A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Mouse Fetal Skin Model of Scarless Wound Repair
* These authors contributed equally
In This Article
Summary
During mammalian development, early gestational skin wounds heal without a scar. Here we detail a reliable and reproducible model of fetal scarless wound healing in the cutaneous dorsum of E16.5 (scarless) and E18.5 (scarring) mouse embryos.
Abstract
Early in utero, but not in postnatal life, cutaneous wounds undergo regeneration and heal without formation of a scar. Scarless fetal wound healing occurs across species but is age dependent. The transition from a scarless to scarring phenotype occurs in the third trimester of pregnancy in humans and around embryonic day 18 (E18) in mice. However, this varies with the size of the wound with larger defects generating a scar at an earlier gestational age. The emergence of lineage tracing and other genetic tools in the mouse has opened promising new avenues for investigation of fetal scarless wound healing. However, given the inherently high rates of morbidity and premature uterine contraction associated with fetal surgery, investigations of fetal scarless wound healing in vivo require a precise and reproducible surgical model. Here we detail a reliable model of fetal scarless wound healing in the dorsum of E16.5 (scarless) and E18.5 (scarring) mouse embryos.
Introduction
Fetal skin wounds heal rapidly and scarlessly until late in gestation1. Fetal scarless wound repair is characterized by regeneration of normal tissue architecture and function. The transition from a scarless to scarring phenotype occurs in the third trimester of pregnancy in humans and around embryonic day 18 (E18) in mice2,3. In comparison to adult, fetal wound repair is characterized by rapid epithelialization, connective tissue deposition, and fibroblast migration.
Many studies have offered possible explanations for the phenomenon of scarless wound healing during early fetal development. Inflammation is a fundamental component of adult wound repair; however, fetal wounds are characterized by a lack of acute inflammation4. Whether this is a consequence of the functional immaturity of the immune system during fetal stages remains unclear. A recent study suggested that differences in the abundance, maturity, and function of mast cells in E15 vs. E18 fetal skin may be responsible for the transition from a scarless phenotype, at least in the mouse3. Other studies posit that differences in the properties and abundance of fetal and adult wound macrophages are responsible for the reformation of normal extracellular matrix (ECM) during fetal wound repair5.
Differences in environmental factors during fetal and adult development may also affect wound repair. Longaker and colleagues showed that wound fluid from the fetus possesses high levels of hyaluronic acid-stimulating activity compared to none in adult wound fluid6. Consequently, higher levels of hyaluronic acid, a glycosaminoglycan that promotes a microenvironment conducive to cell motility and proliferation, in the fetal wound environment may be responsible for the scarless phenotype seen during early fetal development. Other lines of evidence point to the fact that the fetal wound environment is relatively hypoxemic and submerged in sterile amniotic fluid rich in growth factors7. However, no definitive answer has been provided for a critical event or factor during embryogenesis that triggers the transition from scarless regeneration to fibrotic repair.
Understanding the mechanisms responsible for scarless healing in the fetus necessitates a precise and reproducible model. Here we detail a reproducible model of fetal scarless wound healing in the dorsum of E16.5 (scarless) and E18.5 (scarring) mouse embryos. Additionally, minor variations of this model can be utilized to perform a number of further studies, such as gene expression analysis of fetal wounds and skin8,9. Given that precisely timed pregnancies are critical for successful recapitulation of this fetal scarless wound healing model, we also detail our protocol for superovulation timed pregnancies.
Protocol
NOTE: All procedures described in this paper are performed according to guidelines established by the Stanford Administrative Panel on Laboratory Animal Care (APLAC).
1. Timed Pregnancies – Superovulation Technique (Figure 1)
NOTE: Precisely timing the gestational age of mouse embryos for fetal surgery at E16.5 and E18.5 is of critical importance. In this section we detail our protocol for timing mouse pregnancies using pregnant mares serum (PMS) and human chorionic gonadotropin (HCG) injections to induce superovulation.
- Inject female mice (<5 per cage) intraperitoneally (IP) with 3.0-5.0 international units (IU) of PMS in a volume of 100 μl PBS between 1:00 and 3:00 PM for day 1.
- Between 12:00 and 2:00 PM of day 3 (forty-five to 47 hr after PMS injection), inject female mice IP with 3.0-5.0 IU of HCG in a volume of 100 μl PBS.
NOTE: The HCG injection induces ovulation approximately 12 hr post-injection. - Immediately following HCG injections, mate females with males aged 8-16 weeks.
NOTE: We typically place two females into a cage of individual males. - Separate females from males on the morning of day 4 (6:00 – 10:00 AM) and record as fetal age E0.5.
NOTE: Vaginal plugs can be checked at this time; however, the observation of a vaginal plug does not guarantee pregnancy and females can become pregnant when no plug is observed. Given that pregnancy is typically observable by visual inspection and/or palpation at gestational ages E16.5 and E18.5, checking for vaginal plugs on the morning of day 4 is not strictly necessary. In our experience, and depending on the strain, approximately 30-50% of super-ovulated females become pregnant using the technique described here.
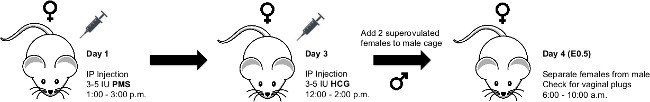
Figure 1. Schematic for Superovulation Technique. Schematic showing procedure for superovulation timed pregnancies in mice. Please click here to view a larger version of this figure.
2. Murine Fetal Surgery (Dorsal Wounding) on E16.5 and E18.5 Embryos (Figure 2)
- Before the procedure, clean all surfaces of the operating room and equipment with 70% isopropyl alcohol. In addition, sterilize all surgical supplies and instruments that will be used in the procedure by autoclaving them. Some institutions may allow subsequent use of hot bead sterilization. Per operation, use sterile packs that include gauze and surgical instruments.
- Induce anesthesia in pregnant mothers (fetal age E16.5 or E18.5) under 2.5% isoflurane/oxygen mixture at 2 L per min followed by maintenance anesthesia at 1 L per min.
- To confirm proper anesthetization, ensure the deep pedal reflexes of the mouse are suppressed and place the mouse in the prone position.
- Apply a vet ophthalmic ointment such as Puralube to prevent eye irritation or dryness during the procedure.
- Prepare abdomen by administering a light application of depilatory cream for no longer than 30 sec (Figure 2A).
- Prepare abdomen for aseptic surgery with povidone-iodine and alcohol (Figure 2B and 2C).
- Perform midline laparotomy under microscope using microsurgical scissors (Figure 2D and 2E).
- Gently expose uterus and fetus selected for surgery (Figure 2F).
- Irrigate surgical field with warm (38 °C) phosphate-buffered saline (PBS) using a blunt-tip needle
NOTE: One can be made by carefully bending the tip of a large-bore needle. (Figure 2G and 2H). - Position the fetus in a manner that allows full access to dorsum.
- Pass a purse string stitch using 7-0 nylon suture through the uterus overlying the site of intended dorsal wounding (Figure 2I). Position purse string over a region of dorsum to the left or right of spinal cord, and in a region of uterine wall devoid of large blood vessels.
- Make a 3 mm incision through uterine wall and amniotic sac in the center of the purse string (Figure 2J).
- Irrigate incision site with warm (38 °C) PBS.
- Using microsurgical scissors, cut a single full-thickness excisional wound, approximately 1 mm in length, in the dorsum of the fetus.
- Gently blot incision site dry with cotton-tip applicator.
- Inject 3 μl volume India ink subcutaneously into the wound site to mark location of wound (Figure 2K).
- Irrigate with warm (38 °C) PBS to ensure ink has been retained within wound site.
- Have surgical assistant inject warm (38 °C) PBS through blunt-tip 10 G syringe into the amniotic sac as the purse string is closed ( Figure 2L). Retract syringe as purse string closure nears completion (Figure 2M).
- Gently return uterus into the abdominal cavity (Figure 2N).
- Evert skin and peritoneum.
- Have surgical assistant irrigate abdominal cavity with warm (38 °C) PBS.
- Close abdomen quickly by stapling skin and peritoneum closed (Figure 2O). The standard closure is performed in two layers; peritoneum and abdominal muscle in one layer, subcutaneous tissue and skin in the second layer. For immediate sacrifice and harvesting of the fetus, our demonstration shows closure in one layer.
- Place the animal under observation in a warm incubator set at 37 °C for 30 min or until the animal regains sufficient consciousness to maintain sternal recumbency.
- Do not return the animal to the company of other animals until it has fully recovered from the procedure.
- Upon awaking from anesthesia and during the subsequent 48 hr, administer subcutaneous injection of buprenorphine (0.05 mg/kg) every 12 hr for analgesia as needed based on pain assessment. Administer carprofen (5 mg/kg) via subcutaneous injection for additional post-operative pain relief as needed.
- Return animals to the cage and provide them with food and water ad libitum.
- Monitor closely for manifestations of pain.
- 48 hr post-surgery, sacrifice pregnant mother with an overdose of isoflurane and harvest wounded fetus. In order to do this, adjust isoflurane concentration to 5% or higher and maintain exposure for 1 min after cessation of breathing. Confirm euthanasia with cervical dislocation. Harvest an unwounded embryo for age-matched control. Late embryos should have a separate method of euthanasia consistent with IACUC recommendations, such as decapitation, cervical dislocation, or chemical injection.
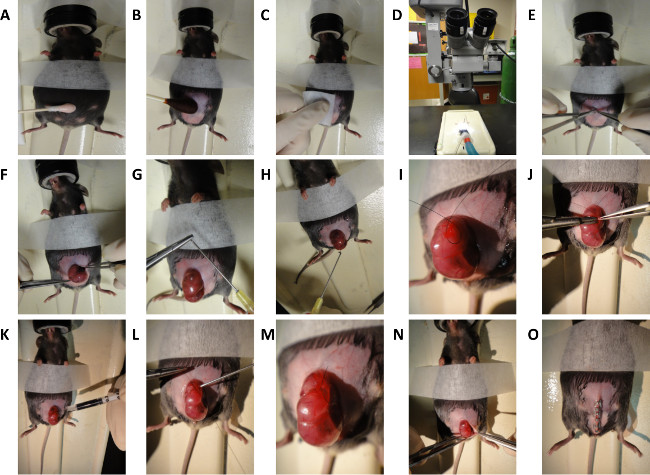
Figure 2. Schematic for Murine Fetal Surgery. General steps for dorsal wounding in E16.5 and E18.5 mouse embryos. (A) Depilation of mouse abdomen. (B and C) Preparation of mouse abdomen. (D) Microscope used for surgical procedure. (E) Midline laparotomy. (F) Exposure of uterus. (G) Creation of blunt-tip needle. (H) Irrigation of uterus with warm saline. (I) Creation of purse string suture. (J) Incision through uterine wall and 1 mm full thickness excisional wound generation. (K) Subcutaneous injection of India ink. (L and M) Closure of purse string suture. (N and O) Closure of abdomen. Please click here to view a larger version of this figure.
Results
For histologic analysis, cutaneous wounds in the dorsal skin of E16.5 and E18.5 mouse embryos should be harvested 48 hr post-wounding, fixed in 4% PFA, and paraffin-embedded. In fluorescent transgenic models, cryopreservation with OCT may be appropriate. There are several stains that may be used to visualize cellular and connective tissue architecture. Hematoxylin and eosin is a two-color stain that stains nuclei blue and eosinophilic structures (i.e., cytoplasm and extracellular collagen) various shades of red,...
Discussion
The surgical protocol presented here describes an excisional model of fetal murine scarless healing first published in 2006 by our laboratory10. In addition to other established models of excisional wounding11, incisional models of fetal murine scarless healing exist as well12,13. Investigations of fetal scarless wound healing in the monkey, lamb, rabbit, opossum, and rat have been reported14-17. However, mice represent an ideal model for exploring fetal scarless wound healing ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by a grant from NIH grant R01 GM087609 (to H.P.L.), a Gift from Ingrid Lai and Bill Shu in honor of Anthony Shu (to H.P.L.), NIH grant U01 HL099776 (to M.T.L.), the Hagey Laboratory for Pediatric Regenerative Medicine and The Oak Foundation (to M.T.L. and H.P.L.). G.G.W. was supported by the Stanford School of Medicine, the Stanford Medical Scientist Training Program, and NIGMS training grant GM07365. M.S.H. was supported by CIRM Clinical Fellow Training Grant TG2-01159. W.X.H. was supported by funding from the Sarnoff Cardiovascular Foundation.
Materials
| Name | Company | Catalog Number | Comments |
| 7-O MONOSOF Suture | eSuture | SN-1647G | |
| Surgical Forceps | Kent Scientific | INS650916 | |
| Micro-scissors | Kent Scientific | INS600127 | |
| Autoclip 9 mm | Texas Scientific Instruments | 205060 | |
| Insulin Syringe | Thermo Fisher Scientific | 22-272-382 | |
| Black Pigment | AIMS | 242 | |
| BD Safety-Lok 3 ml Syringe | BD Biosciences | 309596 | |
| Phosphate Buffered Saline | Life Technologies | 10010-049 | |
| OPMI-MD Surgical Microscope | Carl Zeiss Surgical Inc | ||
| Pregnant Mares Serum (PMS) | Millipore | 367222 | |
| Human Chorionic Gonadotropin (HCG) | Sigma-Aldrich | CG10 | |
| Povidone Iodine Prep Solution | Dynarex | 1415 | |
| Nair (depilatory cream) | Church and Dwight Co. | 22600267058 |
References
- Larson, B. J., Longaker, M. T., Lorenz, H. P. Scarless fetal wound healing: a basic science review. Plastic and reconstructive surgery. 126, 1172-1180 (2010).
- Wilgus, T. A. Regenerative healing in fetal skin: a review of the literature. Ostomy/wound management. 53, 16-31 (2007).
- Wulff, B. C., et al. Mast cells contribute to scar formation during fetal wound healing. The Journal of investigative dermatology. 132, 458-465 (2012).
- Lorenz, H. P., Adzick, N. S. Scarless skin wound repair in the fetus. The Western journal of medicine. 159, 350-355 (1993).
- Longaker, M. T., et al. Wound healing in the fetus. Possible role for inflammatory macrophages and transforming growth factor-beta isoforms. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2, 104-112 (1994).
- Longaker, M. T., et al. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Annals of surgery. 210, 667-672 (1989).
- Colombo, J. A., Napp, M., Depaoli, J. R., Puissant, V. Trophic influences of human and rat amniotic fluid on neural tube-derived rat fetal cells. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 11, 347-355 (1993).
- Colwell, A. S., Longaker, M. T., Peter Lorenz, H. Identification of differentially regulated genes in fetal wounds during regenerative repair. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 16, 450-459 (2008).
- Hu, M. S., et al. Gene expression in fetal murine keratinocytes and fibroblasts. The Journal of surgical research. , (2014).
- Colwell, A. S., Krummel, T. M., Longaker, M. T., Lorenz, H. P. An in vivo mouse excisional wound model of scarless healing. Plastic and reconstructive surgery. 117, 2292-2296 (2006).
- Wilgus, T. A., et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. The American journal of pathology. 165, 753-761 (2004).
- Iocono, J. A., Ehrlich, H. P., Keefer, K. A., Krummel, T. M. Hyaluronan induces scarless repair in mouse limb organ culture. Journal of pediatric surgery. 33, 564-567 (1998).
- Chopra, V., Blewett, C. J., Krummel, T. M. Transition from fetal to adult repair occurring in mouse forelimbs maintained in organ culture. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 5, 47-51 (1997).
- Adzick, N. S., Longaker, M. T. Animal models for the study of fetal tissue repair. The Journal of surgical research. 5, 47-51 (1991).
- Block, M. Wound healing in the new-born opossum (Didelphis virginianam). Nature. 187, 340-341 (1960).
- Longaker, M. T., Dodson, T. B., Kaban, L. B. A rabbit model for fetal cleft lip repair. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 48, 714-719 (1990).
- Longaker, M. T., et al. A model for fetal cleft lip repair in lambs. Plastic and reconstructive surgery. 90, 750-756 (1992).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved