A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Recovery of Adult Zebrafish Hearts for High-throughput Applications
In This Article
Summary
Use of zebrafish for cardiovascular research is expanding towards research on adult hearts. For these applications, quick and simple isolation of cardiac tissues is key to avoid post-mortem changes and to obtain an adequate number of samples. Here, we describe a fast and reproducible method for dissecting adult zebrafish hearts.
Abstract
Use of the zebrafish model system for studying development, regeneration, and disease is expanding toward use of adult hearts for cell dissociation and purification of RNA, DNA, and proteins. All of these applications demand the rapid recovery of significant numbers of zebrafish hearts to avoid gene regulatory, metabolic, and other changes that begin after death. Adult zebrafish hearts are also required for studying heart structure for a variety of mutants and for studying heart regeneration. However, the traditional zebrafish heart dissection is slow and difficult and requires specialized tools, making large-scale dissection of adult zebrafish hearts tedious. Traditional methods also harbor the risk of damaging the heart during the dissection. Here, we describe a method for dissection of adult zebrafish hearts that is fast, reproducible, and preserves heart architecture. Furthermore, this method does not require specialized tools, is painless for the zebrafish, can be performed on fresh or fixed specimens, and can be performed on zebrafish as young as one month old. The approach described expands the use of adult zebrafish for cardiovascular research.
Introduction
Zebrafish are an excellent model for studying heart development and human disease1,2. Specific advantages include the translucent nature of zebrafish embryos, the availability of many genetic mutants and transgenic reporter lines, and the availability of genome editing technologies. In addition to their advantages for studying early heart development, zebrafish are an ideal system for studying vertebrate heart regeneration3.
More recently, adult zebrafish are playing an important part in bioinformatics approaches to studying cardiovascular development and disease, due to their relatively large clutch size and relatively quick and inexpensive breeding compared to other vertebrate models. Promising techniques include ribosome profiling, RNA-Seq, and cell dissociation and FACS sorting4-7. However, for these techniques the quality of the data can depend on obtaining a large number of samples in a rapid, efficient, and reproducible manner, before gene regulatory, metabolic, transcriptional, and other changes occur.
Dissection of adult zebrafish organs has been described in the past8,9. However, previous approaches to dissection of the heart were slow, ran the risk of damaging the heart during dissection, required special tools, and/or required fixation of the zebrafish prior to dissection; for these reasons, past approaches to zebrafish adult heart dissection were not optimized for high-throughput applications and/or applications requiring fresh tissue.
Here, we describe a method for adult zebrafish heart dissection that is simple, fast, efficient, and reproducible, while preserving cardiac morphology. This method does not include cutting into the pericardial space and therefore does not risk damaging the heart during dissection. Instead, this method relies on anatomical landmarks of the zebrafish, and therefore, it is highly reproducible. This dissection method is also versatile in that it can be used on fresh or fixed fish, and on zebrafish as young as one month old. Finally, this method results in minimal suffering to the zebrafish because after anesthesia and/or rapid cooling, the fish is additionally decapitated and pithed in the course of the dissection procedure.
Protocol
NOTE: Always be sure that IACUC or ethics committee approval is in place before beginning any experimental procedure using zebrafish.
1. Prepare reagents and setup
- Prepare the following solutions. Find the recipes for all of these solutions in standard zebrafish manuals10.
- 500 ml of Egg Water
- 200 ml of 0.03% Tricaine in Egg Water
- 100 ml of 1x Phosphate-Buffered Saline (PBS)
- RBC Lysis Buffer (optional)
- Destination buffer of choice (fixative, Trizol, PBS, etc.) in a 1.5 ml microfuge tube on ice
NOTE: Fixative and Trizol are toxic if they come into contact with skin. Wear gloves when handling these substances.
- Prepare dissection setup:
- Position the gooseneck light source over the dissecting microscope. Place the lid of a 9 cm Petri dish on the dissecting microscope stage, and pour a thin layer of 1x PBS into it (the lid is used because the dish itself is too deep for an easy dissection).
- Place the Petri dish itself (not the lid) on the countertop near the dissecting scope and pour approximately 15 ml of 1x PBS in it. Use this to wash the heart after dissection. Alternatively, use RBC Lysis Buffer.
- Place razor blade, two pairs of forceps, microscissors (optional), and a transfer pipette beside the microscope. Obtain a container of ice if euthanasia by rapid cooling is chosen.
- Pour 200 ml of 0.03% Tricaine in Egg Water into a 250 ml glass beaker if euthanasia by Tricaine is chosen. Prepare a small biohazard bag for disposal of zebrafish carcasses.
2. Prepare Zebrafish
- For fixed fish, bring fixed fish, rinsed in PBS, to the microscope setup.
- Euthanize the whole adult zebrafish by rapid cooling with ice for >10 min.
- Use forceps to make a hole in the skin over the peritoneum (away from the heart) to aid penetration of fixative, and then put them in 4% paraformaldehyde in Fix Buffer10 for 2 hr at RT or O/N at 4 °C.
- Alternatively, for fresh fish, place fish to be euthanized in a small holding tank and bring to the microscope setup. Depending on the individual facility’s fish protocol and the downstream uses for the dissected fish hearts, anesthetize fish with Tricaine or euthanize by rapid cooling before decapitation.
- To use ice, pick up one fish with the fish net and place in ice water until the fish stops moving, approximately 5 min.
- Alternatively, to use Tricaine, pick up one fish with the fish net and place in the beaker of the solution until gill movements stop.
- Quickly pick up the euthanized fish by its tail fin and lay it on its side in the Petri dish cover that was filled with 1x PBS. Use the forceps to lift a pectoral fin with one hand, while using the razor blade to decapitate the fish with the other hand, just posterior to the attachment of the pectoral fin (Figure 1A). Perform this step either by looking through the microscope or by just directly visualizing the fish on the microscope stage.
NOTE: Freshly euthanized fish will still bleed after decapitation.
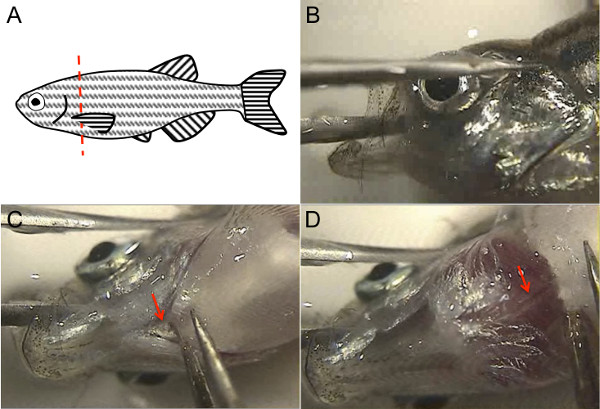
Figure 1. Zebrafish adult heart dissection utilizes zebrafish anatomical landmarks. (A) To decapitate the fish, lift the pectoral fin with a forceps and use a sharp clean razor blade along the red dotted line as shown. (B) To steady the fish head, place one tine of the forceps in the fish mouth while the other tine lies across the eye, and then turn the fish head so that the ventral surface is up and both tines of the forceps are stable against the bottom of the Petri dish. (C) Use the free forceps to cut the attachment of the operculum (arrow). (D) Lifting this, the dorsal aorta is visible as a white structure with a pink stripe denoting luminal blood (arrow). Please click here to view a larger version of this figure.
3. Dissect the Heart
- Visualize the fish head under the microscope. With one forceps, steady the fish head by placing one tine of the forceps in the fish mouth, through the brain, while the other tine is outside the head, across the eye (Figure 1B). Ensure both tines of the forceps are steady against the bottom of the petri dish. Holding the fish head this way, its ventral surface should be facing upwards.
- With the other hand, use the second forceps to cut the ventral attachment of the operculum to the body (arrow, Figure 1C). Lifting this slightly with the forceps, the dorsal aorta will appear underneath as a white structure with a red stripe of blood in the aortic lumen (arrow, Figure 1D). Cut the dorsal aorta with the forceps by pinching the aorta and pulling upwards; alternatively, use microscissors to cut the dorsal aorta.
- Now, use the second forceps to grasp the pectoral fin from its base, including the body cartilage at the base of the fin, and lift this off. Repeat with the other pectoral fin. Occasionally, the heart comes out with the pectoral fins, so examine these pieces to make sure the heart is not attached before discarding them.
NOTE: At this point, the heart should be visible, still intact and connected to the remaining fish head (although occasionally it comes off with the pectoral fins). The heart can be identified by its shape, its pink color, and its being surrounded by pigmented pericardium. Because euthanasia of the fish is performed quickly, freshly euthanized fish hearts may still be beating slowly. - Let go of the fish head with the first forceps, so that both hands hold forceps and are free. Use both forceps to tease the heart away from the pericardium. Grasp the heart by the remainder of the dorsal aorta while the remaining pericardium is pulled away.
NOTE: Whether fixed or fresh, the heart is robust to gentle pulling while the surrounding connective tissue is more friable. Therefore, any surrounding pericardial tissue can be dissected away with the heart remaining intact. - Discard the leftover fish carcass in the biohazard bag.
4. Prepare the Heart for Downstream Applications
- Using forceps, grasp the heart by the edge of the dorsal aorta/bulbus arteriosus and place the heart in the fresh dish of 1x PBS. Alternatively, use a transfer pipette. Move the heart back and forth in the PBS about 10x to wash away as much blood as possible.
- For applications in which it is important to remove all possible blood cells, wash in a 9 cm Petri dish with 15 ml RBC Lysis Buffer instead of PBS, using the same back-and-forth motion. If preserving the structure of the heart is not important for the downstream application (e.g., making RNA), use the forceps or microscissors to open the heart cavity and facilitate rinsing away of blood cells.
- For applications in which separate heart chambers are desired, use forceps or microscissors to dissect the bulbus arteriosus, atrium, and ventricle apart from each other.
- Transfer the heart, or separate chambers, to the destination buffer on ice.
NOTE: Common destinations include buffers for cell dissociation, 4% paraformaldehyde, or Trizol.
Results
Using this method, an adult zebrafish heart can be dissected in less than 1 min, compared to over 5 min using traditional methods8. Hearts dissected using this method are reliably intact (Figure 2A), while traditional methods8 require cutting blindly into the pericardium and therefore commonly cause damage or loss of the atrium or bulbus arteriosus (Figure 2B). Hearts dissected maintain their structural integrity and are suitable for histology (Figure 2C
Discussion
While methods for dissecting the adult zebrafish heart have been described, these methods were time-consuming and commonly caused damage to the heart during dissection. To perform experiments where a large number of adult hearts may be needed, and/or when avoiding degradation of heart tissue is important for downstream applications, the time required using traditional dissection techniques is prohibitive. Similarly, reproducibly obtaining undamaged, intact hearts is important for study of heart structure and for immunohi...
Disclosures
The authors have no disclosures.
Acknowledgements
The authors would like to thank Dr. Shaun Coughlin for hosting the filming of this procedure in his laboratory, and for general support. R.A. was supported by the NIH (F32HL110489) and the Sarnoff Cardiovascular Research Foundation. S.R. was supported by a Research Fellowship of the Deutsche Forschungsgemeinschaft (DFG) and the American Heart Association (AHA). D.Y.R.S was supported by the NIH (RO1HL54737), the Packard Foundation, and the Max Planck Society.
Materials
| Name | Company | Catalog Number | Comments |
| Small tank for transporting fish | Aquaneering | ZHCT100 | |
| Fish net | Petsmart | 36-16731 | |
| 250 ml glass beaker | Kimble | 14005-250 | |
| 9 cm polystyrene Petri dish | Nunc | 172958 | |
| Razor blade | Personna American Safety Razor Company | 94-120-71 | |
| 2 Dumont #5SF forceps | Fine Science Tools | 11252-00 | |
| Dissecting microscope | Olympus | SZX16 | |
| Tricaine | Sigma | A-5040 | |
| Plastic transfer pipette | Thermo Scientific | 202-20S | |
| Gooseneck light source | Dolan-Jenner Industries, Inc | Fiber-Lite 180 Illuminator, 181 Dual Gooseneck System | |
| Fluorescent light source | Lumen Dynamics | X-Cite 120Q | optional |
| Micro-scissors | Biomedical Research Instruments, Inc | 11-1000 | optional |
| RBC lysis buffer | eBioscience | 00-4333-57 | optional |
References
- Arnaout, R., et al. Zebrafish model for human long QT syndrome. Proceedings of the National Academy of Sciences of the United States of America. 104 (27), 11316-11321 (2007).
- Chi, N. C., et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biology. 6 (5), 109 (2008).
- Poss, K. D. Getting to the heart of regeneration in zebrafish. Seminars in Cell & Developmental Biology. 18 (1), 36-45 (2007).
- Fang, Y., et al. Translational profiling of cardiomyocytes identifies an early Jak1/Stat3 injury response required for zebrafish heart regeneration. Proceedings of the National Academy of Sciences of the United States of America. 110 (33), 13416-13421 (2013).
- Manoli, M., Driever, W. Fluorescence-activated cell sorting (FACS) of fluorescently tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harbor Protocols. 2012 (8), (2012).
- Cannon, J. E., et al. Global analysis of the haematopoietic and endothelial transcriptome during zebrafish development. Mechanisms of Development. 130 (2-3), 122-131 (2013).
- Wang, Z., Gerstein, M., Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nature Reviews Genetics. 10 (1), 57-63 (2009).
- Gupta, T., Mullins, M. C. Dissection of organs from the adult zebrafish. Journal of Visualized Experiments. 37, (2010).
- Singleman, C., Holtzman, N. G. Heart dissection in larval, juvenile and adult zebrafish, Danio rerio. Journal of Visualized Experiments. 55, (2011).
- Westerfield, M. . The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio. , (1993).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved