitted helminth (STH) infections are common. Indeed, more than 1 billion people are affected, mainly in the developing world where poverty prevails and hygiene behavior, water supply, and sanitation are often deficient1,2. Ascaris lumbricoides, Trichuris trichiura, and the two hookworm species, Ancylostoma duodenale and Necator americanus, are the most prevalent STHs3. The estimated global burden due to hookworm disease, ascariasis, and trichuriasis is 22.1, 10.5, and 6.4 million disability-adjusted life years (DALYs), respectively4. Furthermore, an estimated 30-100 million people are infected with Strongyloides stercoralis, the most neglected STH species of global significance which arguably also causes a considerable public health impact5,6. Multiple-species infections (i.e., different STHs harbored in a single individual) are common, and infections have been linked to lowered productivity and thus economic outlook of developing countries1,3.
For the diagnosis of common STHs, the World Health Organization (WHO) recommends the Kato-Katz technique7,8, which is a relatively straightforward method for determining the prevalence and intensity of such infections. It facilitates the detection of parasite eggs that infected subjects pass in their feces.
With regard to the diagnosis of S.stercoralis, there is currently no simple and accurate tool available. The Baermann technique is the most widely employed method for its diagnosis. The principle behind the Baermann technique is that active S.stercoralis larvae migrate out of an illuminated fresh fecal sample as the larvae are phototactic9. It requires less sophisticated laboratory materials and is less time consuming than culture and immunological methods5.
Morbidities associated with STH infections range from acute but common symptoms, such as abdominal pain, diarrhea, and pruritus, to chronic symptoms, such as anemia, under- and malnutrition, and cognitive impairment10. Since the symptoms are generally unspecific and subtle, they often go unnoticed, are considered a normal condition by affected individuals, or are treated as symptoms of other diseases that might be more common in a given setting. Hence, it is conceivable that the true burden of STH infections is underestimated by assessment tools relying on self-declared signs and symptoms as is usually the case in population-based surveys.
In the late 1980s and early 1990s, Stephenson and colleagues highlighted the possibility of STH infections lowering the physical fitness of boys aged 6-12 years11,12. This line of scientific inquiry gained new momentum recently13,14,15. The 20-meter (m) shuttle run test was developed and validated by Léger et al.16 and is used worldwide to measure the aerobic fitness of children17. The test is easy to standardize and can be performed wherever a 20-m long and flat running course and an audio source are available, making its use attractive in resource-constrained settings13. To facilitate and standardize attempts at assessing whether STH infections have an effect on the physical fitness of school-aged children, we present methodologies that diagnose STH infections or measure physical fitness that are simple to execute and yet, provide accurate and reproducible outcomes. This will help to generate new evidence regarding the health impact of STH infections.
Protocol
1. Kato-Katz Technique
- Place a standard Kato-Katz template with a hole for holding 41.7 milligrams (mg) of stool on a microscope slide.
- Scoop 2-3 grams (g) of a fresh fecal sample onto a piece of newspaper or aluminum foil, and press a piece of wire or plastic mesh on top to sieve it.
- Using a small plastic spatula, scrape the sieved material off the mesh and completely fill the hole in the Kato-Katz template with it. To remove excess fecal material, level the content of the hole with the spatula.
- Vertically remove the template without disturbing the fecal material now adhering to the microscope slide. The template and spatula can be cleaned in water with detergent, and reused.
- Place a piece of cellophane, pre-soaked in glycerine-malachite green solution for at least 24 hours (hr), over the fecal sample on the microscope slide.
- To spread the fecal material into a thick smear, gently press a clean microscope slide against the sample slide, evenly distributing the material within a circle of a diameter slightly smaller than the breadth of the microscope slide.
- Allow the slide to clear for 30-60 minutes (min), during which the slides must be kept away from direct sunlight. Then, systematically examine the thick smear under a light microscope (40-100x magnification). Count the number of STH eggs and stratify them by species (i.e., A.lumbricoides, T.trichiura, and hookworm). Of note, other helminth eggs (e.g. Schistosoma mansoni) can also be detected by this method and should be counted and recorded separately.
- To get a standardized estimate of the intensity of infection, conventionally expressed as number of eggs per gram of stool (EPG), multiply the egg count by 24 (24 x 41.7 mg ≈ 1 g).
2. Baermann Technique
- Set up a stand that can hold a glass funnel and attach a rubber tube, closed with a clip, to the bottom of the funnel.
- Place a sieve or a piece of wire screen shaped like a cone, in the funnel and layer it with a piece of medical gauze.
- Fill up the funnel with tap water and ensure that the whole set-up does not leak.
- Scoop 20-30 g of fresh stool onto the middle of the medical gauze and ensure that it is completely submerged in water. Add more water if necessary. Fold the medical gauze over the sample.
- From below, shine artificial light at the funnel for 2 hr.
- Drain 50 milliliters (ml) of the lower portion of the water in the funnel into a centrifuge tube by gently releasing the clip on the rubber tube.
- Centrifuge the collected water at 600 x g (relative centrifugal force) for 5 min and carefully pour out the supernatant without disturbing the sediment. Retain the last few drops in the tube.
- Re-suspend the sediment and pipette 2 drops of the solution onto a microscope slide and examine the slide under a light microscope (40x magnification for detection and 100-400x magnification for species confirmation). First-stage larvae (L1) of S. stercoralis are alive and actively move under the microscope.
3. 20-m Shuttle Run Test
- Measure a 20-m flat and straight running course and mark it with cones.
- Help the test participant wear a heart rate monitor on the wrist and the corresponding transmitter around the chest18. This equipment allows the heart rate of the participant to be measured before and after the test and thus monitors whether the participant has exerted maximal effort.
- Instruct the participant to run back and forth on the course by following the pace of pre-recorded sound signals delimiting 20-m laps. Starting with a running speed of 8.5 kilometers per hour (km/hr), the frequency of the signals indicating 20-m intervals increases by an equivalent of 0.5 km/hr every min.
- Ensure that the participant starts a new 20-m interval each time a signal is sounded and finishes it before the next signal arrives.
- Stop the participant if he/she fails to follow the pace for two consecutive 20-m intervals.
- Record the highest running speed indicated for the last interval which the participant completed fully, along with the age and sex of the participant.
- Verify that the heart rate achieved at the end of the test is the child's maximal heart rate19. If this heart rate is not reached, the child might not have exerted maximal effort, and hence the test should be repeated.
- Estimate the maximum aerobic capacity (VO2 max) of the participant, using an equation put forth by Léger and co-workers: VO2 max = 31.025 + 3.238 * speed (in km/hr) - 3.248 * age (in years) + 0.1536 * speed (in km/hr) * age (in years).
4. Representative Results
In terms of STH diagnosis with the Kato-Katz technique, unique characteristics of the different helminth eggs allow for the identification of most species20. The eggs of A.lumbricoides (approximately 45-75 micrometer (μm) long and 35-50 μm wide, Figure 1) have thicker shell walls than the eggs of T.trichiura (Figure 2) and hookworm (Figure 3). A fertilized egg of A.lumbricoides (Figure 1) has an outer albuminous coat, which makes it look golden brown, while a non-fertilized egg lacks this outer coat and is slightly elongated and larger overall. The eggs of T.trichiura (approximately 50 μm long and 22 μm wide, Figure 2) are elongated and have distinct polar plugs. Hookworm eggs (approximately 64-76 μm long and 36-40 μm wide, Figure 3), on the other hand, have a very thin wall, followed by a clear ring around a dense cluster of cells in the center of the egg.
S.stercoralis L1 larvae (Figure 4) are approximately 180-380 μm long and have a very short buccal cavity. A distinct genital primordium (highlighted with a blue arrow in Figure 4) can be observed and the tail of the larvae (highlighted with a red arrow in Figure 4) has a sharp end.
As the true impact of STH infections on the physical fitness of children is still being debated13,14,15, there is no representative result for their impact at this point. Interpretation of 20-m shuttle run test results can be performed in various ways. The physical fitness of children can be compared between infected and non-infected subjects, who are similar in terms of age and sex, other potentially relevant health conditions, socioeconomic status, and cultural habits. This can be done in a cross-sectional survey13,14,15 where the STH infection status and physical fitness of infected and non-infected children are assessed at a particular time point. The strength of the evidence will be higher when following a randomized controlled trial design where the physical fitness of school-aged children is assessed before and after anthelminthic/placebo treatment, allowing for both short- and long-term evaluation. One can further try to identify a possible dose-response relationship between the intensity of STH infections and physical fitness and compare different levels of multiparasitism1 with physical fitness. In the work presented by Yap and co-workers15, physical fitness, as depicted by VO2 max, was found to be significantly reduced in children infected with T.trichiura compared to children who were not infected with T.trichiura (Figure 5).
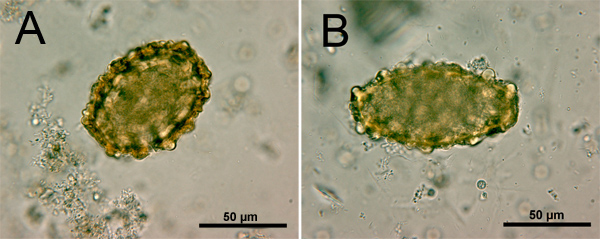
Figure 1. Microscopic images of A.lumbricoides eggs: fertilized (A) and non-fertilized (B) under 500x magnification. Scale bar = 50 μm.
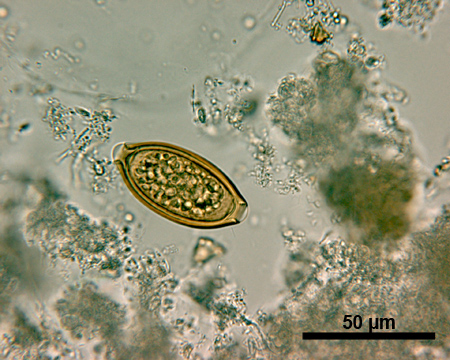
Figure 2. Microscopic image of a T.trichiura egg under 500x magnification. Scale bar = 50 μm.
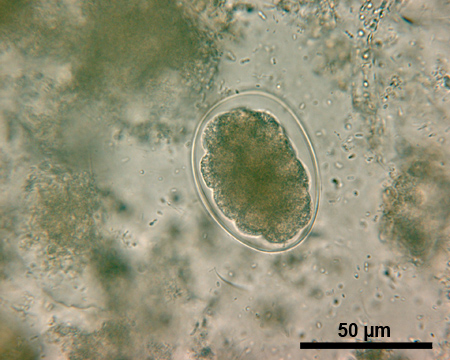
Figure 3. Microscopic image of a hookworm egg under 500x magnification. Scale bar = 50 μm.
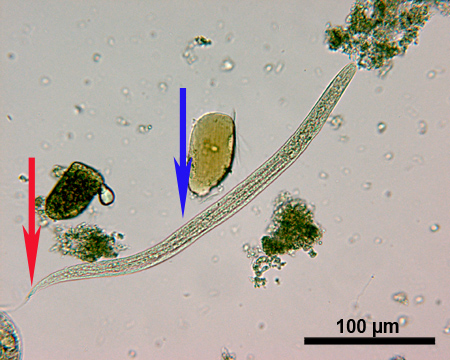
Figure 4. Microscopic image of a S.stercoralis L1 larva under 250x magnification. Arrows indicate tail (red) and genital primordium (blue). Scale bar = 100 μm.
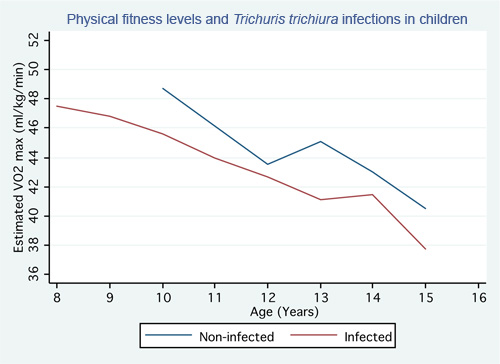
Figure 5. Graph showing the physical fitness level of children infected with T.trichiura compared to the physical fitness level of children not infected with T. trichiura.
Discussion
The three protocols described in this paper have been tested and executed in the south-western part of Yunnan province, People's Republic of China among members of the Bulang ethnic minority21 and in different parts of Africa6,13,14.
There are several templates available for the Kato-Katz technique. Each distinct hole-size will deliver a distinct amount of fecal material. For the calculation of EPGs, it is therefore necessary to know the unit of the template, so as to mul...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors are grateful to the communities in Yunnan province, People's Republic of China and Agboville, Côte d'Ivoire, for their participation in the studies described above. We are indebted to the local field teams that have assisted in the testing and execution of the protocols. We are particularly grateful to the following colleagues from the Swiss Tropical and Public Health Institute: Yvette Endriss for providing the microscopic images, Hanspeter Marti and his team for laboratory support and Stefanie Krauth for her assistance during the video filming. Finally, we thank the two anonymous referees for a series of useful comments.
Materials
| Name | Company | Catalog Number | Comments |
Note: Materials in this list are generic and can be obtained from different sources. | |||
| Kato-Katz kit [400 plastic templates with a hole of 6 millimeter (mm) (diameter) in a 1.5 mm thick template; 400 plastic spatula; a 20-m role of nylon screen of 80 mesh size; a 20-m role of hydrophilic cellophane, 34 μm thick.] | Vestergaard Frandsen | Please note that the glycerin-malachite green solution is not included in this kit but can be bought from any chemical supplier. | |
| Glass funnel [about 8 centimeter (cm) wide and 6 cm deep] | The glass funnel for the Baermann technique should be able to hold about 60 mL of water. The size of the rubber hose and gauze is dependent on the type of glass funnel used. | ||
| Biological microscope | Olympus | CX21LED/CX21 | |
| Centrifuge | Shanghai Medical Instruments Group | 80 - 2T | |
| Pre-recorded signals | Bitworks Design | Team Bleep Test Version 1.3.1 | iPhone application |
| Heart rate monitor | POLAR | FT1 | Watch and transmitter |
References
- Steinmann, P., Utzinger, J., Du, Z. W., Zhou, X. N. Multiparasitism: a neglected reality on global, regional and local scale. Advances in Parasitology. 73, 21-50 (2010).
- Bethony, J., Brooker, S., Albonico, M., Geiger, S., Loukas, A., Diemert, D., Hotez, P. J. The soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 367, 1521-1532 (2006).
- Hotez, P. J., Molyneux, D. H., Fenwick, A., Kumaresan, J., Ehrlich Sachs, S., Sachs, J. D., Savioli, L. Control of neglected tropical diseases. New England Journal of Medicine. 357, 1018-1027 (2007).
- Chan, M. S. The global burden of intestinal nematode infections-fifty years on. Parasitology Today. 13, 438-443 (1997).
- Olsen, A., van Lieshout, L., Marti, H., Polderman, T., Polman, K., Steinmann, P., Stothard, R., Thybo, S., Verweij, J. J., Magnussen, P. Strongyloidiases - the most neglected of the neglected tropical diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene. 103, 967-972 (2009).
- Becker, S. L., Sieto, B., Silue, K. D., Adjossan, L., Kone, S., Hatz, C., Kern, W. V., N'Goran, E. K., Utzinger, J. Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Neglected Tropical Diseases. 5, e1292 (2011).
- World Health Organization. Action against worms. WHO Newsletter. (11), (2008).
- Montresor, A., Crompton, D. W. T., Hall, A., Bundy, D. A. P., Savioli, L. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. World Health Organization. , (1998).
- García, L. S., Bruckner, D. A. . Diagnostic medical parasitology. , (2001).
- Brooker, S. Estimating the global distribution and disease burden of intestinal nematode infection: adding up the numbers - a review. International Journal for Parasitology. 40, 1137-1144 (2010).
- Stephenson, L. S., Latham, M. C., Kinoti, S. N., Kurz, K. M., Brigham, H. Improvements in physical fitness of Kenyan schoolboys infected with hookworm, Trichuris trichiura and Ascaris lumbricoides following a single dose of albendazole. Transactions of the Royal Society of Tropical Medicine and Hygiene. 84, 277-282 (1990).
- Stephenson, L. S., Latham, M. C., Adams, E. J., Kinoti, S. N., Pertet, A. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. Journal of Nutrition. 123, 1036-1046 (1993).
- Bustinduy, A. L., Thomas, C. L., Fiutem, J. J., Parraga, I. M., Mungai, P. L., Muchiri, E. M., Mutuku, F., Kitron, U., King, C. H. Measuring fitness of Kenyan children with polyparasitic infections using the 20-meter shuttle run test as a morbidity metric. PLoS Neglected Tropical Diseases. 5, e1213 (2011).
- Müller, I., Coulibaly, J. T., Fürst, T., Knopp, S., Hattendorf, J., Krauth, S. J., Stete, K., Righetti, A. A., Glinz, D., Yao, A. K., Pühse, U., N'Goran, E. K., Utzinger, J. Effect of schistosomiasis and soil-transmitted helminth infections on physical fitness of school children in Côte d'Ivoire. PLoS Neglected Tropical Diseases. 5, e1239 (2011).
- Yap, P., Du, Z. W., Chen, R., Zhang, L. P., Wu, F. W., Wang, J., Wang, X. Z., Zhou, H., Zhou, X. N., Utzinger, J., Steinmann, P. Soil-transmitted helminth infections and physical fitness in school-aged Bulang children of southwest China: results from a cross-sectional survey. Parasites and Vectors. 5, 50 (2012).
- Léger, L. A., Mercier, D., Gadoury, C., Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. Journal of Sports Sciences. 6, 93-101 (1988).
- Tomkinson, G. R., Léger, L. A., Olds, T. S., Cazorla, G. Secular trends in the performance of children and adolescents (1980-2000): an analysis of 55 studies of the 20 m shuttle run test in 11 countries. Sports Medicine. 33, 285-300 (2003).
- Mahon, A. D., Marjerrison, A. D., Lee, J. D., Woodruff, M. E., Hanna, L. E. Evaluating the prediction of maximal heart rate in children and adolescents. Research Quarterly for Exercise and Sport. 81, 466-471 (2010).
- Bogitsh, B. J., Carter, C. E., Oeltmann, T. N. . Human Parasitology. , (2011).
- Steinmann, P., Zhou, X. N., Du, Z. W., Jiang, J. Y., Wang, L. B., Wang, X. Z., Li, L. H., Marti, H., Utzinger, J. Occurence of Strongyloides stercoralis in Yunnan province, China, and comparison of diagnostic methods. PLoS Neglected Tropical Diseases. 1, e75 (2007).
- Martin, L. K., Beaver, P. C. Evaluation of Kato thick-smear technique for quantitative diagnosis of helminth infections. American Journal of Tropical Medicine and Hygiene. 17, 382-391 (1968).
- Dacombe, R. J., Crampin, A. C., Floyd, S., Randall, A., Ndhlovu, R., Bickle, Q., Fine, P. E. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 101, 140-145 (2007).
- Knopp, S., Mgeni, A. F., Khamis, I. S., Steinmann, P., Stothard, J. R., Rollinson, D., Marti, H., Utzinger, J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PloS Neglected Tropical Diseases. 2, e331 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved