A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Measuring Protein Stability in Living Zebrafish Embryos Using Fluorescence Decay After Photoconversion (FDAP)
In This Article
Summary
Protein levels in cells and tissues are often tightly regulated by the balance of protein production and clearance. Using Fluorescence Decay After Photoconversion (FDAP), the clearance kinetics of proteins can be experimentally measured in vivo.
Abstract
Protein stability influences many aspects of biology, and measuring the clearance kinetics of proteins can provide important insights into biological systems. In FDAP experiments, the clearance of proteins within living organisms can be measured. A protein of interest is tagged with a photoconvertible fluorescent protein, expressed in vivo and photoconverted, and the decrease in the photoconverted signal over time is monitored. The data is then fitted with an appropriate clearance model to determine the protein half-life. Importantly, the clearance kinetics of protein populations in different compartments of the organism can be examined separately by applying compartmental masks. This approach has been used to determine the intra- and extracellular half-lives of secreted signaling proteins during zebrafish development. Here, we describe a protocol for FDAP experiments in zebrafish embryos. It should be possible to use FDAP to determine the clearance kinetics of any taggable protein in any optically accessible organism.
Introduction
The levels of proteins in cells and organisms are determined by their rates of production and clearance. Protein half-lives can range from minutes to days1-4. In many biological systems, the stabilization or clearance of key proteins has important effects on cellular activity. Modulation of intracellular protein stability is required for cell cycle progression5,6, developmental signaling7-9, apoptosis10, and normal function and maintenance of neurons11,12. Extracellular protein stability affects the distribution and availability of secreted proteins, such as morphogens13,14, within a tissue.
Over the last few decades, protein stability has mainly been assessed in cell culture using radioactive pulse-labeling or cycloheximide chase experiments15. In such pulse-chase experiments, cells are either transiently exposed to a “pulse” of radioactive amino acid precursors that are incorporated into newly synthesized proteins, or they are exposed to cycloheximide, which inhibits protein synthesis. Cultured cells are then collected at different time points, and either immunoprecipitation followed by autoradiography (in radioactive pulse-chase experiments) or western blotting (in cycloheximide experiments) is used to quantify the clearance of protein over time.
Conventional protein stability assays have several shortcomings. First, proteins in these assays are often not expressed in their endogenous environments, but rather in tissue culture and sometimes in cells from different species. For proteins whose stability is context-dependent, this approach is problematic. Second, it is not possible to follow protein clearance in individual cells or organisms over time, and the data from these assays reflects an average of different populations of cells at different time points. Since individual cells may have started with different amounts of protein, may have taken up the radioactive label or cycloheximide at different times, or may have different clearance kinetics, such aggregate data could be misleading. Finally, in the case of cycloheximide chase experiments, addition of the protein synthesis inhibitor may have unintended physiological effects that could artificially alter protein stability16-18. These shortcomings can be avoided by using Fluorescence Decay After Photoconversion (FDAP), a technique that utilizes photoconvertible proteins to measure protein clearance dynamically in living organisms19-25 (see Discussion for limitations of the FDAP technique).
Photoconvertible proteins are fluorescent proteins whose excitation and emission properties change after exposure to specific wavelengths of light26. One commonly used variant is Dendra2, a “green-to-red” photoconvertible protein that initially has excitation and emission properties similar to green fluorescent proteins, but after exposure to UV light—“photoconversion”—its excitation/emission properties become similar to those of red fluorescent proteins23,27. Importantly, new protein produced after photoconversion will not have the same excitation/emission properties as the photoconverted protein, allowing decoupling of production and clearance upon photoconversion and observation of only a pool of photoconverted protein. Tagging proteins of interest with photoconvertible proteins thus provides a convenient way to pulse-label proteins in intact, optically accessible living organisms.
In FDAP assays (Figure 1A), a protein of interest is tagged with a photoconvertible protein and expressed in a living organism (Figure 1B). The fusion protein is photoconverted, and the decrease in photoconverted signal over time is monitored by fluorescence microscopy (Figure 1C). The data is then fitted with an appropriate model to determine the half-life of the fusion protein (Figure 1D).
The FDAP assay described here was designed to determine the extracellular half-lives of secreted signaling proteins in zebrafish embryos during early embryogenesis19. However, this approach can be adapted to any transparent model organism that tolerates live imaging, and could be used to monitor the clearance of any taggable intracellular or extracellular protein. Variations of the technique described here have been performed in cultured cells20,23 and Drosophila22 and mouse21 embryos.
Protocol
1. Generating a Photoconvertible Fusion Construct and Injecting Dechorionated Zebrafish Embryos
- Generate a functional construct containing the protein of interest fused to a green-to-red photoconvertible protein (see Discussion), then use in vitro transcription to generate capped mRNA encoding the fusion protein as in Müller et al., 201219.
- Use pronase to remove the chorions from about 30 zebrafish embryos at the one-cell stage. Alternatively, manually dechorionate embryos using forceps28.
Note: Embryos must be dechorionated for subsequent imaging. If desired, embryos can be injected through the chorion and dechorionated later, just prior to imaging.- Make a 5 mg/ml stock solution of pronase from Streptomyces griseus in standard zebrafish embryo medium19. Rock the solution gently at RT for 10 min to allow the protease to dissolve. Aliquot 2 ml into microcentrifuge tubes and freeze at -20 °C.
- Transfer one-cell stage embryos to a 5 cm diameter glass or agarose-coated plastic Petri dish containing ~8 ml embryo medium. Add 2 ml of thawed pronase stock solution to the dish and incubate at RT for 5 to 10 min.
- Avoid exposing embryos to air or plastic, as contact with either will cause dechorionated embryos to rupture. Fill a 200 ml glass beaker with embryo medium. Transfer the embryos to the beaker by tilting the Petri dish while submerging it in the medium.
- After the embryos have settled to the bottom of the beaker, pour out most of the embryo medium, then pour fresh embryo medium into the beaker. The mild swirling of the medium pouring into the beaker causes embryos to lose their weakened chorions.
- Repeat step 1.2.4.
- Transfer the dechorionated embryos to an agarose-coated injection dish29 using a glass Pasteur pipette with a flamed tip. Flaming the pipette tip prevents jagged edges from injuring embryos.
- Co-inject the mRNA and a 3 kDa Alexa488-dextran conjugate29,30 (Figure 1B; see Discussion for suggested mRNA and Alexa488-dextran amounts). Inject directly into the center of the cell (not the yolk) to ensure even distribution of mRNA and fluorescent dye once cleavage commences.
Note: The Alexa488 signal will be used during data analysis to generate compartmental masks in order to distinguish between intracellular and extracellular fluorescence. - Transfer injected embryos to a 1–2% agarose-coated well of a six-well plastic dish filled with embryo medium. Incubate in the dark at 28 °C until embryos have reached late sphere stage31 (approximately 5 hr post fertilization). Check embryos every one to 2 hr under a stereomicroscope and remove any debris generated by embryos that have died.
2. Mounting Zebrafish Embryos for Photoconversion and Imaging on an Inverted Confocal Microscope
- Use a stereomicroscope to identify one to five healthy embryos, and use a glass Pasteur pipette with a flamed tip to remove them from the dish.
- Gently eject the embryos into a microcentrifuge tube containing ~1 ml of melted 1% low melting point agarose in 1x Danieau’s embryo medium (see Materials List) (Figure 2A).
Note: Agarose should have a temperature between 40 and 42 °C; higher temperatures could damage the embryos. - Draw the embryos back into the pipette along with some agarose. Gently eject the agarose and embryos onto the cover glass of a glass-bottom dish (Figure 2B). Ensure that the thickness of the cover glass is compatible with the objective on the confocal microscope.
- Re-use the glass pipette if desired. To clean the residual agarose out of the pipette and prevent clogging, quickly pipette embryo medium up and down. Place a 15 ml tube filled with ~5 ml of embryo medium next to the stereomicroscope for this purpose.
- Use a metal probe to position the embryos so that the animal pole (blastoderm) faces the cover glass. Work quickly since the agarose will solidify in 20–30 sec. Use the stereomicroscope to monitor the embryos’ positions and readjust as necessary until the agarose hardens.
- Repeat steps 2.1–2.4 until the desired number of embryos has been mounted.
Note: In a typical experiment, four agarose drops containing four or five embryos each will fit easily on the cover glass. About 16 embryos can be imaged during a single ideal experiment (Figure 2C). - When the agarose has solidified, fill the glass-bottom dish with 1x Danieau’s embryo medium.
3. Photoconverting and Measuring the Decrease of the Photoconverted Signal
A 25X or 40X water objective is appropriate for the size and refractive index of zebrafish embryos. It is best to use immersion oil with the same refractive index as water rather than actual water, since water will evaporate during the course of the five-hour experiment. Ensure that the immersion oil is designed to be used with a water (not oil) objective.
- Place a large drop of immersion oil on the objective to ensure that the oil film between the objective and cover glass will not break as the stage moves to different embryo positions during imaging. Securely place the glass-bottom dish onto the stage so that the dish will not shift when the stage moves. If possible, use a heated stage at 28 °C, the optimal temperature for zebrafish development.
- Define each embryo’s position in the confocal microscope’s software package. Adjust the z-depth for each embryo, and attempt to target roughly the same plane in each embryo.
Note: About 30 μm from the animal pole is a good depth since at this depth the enveloping layer of the embryo can be avoided, imaging area is maximized, and light scattering is minimal. A single optical slice with a thickness of ~3.3 μm provides sufficient data; there is no need to acquire a z-stack (see Section 5). - Collect two signals during the experiment: the “green” signal from the Alexa488-dextran conjugate—which will be used during data analysis to isolate extracellular and intracellular fluorescence—and the “red” signal from the fusion protein after it is photoconverted.
- Excite Alexa488 using a 488 nm laser, and collect emitted fluorescence between ~500 and 540 nm. Note: After photoconversion, many green-to-red photoconvertible proteins (e.g., Dendra2) can be excited with a 543 nm laser and emit fluorescence between ~550 and 650 nm. Adjust as necessary based on the photoconvertible protein used.
- Acquire “pre-photoconversion” images, and configure the confocal microscope’s software to image each of the previously defined positions (from step 3.2) with the appropriate imaging conditions every 10 or 20 min for a five-hour time course (see Section 5 and Discussion).
- To photoconvert the fusion protein, switch to a 10X objective and expose groups of embryos to UV light from a mercury arc lamp with a ~300–400 nm excitation filter at 100% output for 2 min. Shift the focus along the z-axis to promote uniform photoconversion (see Section 5). Ensure that the immersion oil does not drip onto the 10x objective during photoconversion.
Note: The shifting of focus during photoconversion could be automated in order to avoid variability among experimenters. - Switch back to the 25X or 40X objective immediately after photoconversion. Ensure that the previously defined positions from step 3.2 are still accurate. If the dish shifted during photoconversion, re-define the positions of the embryos.
- Start the program created in step 3.4 and allow imaging to continue for 5 hr. Note the time elapsed between photoconversion and the start of imaging for each embryo.
- Occasionally check on the experiment. Monitor the level of Danieau’s medium and add more if necessary. Restart the software if it has stalled.
- In order to determine the background fluorescence values that will be used during data analysis to estimate the asymptote of an exponentially decreasing model, include some embryos that have been injected with Alexa488-dextran but not mRNA in the experiment. To determine the instrument noise, which will also be used during subsequent data analysis, acquire an image in the absence of a sample.
4. Analyzing the Data Using PyFDAP
- Visually inspect the time course data sets from each embryo. Discard data sets from embryos that died during imaging, that shifted significantly, that have very low levels of photoconverted signal, or that contain regions of cells that look unusual and have stopped moving and dividing (typical of injured or sick embryos).
Note: Occasionally, bubbles in the immersion oil or other artifacts will appear in one or two images in an otherwise usable data set. Note any images that contain artifacts; they will be discarded later, and the remaining time points from such a data set can still be analyzed. - Use the Python-based software package PyFDAP to analyze the FDAP data. PyFDAP calculates half-lives by determining the average intracellular and extracellular red fluorescence intensity in each image and fitting the data with an exponentially decreasing function56 (Figure 3).
- Download PyFDAP (see Materials List).
- Use PyFDAP to separate intracellular and extracellular photoconverted signal (Figure 3A,B). Use the Alexa488 signal, which is strictly intracellular, to create an intracellular mask. Apply this mask to the corresponding red channel image to prevent intracellular pixels from being considered when calculating average extracellular intensity. To measure average intracellular intensity, invert the mask.
- In PyFDAP, display the masked images generated in step 4.2.2. Visually inspect these images and discard data sets in which masks do not accurately distinguish intracellular from extracellular space (this should be rare; note that cell membranes are included in images in which extracellular space has been masked, but they could be removed by altering the thresholding algorithm or by introducing a membrane mask (e.g., using membrane-CFP)). Also discard any single images containing artifacts (e.g., bubbles in the immersion oil) identified in step 4.1.
- Use PyFDAP to calculate average extracellular and intracellular fluorescence intensities for each image. PyFDAP calculates these averages by summing the intensities of pixels that fall outside of the mask and dividing by the total number of pixels summed.
- Fit the fluorescence data (Figure 3C) with the following exponential function:
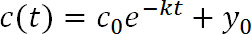
where t is time post-photoconversion, c(t) is intensity at a given value of t, c0 is the intensity at t = 0, k is the clearance rate constant, and y0 is the asymptote that the function approaches as fluorescence decreases (Figure 1D). y0 can be constrained based on the measurements in step 3.819. - Use PyFDAP to calculate the extracellular and intracellular protein half-lives (τ) from the clearance rate constants (k) using the following relationship:
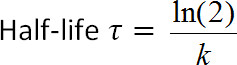
5. Control Experiments to Assess Photobleaching, Inadvertent Photoconversion, and Photoconversion Uniformity
- Assessing photobleaching
Note: Photobleaching could cause an artifactual decrease in fluorescence intensity that reflects the bleaching properties of the fluorescent protein in addition to the clearance of the protein of interest.- To assess possible photobleaching, perform one set of FDAP experiments with 10 min intervals between imaging and a second set with 20 min intervals between imaging (Figure 4). Analyze the data from both sets of experiments using PyFDAP as described in Section 4.
- Compare the half-lives from the 10 and 20 min interval experiments. Longer half-lives from 20 min interval experiments indicate significant photobleaching. If the half-lives from both experiments are identical, photobleaching is not a significant concern.
- Alternatively, assess photobleaching by acquiring a series of ~30 images immediately after photoconversion. A significant decrease in fluorescence intensity indicates significant photobleaching.
- If photobleaching is detected, use lower laser power, decrease imaging time, or consider using a more photostable photoconvertible protein32.
- Assessing inadvertent photoconversion.
Note: Dendra2 can be photoconverted using 488 nm illumination27. When exciting Alexa488 with the 488 nm laser as described in step 3.3.1, inadvertent photoconversion and therefore an artifactual increase in the apparent half-life of the protein of interest is possible. However, we and others33 have found that 488 nm illumination is an inefficient method of photoconversion in zebrafish embryos.- Use the control experiment described in step 5.1.1 to detect inadvertent photoconversion. Compare the half-lives from the 10 and 20 min interval experiments. Shorter half-lives from 20 min interval experiments indicate significant inadvertent photoconversion. If the half-lives from both experiments are identical, inadvertent photoconversion is not a significant concern.
- If inadvertent photoconversion is detected, use a lower 488 nm laser power and shorter imaging times to avoid inadvertently photoconverting Dendra2.
- Assessing photoconversion uniformity.
Note: If photoconversion is biased toward the animal pole of the embryo, the decrease in fluorescence will be influenced by protein diffusion or cell movement into deeper planes (Figure 5A).- To determine whether photoconversion is uniform, express a secreted photoconvertible protein (for experiments with extracellular fusion proteins) or a cytoplasmic photoconvertible protein (for experiments with intracellular fusion proteins). Photoconvert as usual, then acquire a z-stack encompassing most of the blastoderm every 20 min for 80 min.
- If photoconversion is biased toward the animal pole, the fluorescence intensity in deeper planes will increase over time due to diffusion or cell movement (Figure 5B). If non-uniform photoconversion is detected, focus deeper into the embryos during photoconversion.
Results
FDAP has been used to determine the half-lives of extracellular signaling proteins in zebrafish embryos19. One of these proteins, Squint, induces expression of mesendodermal genes during embryogenesis34. Squint-Dendra2 activates expression of mesendodermal genes at levels similar to untagged Squint, as demonstrated by qRT-PCR and in situ hybridization assays19. Embryos were co-injected with Alexa488-dextran and mRNA encoding Squint-Dendra2 and subjected to the FDAP assay. A decre...
Discussion
The success of an FDAP experiment relies on the generation of a functional photoconvertible fusion protein. Tagging a protein can affect its biological activity and/or biophysical properties, including its localization, solubility, and stability36-41. Be prepared to test the activity of several different fusion constructs in order to find one that is active. We have found that changing the position of the photoconvertible protein relative to the protein of interest or using longer linkers (e.g., using...
Disclosures
The authors have no conflicts to disclose.
Acknowledgements
The authors would like to thank Jeffrey Farrell, James Gagnon, and Jennifer Bergmann for comments on the manuscript. KWR was supported by the National Science Foundation Graduate Research Fellowship Program during the development of the FDAP assay. This work was supported by grants from the NIH to AFS and by grants from the German Research Foundation (Emmy Noether Program), the Max Planck Society, and the Human Frontier Science Program (Career Development Award) to PM.
Materials
| Name | Company | Catalog Number | Comments |
| PyFDAP (download from the following website: http://people.tuebingen.mpg.de/mueller-lab) | Install and operate using the instructions provided on the PyFDAP website; PyFDAP is compatible with Linux, Mac, and Windows operating systems. | ||
| mMessage mMachine Sp6 Transcription Kit | Life Technologies | AM1340 | To generate capped mRNA for injection into embryos |
| Alexa488-dextran conjugate, 3 kDa | Life Technologies | D34682 | Co-inject with mRNA to create intracellular and extracellular masks |
| 6-well plastic dish | BD Falcon | Incubate embryos in agarose-coated wells until ready for mounting | |
| Embryo medium | 250 mg/L Instant Ocean salt, 1 mg/L methylene blue in reverse osmosis water adjusted to pH 7 with NaHCO3 | ||
| Protease from Streptomyces griseus | Sigma | P5147 | Make a 5 mg/ml stock and use at 1 mg/ml to dechorionate embryos at the one-cell stage |
| 5 cm diameter glass Petri dish | For embryo dechorionation | ||
| 200 ml glass beaker | For embryo dechorionation | ||
| Microinjection apparatus | For injection of mRNA and dye into embryos at the one-cell stage | ||
| Stereomicroscope | For injecting and mounting embryos | ||
| 1x Danieau's medium | Dilute low melting point agarose and perform imaging in this medium; recipe: 0.2 mm filtered solution of 58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.3 mM CaCl2, 5 mM HEPES pH 7.2 | ||
| UltraPure low melting point agarose | Invitrogen | 16520-100 | For mounting embryos; use at a concentration of 1% in Danieau's medium: add 200 mg to 20 ml Danieau's medium, microwave until dissolved, then aliquot 1 ml into microcentrifuge tubes; aliquots can be stored at 4 °C, re-melted at 70 °C, and cooled to 40–42 °C when ready to use |
| Glass Pasteur pipette | Kimble Chase (via Fisher) | 63A53WT | For mounting embryos; flame the tip to prevent jagged edges from injuring embryos |
| Metal probe | For positioning embryos during mounting | ||
| Glass bottom dishes | MatTek | P35G-1.5-14-C | Use the appropriate cover glass thickness for your objective; part number listed here is for cover glass No. 1.5 |
| 15 ml tube filled with ~5 ml embryo medium | BD Falcon | For rinsing residual agarose from the Pasteur pipette | |
| Inverted laser scanning confocal microscope | A mercury arc lamp, 488 nm laser, 543 nm laser, and the appropriate filter sets are required | ||
| Heated stage | To maintain embryos at the optimal temperature of 28 °C during the experiment | ||
| Confocal software capable of time-lapse imaging | Must be able to define multiple positions and automatically image them at defined intervals | ||
| 25X or 40X water objective | Objective for imaging | ||
| 10X air objective | Objective for photoconversion | ||
| Immersion oil | Immersion oil with the same refractive index as water |
References
- Schwanhäusser, B., et al. Global quantification of mammalian gene expression control. Nature. 473 (7347), 337-342 (2011).
- Boisvert, F. M., et al. A Quantitative Spatial Proteomics Analysis of Proteome Turnover in Human Cells. Molecular & Cellular Proteomics. 11 (3), 011429 (2012).
- Belle, A., Tanay, A., Bitincka, L., Shamir, R., O'Shea, E. K. Quantification of protein half-lives in the budding yeast proteome. Proceedings of the National Academy of Sciences of the United States of America. 103 (35), 13004-13009 (2006).
- Eden, E., et al. Proteome Half-Life Dynamics in Living Human Cells. Science. 331 (6018), 764-768 (2011).
- Parry, D. H., O'Farrell, P. H. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Current Biology. 11 (9), 671-683 (2001).
- Holloway, S. L., Glotzer, M., King, R. W., Murray, A. W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 73 (7), 1393-1402 (1993).
- Dharmasiri, N., Estelle, M. Auxin signaling and regulated protein degradation. Trends Plant Sci. 9 (6), 302-308 (2004).
- MacDonald, B. T., Tamai, K., He, X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental Cell. 17 (1), 9-26 (2009).
- Chen, X., et al. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. The Journal of Cell Biology. 192 (5), 825-838 (2011).
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicologic Pathology. 35 (4), 495-516 (2007).
- Yi, J. J., Ehlers, M. D. Emerging Roles for Ubiquitin and Protein Degradation in Neuronal Function. Pharmacological Reviews. 59 (1), 14-39 (2007).
- Rubinsztein, D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 443 (7113), 780-786 (2006).
- Müller, P., Schier, A. F. Extracellular Movement of Signaling Molecules. Developmental Cell. 21 (1), 145-158 (2011).
- Eldar, A., Rosin, D., Shilo, B. -. Z., Barkai, N. Self-enhanced ligand degradation underlies robustness of morphogen gradients. Developmental Cell. 5 (4), 635-646 (2003).
- Zhou, P. Determining protein half-lives. Methods In Molecular Biology. 284, 67-77 (2004).
- Woodside, K. H. Effects of cycloheximide on protein degradation and gluconeogenesis in the perfused rat liver. Biochim Biophys Acta. 421 (1), 70-79 (1976).
- Schimke, R. T., Doyle, D. Control of enzyme levels in animal tissues. Annual Review of Biochemistry. 39, 929-976 (1970).
- Kenney, F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 156 (3774), 525-528 (1967).
- Müller, P., et al. Differential Diffusivity of Nodal and Lefty Underlies a Reaction-Diffusion Patterning System. Science. 336 (6082), 721-724 (2012).
- Kiuchi, T., Nagai, T., Ohashi, K., Mizuno, K. Measurements of spatiotemporal changes in G-actin concentration reveal its effect on stimulus-induced actin assembly and lamellipodium extension. The Journal of Cell Biology. 193 (2), 365-380 (2011).
- Plachta, N., Bollenbach, T., Pease, S., Fraser, S. E., Pantazis, P. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nature Cell Biology. 13 (2), 17-123 (2011).
- Drocco, J. A., Grimm, O., Tank, D. W., Wieschaus, E. Measurement and Perturbation of Morphogen Lifetime: Effects on Gradient Shape. Biophys J. 101 (8), 1807-1815 (2011).
- Zhang, L., et al. Method for real-time monitoring of protein degradation at the single cell level. BioTechniques. 42 (4), 446-450 (2007).
- Miyawaki, A. Proteins on the move: insights gained from fluorescent protein technologies. Nat Rev Mol Cell Biol. 12 (10), 636-668 (2011).
- Pantazis, P., Supatto, W. Advances in whole-embryo imaging: a quantitative transition is underway. Nat Rev Mol Cell Biol. 15 (5), 327-339 (2014).
- Lukyanov, K. A., Chudakov, D. M., Lukyanov, S., Verkhusha, V. V. Innovation: Photoactivatable fluorescent proteins. Nat Rev Mol Cell Biol. 6 (11), 885-891 (2005).
- Gurskaya, N. G., et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nature Biotechnology. 24 (4), 461-465 (2006).
- Zou, J., Wei, X. Transplantation of GFP-expressing Blastomeres for Live Imaging of Retinal and Brain Development in Chimeric Zebrafish Embryos. Journal of Visualized Experiments. (41), (2010).
- Yuan, S., Sun, Z. Microinjection of mRNA and Morpholino Antisense Oligonucleotides in Zebrafish Embryos. Journal of Visualized Experiments. (27), (2009).
- Rosen, J. N., Sweeney, M. F., Mably, J. D. Microinjection of Zebrafish Embryos to Analyze Gene Function. Journal of Visualized Experiments. (25), (2009).
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., Schilling, T. F. Stages of embryonic development of the zebrafish. Developmental Dynamics. 203 (3), (1995).
- McKinney, S. A., Murphy, C. S., Hazelwood, K. L., Davidson, M. W., Looger, L. L. A bright and photostable photoconvertible fluorescent protein. Nature Methods. 6 (2), 131-133 (2009).
- Dempsey, W. P., Qin, H., Pantazis, P. In Vivo Cell Tracking Using PhOTO Zebrafish. Methods in Molecular Biology. 1148, 217-228 (2014).
- Schier, A. F. Nodal Morphogens). Cold Spring Harbor Perspectives in Biology. 1 (5), a003459-a003459 (2009).
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 9 (1), 62-66 (1979).
- Pédelacq, J. -. D., Cabantous, S., Tran, T., Terwilliger, T. C., Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nature Biotechnology. 24 (1), 79-88 (2005).
- Swulius, M. T., Jensen, G. J. The Helical MreB Cytoskeleton in Escherichia coli MC1000/pLE7 Is an Artifact of the N-Terminal Yellow Fluorescent Protein Tag. Journal of Bacteriology. 194 (23), 6382-6386 (2012).
- Landgraf, D., Okumus, B., Chien, P., Baker, T. A., Paulsson, J. Segregation of molecules at cell division reveals native protein localization. Nature Methods. 9 (5), 480-482 (2012).
- Stadler, C., et al. Immunofluorescence and fluorescent-protein tagging show high correlation for protein localization in mammalian cells. Nature Methods. 10 (4), 315-323 (2013).
- Quattrocchio, F. M., Spelt, C., Koes, R. Transgenes and protein localization: myths and legends. Trends Plant Sci. 18 (9), 473-476 (2013).
- Morimoto, Y. V., Kojima, S., Namba, K., Minamino, T. M153R Mutation in a pH-Sensitive Green Fluorescent Protein Stabilizes Its Fusion Proteins. PLoS ONE. 6 (5), e19598 (2011).
- Shaner, N. C., Steinbach, P. A., Tsien, R. Y. A guide to choosing fluorescent proteins. Nature Methods. 2 (12), 905-909 (2005).
- Waters, J. C. Accuracy and precision in quantitative fluorescence microscopy. The Journal of Cell Biology. 185 (7), 1135-1148 (2009).
- Moll, U. M., Petrenko, O. The MDM2-p53 interaction. Mol Cancer Res. 1 (14), 1001-1008 (2003).
- Auer, T. O., Duroure, K., De Cian, A., Concordet, J. P., Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Research. 24 (1), 142-153 (2014).
- Bedell, V. M., et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 491 (7422), 114-118 (2012).
- Hruscha, A., et al. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development. 140 (24), 4982-2987 (2013).
- Hwang, W. Y., et al. Heritable and Precise Zebrafish Genome Editing Using a CRISPR-Cas System. PLoS ONE. 8 (7), 68708 (2013).
- Hwang, W. Y., et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature Biotechnology. 31 (3), 227-229 (2013).
- Zu, Y., et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nature Methods. 10 (4), 329-331 (2013).
- Keller, P. J., Schmidt, A. D., Wittbrodt, J., Stelzer, E. H. K. Reconstruction of Zebrafish Early Embryonic Development by Scanned Light Sheet Microscopy. Science. 322 (5904), 1064-1069 (2008).
- Blanchet, M. H., et al. Cripto Localizes Nodal at the Limiting Membrane of Early Endosomes. Science Signaling. 1 (45), ra13-ra13 (2008).
- Jullien, J., Gurdon, J. Morphogen gradient interpretation by a regulated trafficking step during ligand-receptor transduction. Genes & Development. 19 (22), 2682-2694 (2005).
- Incardona, J. P., et al. Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proceedings of the National Academy of Sciences of the United States of America. 97 (22), 12044-12049 (2000).
- Scholpp, S., Brand, M. Endocytosis Controls Spreading and Effective Signaling Range of Fgf8 Protein. Current Biology. 14 (20), 1834-1841 (2004).
- Bläßle, A., Müller, P. . PyFDAP: Automated analysis of Fluorescence Decay After Photoconversion (FDAP) experiments. Bioinformatics. In Press, (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved