A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Supercritical Nitrogen Processing for the Purification of Reactive Porous Materials
In This Article
Summary
Nitrogen is an effective supercritical fluid for extraction or drying processes due to its small molecular size, high density in the near-liquid supercritical regime, and chemical inertness. We present a supercritical nitrogen drying protocol for the purification treatment of reactive, porous materials.
Abstract
Supercritical fluid extraction and drying methods are well established in numerous applications for the synthesis and processing of porous materials. Herein, nitrogen is presented as a novel supercritical drying fluid for specialized applications such as in the processing of reactive porous materials, where carbon dioxide and other fluids are not appropriate due to their higher chemical reactivity. Nitrogen exhibits similar physical properties in the near-critical region of its phase diagram as compared to carbon dioxide: a widely tunable density up to ~1 g ml-1, modest critical pressure (3.4 MPa), and small molecular diameter of ~3.6 Å. The key to achieving a high solvation power of nitrogen is to apply a processing temperature in the range of 80-150 K, where the density of nitrogen is an order of magnitude higher than at similar pressures near ambient temperature. The detailed solvation properties of nitrogen, and especially its selectivity, across a wide range of common target species of extraction still require further investigation. Herein we describe a protocol for the supercritical nitrogen processing of porous magnesium borohydride.
Introduction
Supercritical fluid extraction (SFE) and drying (ScD) methods are well established in a wide range of practical applications, especially in the food and petroleum industries, but also in chemical synthesis, analysis, and materials processing.1-6 The use of drying or extraction media at conditions above their critical points is often faster, cleaner, and more efficient than traditional (liquid) techniques, and has the added advantage of being highly tunable with respect to the solvation power of the fluid by slight adjustment of the operating conditions.3,7 A simple ScD method consists of three basic steps. The first step is exposing the solid (or perhaps liquid) starting material which contains the target impurity compound to an appropriately chosen ScD fluid in its liquid (or near-liquid supercritical) phase, where its high density corresponds to a high (and perhaps selective7) solvent power with respect to the target species. The second step is heating and compressing the system above the chosen ScD fluid’s critical point in a closed container so that the fluid and its dissolved target species do not pass a phase boundary which might result in separation. The final step is slowly reducing the pressure of the ScD fluid to vacuum at a temperature above the critical temperature, allowing the fluid solution containing the target species to escape, again without encountering a phase boundary or any detrimental surface tension effects along the way.
The starting material is left depleted of the target species and may be subjected to iterated treatments if necessary. In cases of supercritical fluid extraction, the target solute species is the desired product, and is collected from solution for further use.8,9 In other cases, the dried or purified starting material is the desired product, and the extracted impurities are discarded. This latter scenario, referred to herein as the ScD approach, was discovered to be an effective strategy for the pretreatment of high surface area, microporous materials such as metal-organic frameworks (MOFs), where traditional heat-treatment methods under vacuum are in many cases not sufficient in clearing the pores of all unwanted guests, or result in pore collapse.10 Carbon dioxide ScD (CScD) processing is now a routine post-synthetic process for MOFs,11 leading to increases in nitrogen-accessible surface areas over untreated materials of up to 1,000%12 and other improvements, such as in catalytic activity.13 Other notable supercritical fluid applications are as a widely tunable medium for chemical reactions,14-16 supercritical fluid chromatography (SCFC)6,17,18 and synthesis of aerogels and advanced composite materials.19-22
For drying applications, a ScD fluid is chosen based on two criteria: a) the proximity of its critical point to ambient conditions (for convenience and to reduce energy costs or process complexity) and b) its solvation power with respect to the target species. Carbon dioxide (CO2) has proven to be a convenient ScD fluid in many applications since it is nontoxic, nonflammable, and cheap, and can be tuned to exhibit a high solvation power toward a number of common organic target species in its near-liquid state (at pressures of <10 MPa and temperatures of 273-323 K).1-3,7-9 Other common supercritical solvents (or co-solvents) include water (spanning a remarkable range of solvent properties between its ambient and supercritical state23), acetone, ethylene, methanol, ethanol, and ethane, covering the spectrum from polar (protic and aprotic) to nonpolar, and having critical points relatively near to ambient conditions.
Carbon dioxide is by far the most common ScD fluid used. In established CScD methods, the reactivity of the starting material is not an inhibitive factor since CO2 is only very weakly reactive at temperatures near its critical point. However, certain classes of materials such as so-called complex hydrides (e.g., alanates and borohydrides) present unique challenges in handling due to their strong reactivity in the presence of water or CO2 in addition to their (perhaps intentionally tailored) instability under heating.24-26 Moreover, there is great international interest in such materials as high-density hydrogen storage compounds,27-30 and therefore also in nanostructured and/or porous varieties31-33. For the effective purification of such reactive, unstable, and nanostructured materials, ScD methods are a promising strategy.34 A ScD fluid must be used which has a small molecular diameter appropriate for penetration into narrow cavities and which also has a high solvation power toward the target impurities, while remaining unreactive toward the starting material itself. Herein, the use of supercritical nitrogen (N2) as an effective fluid for such extraction and especially drying applications is presented. A specific supercritical nitrogen drying (NScD) methodology is described below for the purification of γ-phase magnesium borohydride where the target species include both diborane and an n-butyl compound (similar to but not specifically identifiable as n-butane). The following protocol can be easily modified for general extension to other supercritical nitrogen drying or extraction processes.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Apparatus
- Use a basic supercritical drying (ScD) apparatus comprised of four primary components connected by high pressure gas tubing: the gas supply, a vacuum system, sensors (temperature and pressure), and the sample environment (which can be submersed in a bath). Ensure that the construction is of high-quality stainless steel valves, fittings, and tubing, pressure-rated to at least 10 MPa within the temperature range between 80-300 K.
Note: A schematic is shown in Figure 1. - For nitrogen ScD (NScD) treatments, ensure that the gas supply is research purity (>99.999%) nitrogen gas fitted with a pressure regulator for outlet pressure control between 0-10 MPa. Attach a 50 L bottle (20 MPa) to the apparatus, and purge the system with pure nitrogen multiple times before use.
- Ensure that the vacuum system is capable of achieving vacuum pressures down to <0.1 Pa and is connected to the apparatus with a fine-control needle valve. Preferably, use an oil-free roughing pump and a molecular-drag turbo pump, placed in series.
- Use at least two pressure sensors for accurate measurement of the pressure during ScD treatments: a low pressure sensor for vacuum measurement and a high pressure sensor to achieve a total measurable pressure range between 0.1-107 Pa.
- Use at least two temperature sensors for the minimal accuracy necessary to perform typical ScD treatments: a sensor in thermal contact with the sample and a sensor within the primary gas dosing manifold for accurate measurements between 77-300 K (e.g., K-type thermocouples).
- Check that the sample holder has an appropriate inner volume to contain the amount of sample necessary for treatment, and is constructed of stainless steel.
Note: A lengthened cylindrical design aids in thermal contact with the bath. - Ensure that the fitting that closes the sample container is appropriate for high pressures and repeated use (e.g., Swagelok VCR). Connect the sample container volume to a valve for isolation from the outside environment via an appropriate length of tubing (the dip tube) for complete immersion of the sample holder into the bath.
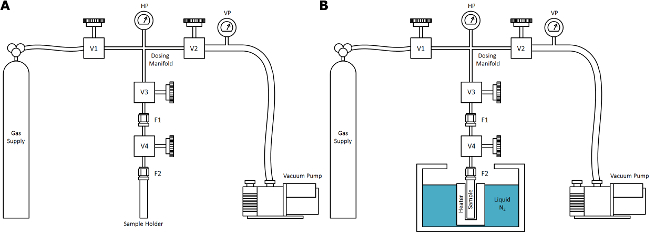
Figure 1. Supercritical Nitrogen Drying (NScD) Apparatus. A schematic depiction of the simplified NScD apparatus for use in the typical laboratory: (A) at ambient temperature and (B) after submersion of the sample in the bath. The gas supply for the process described in this work is nitrogen, but this general apparatus is generalizable to other ScD fluids with a critical point lying within a practical range of temperature and pressure, such as CO2. The components are labeled for consistency with the protocol described herein. Please click here to view a larger version of this figure.
2. Preparation
- Load 0.2-0.5 g of sample (γ-Mg(BH4)2, in powder form, following wet-chemical synthesis and standard drying methods) into the sample holder under appropriate conditions, typically in an inert atmosphere such as an argon glovebox, at ambient temperature or below. Close the sample holder (fitting F2) with a filter gasket and then close the valve (valve V4). Transfer the sample holder to the apparatus and attach (fitting F1).
- Open the dosing manifold to vacuum via V2 and evacuate. Open V3 and evacuate. Purge the apparatus with nitrogen via V1 and evacuate via V2. Open V4 and evacuate the sample at RT for up to 24 hr, to reach the minimum pressure of the system (<0.1 Pa).
- Install the sample bath (see Figure 2) around the sample holder. Perform this by raising the bath into position on a scissor lift, or similar mechanism.
- Set the heater to the desired future liquid temperature (Tl, see step 3.1) of 110 K, and continue to evacuate the apparatus until the temperature equilibrates.
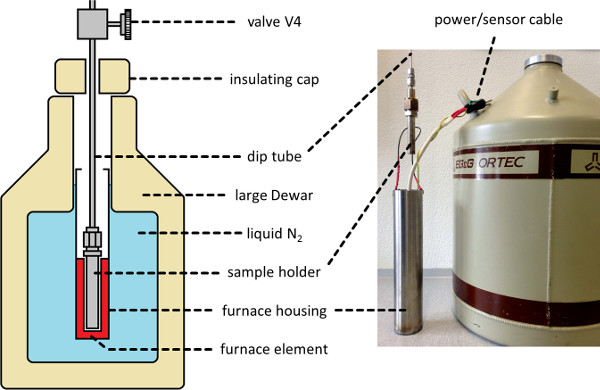
Figure 2. Cryogenic Furnace Sample Bath. A schematic depiction (left) and photograph (right) of a cryostatic thermal bath environment appropriate for containing the sample holder during NScD processing, permitting measurement and control of the sample temperature between 77-298 K. Please click here to view a larger version of this figure.
3. Supercritical Nitrogen Exposure
- For NScD processing of γ-Mg(BH4)2, (the subject of demonstration in this protocol) select a liquid temperature (Tl) of 110 K. This corresponds to a moderate liquid solvent density (~0.6 g ml-1); Adjust as necessary for the application of this protocol to other NScD treatment processes (see the Note below).
- Close off the system to vacuum by closing V2. Throttle open V1 slowly, allowing the pressure to increase into the liquid region of the phase diagram. Equilibrate the system at 2 MPa and Tl.
- Soak the sample in liquid N2 for 4 hr.
- Set the heater to 150 K with a ramp ≤2 K min-1. Allow the pressure to increase no higher than the maximum rated pressure of the apparatus (this Pmax should be ≥10 MPa); if necessary, carefully vent the excess pressure to vacuum via V2. Equilibrate the system at Pmax and 150 K.
- Soak the sample in supercritical N2 for 1 hr.
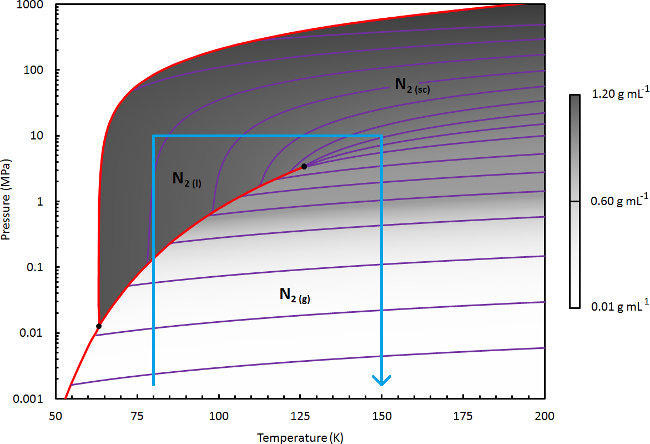
Figure 3. Phase Diagram of Nitrogen. A detailed phase diagram of nitrogen, where the fluid density (shown in linear greyscale) is calculated using Refprop (a modified Webb-Benedict-Rubin equation of state).41 Various lines of constant density are shown in purple. The solid phase boundaries and boiling transition lines are shown in red. The blue lines indicate the boundary of the region of the phase diagram that is relevant for drying or extraction processing using N2. Please click here to view a larger version of this figure.
Note: For the application of this protocol to other materials, establish an appropriate NScD treatment scheme by choosing the conditions necessary for effective solvation of the target species. Refer to the phase diagram of N2, shown in Figure 3. To achieve a high fluid density in the liquid phase (e.g., 0.8-1 g ml-1), select a Tl of 80-90 K. For moderate liquid density (e.g., 0.6-0.8 g ml-1), select a Tl of 90-115 K. A trial and error approach may be necessary.
4. Supercritical Nitrogen Release
- Carefully crack the system to vacuum by throttling V2, allowing the pressure to decrease as slowly as possible. Repeatedly crack the system to higher vacuum rates as necessary to achieve a gradual decline to high vacuum (<0.1 Pa) in the approximate time span of 12-24 hr.
- Remove the sample bath and fully open V2 to completely evacuate the sample. Equilibrate at RT and high vacuum (<0.1 Pa).
- Degas the sample at RT and <0.1 Pa for 1-24 hr, as desired.
5. Post Treatment
- Close valves V3 and V4, and remove the sample holder from the apparatus (fitting F1).
- Transfer the sample holder to an inert environment for handling, such as an argon-filled glovebox. Remove the sample from the sample holder (fitting F2) and store in a sealed container at ambient temperature or below.
Access restricted. Please log in or start a trial to view this content.
Results
Alkali and alkaline earth metal borohydrides are potential hydrogen storage materials, which deliver a large content of gaseous hydrogen upon decomposition.27,29 Other decomposition products such as diborane have also sometimes been detected in the gas desorbed, but their origin is not a priori clear; it is possible they are products of the pure phase decomposition, but may also be impurities or products of reactions of impurities leftover from chemical synthesis.35 The porous phase of magnesium bor...
Access restricted. Please log in or start a trial to view this content.
Discussion
Perhaps due to its relatively low critical temperature (126 K), N2 has historically been overlooked as an effective ScD solvent. In earlier reports,3,17,42,43 it has only been alluded to in the context of processing temperatures at or above ambient, where it exhibits only modest solvation power due to its low fluid density in this region of its phase diagram (except at extremely high pressures43). The key step in realizing the practical utility of N2 as a supercritical solvent ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the European Fuel Cells and Hydruogen Joint Undertaking under collaborative project BOR4STORE (Grant Agreement No. 303428) and infrastructure program H2FC (Grant Agreement No. FP7-284522).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Compressed Nitrogen Gas | Messer Schweiz AG | 50 L bottle, purity > 99.999%, <3 ppmv H2O | |
| Liquid Nitrogen | Pan Gas AG | Bulk storage, on site | |
| Custom Supercritical Drying Apparatus | Empa | Swagelok (compression fitting and VCR) components | |
| Custom Cryogenic Furnace Bath | Empa | ||
| Custom Labview Interface | Empa |
References
- McHugh, M. A., Krukonis, V. J. Supercritical Fluid Extraction. , 1st ed, Butterworth. Stoneham, MA. (1986).
- Schneider, G. M. Physicochemical Principles of Extraction with Supercritical Gases. Angew. Chem. lnt. Ed. 17, 716-727 (1978).
- Williams, D. F. Extraction with Supercritical Gases. Chem. Eng. Sci. 36 (11), 1769-1788 (1981).
- Eckert, C. A., Knutson, B. I., Debenedetti, P. G. Supercritical fluids as solvents for chemical and materials processing. Nature. 383, 313-318 (1996).
- Cooper, A. I. Polymer synthesis and processing using supercritical carbon dioxide. J. Mater. Chem. 10, 207-234 (2000).
- Klesper, E., Corwin, A. H., Turner, D. A. High pressure gas chromatography above critical temperatures. J. Org. Chem. 27, 700-701 (1962).
- Tucker, S. C. Solvent Density Inhomogeneities in Supercritical Fluids. Chem. Rev. 99 (2), 391-418 (1999).
- Hubert, P., Vitzthum, O. G. Fluid Extraction of Hops, Spices, and Tobacco with Supercritical Gases. Angew. Chem. Int. Ed. 17 (10), 710-715 (1978).
- Zosel, K. Separation with Supercritical Gases: Practical Applications. Angew. Chem. Int. Ed. 17 (10), 702-709 (1978).
- Nelson, A. P., Farha, O. K., Mulfort, K. L., Hupp, J. T. Supercritical Processing as a Route to High Internal Surface Areas and Permanent Microporosity in Metal−Organic Framework Materials. J. Am. Chem. Soc. 131, 458-460 (2009).
- Liu, B., Wong-Foy, A. G., Matzger, A. J. Rapid and enhanced activation of microporous coordination polymers by flowing supercritical CO2. Chem. Commun. 49, 1419-1421 (2013).
- Cooper, A. I., Rosseinsky, M. J. Metal–organic frameworks: improving pore performance. Nat. Chem. 1, 26-27 (2009).
- Totten, R. K., et al. Enhanced Catalytic Activity through the Tuning of Micropore Environment and Supercritical CO2 Processing: Al(Porphyrin)-Based Porous Organic Polymers for the Degradation of a Nerve Agent Simulant. J. Am. Chem. Soc. 135, 11720-11723 (2013).
- Savage, P. E., Gopalan, S., Mizan, T. I., Martino, C. J., Brock, E. E. Reactions at supercritical conditions: Applications and fundamentals. AIChE J. 41 (7), 1723-1778 (1995).
- Baiker, A. Supercritical Fluids in Heterogeneous Catalysis. Chem. Rev. 99 (2), 453-474 (1999).
- Jessop, P. G., Ikariya, T., Noyori, R. Homogeneous Catalysis in Supercritical Fluids. Chem. Rev. 99 (2), 475-494 (1999).
- Giddings, J. C., Myers, M. N., McLaren, L., Keller, R. A. High Pressure Gas Chromatography Of Nonvolatile Species. Science. 162 (3849), 67-73 (1968).
- Gere, D. R. Supercritical Fluid Chromatography. Science. 222, 253-259 (1983).
- Kistler, S. S. Coherent Expanded Aerogels and Jellies. Nature. 127, 741-741 (1931).
- Biener, J., et al. Advanced carbon aerogels for energy applications. Energy Environ. Sci. 4, 656-667 (2011).
- Morère, J., et al. Deposition of Pd into mesoporous silica SBA-15 using supercritical carbon dioxide. J. Supercrit. Fluids. 56 (2), 213-222 (2011).
- Sathish, M., Mitani, S., Tomai, T., Honma, I. Supercritical fluid assisted synthesis of N-doped graphene nanosheets and their capacitance behavior in ionic liquid and aqueous electrolytes. J. Mater. Chem. A. 2, 4731-4738 (2014).
- Toews, K. L., Shroll, R. M., Wai, C. M., Smart, N. G. pH-Defining Equilibrium between Water and Supercritical CO2. Influence on SFE of Organics and Metal Chelates. Anal. Chem. 67 (22), 4040-4043 (1995).
- Barbaras, G., Barbaras, G. D., Finholt, A. E., Schlesinger, H. I. Cause Of Explosions Occasionally Observed During Evaporation Of Solutions Of Aluminum Hydride And Related Compounds. J. Am. Chem. Soc. 70, 877(1948).
- Burr, J. G., Brown, W. G., Heller, H. E. The Reduction of Carbon Dioxide to Formic Acid. J. Am. Chem. Soc. 72 (6), 2560-2562 (1950).
- Hugelshofer, C. L., et al. Gas−Solid Reaction of Carbon Dioxide with Alanates. J. Phys. Chem. C. 118, 15940-15945 (2014).
- Orimo, S. I., Nakamori, Y., Eliseo, J. R., Züttel, A., Jensen, C. M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 107 (10), 4111-4132 (2007).
- Gross, K. J., Thomas, G. J., Jensen, C. M. Catalyzed alanates for hydrogen storage. J. Alloys Compd. 330-332, 683-690 (2002).
- Li, H. W., Yan, Y., Orimo, S. I., Züttel, A., Jensen, C. M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies. 4 (1), 185-214 (2011).
- Frankcombe, T. J. Proposed Mechanisms for the Catalytic Activity of Ti in NaAlH4. Chem. Rev. 112, 2164(2012).
- Vajo, J. J., Olson, G. L. Hydrogen storage in destabilized chemical systems. Scr. Mater. 56, 829(2007).
- Zhang, Y., et al. LiBH4 nanoparticles supported by disordered mesoporous carbon: Hydrogen storage performances and destabilization mechanisms. Int. J. Hyd. Energ. 32 (16), 3976-3980 (2007).
- Christian, M. L., Aguey-Zinsou, K. F. Core–Shell Strategy Leading to High Reversible Hydrogen Storage Capacity for NaBH4. ACS Nano. 6 (9), 7739-7751 (2012).
- Stadie, N. P., et al. Supercritical N2 Processing as a Route to the Clean Dehydrogenation of Porous Mg(BH4)2. J. Am. Chem. Soc. 136 (23), 8181-8184 (2014).
- Borgschulte, A., et al. Impurity Gas Analysis of the Decomposition of Complex Hydrides. J. Phys. Chem. C. 115, 17220-17226 (2011).
- Filinchuk, Y., et al. Porous and Dense Magnesium Borohydride Frameworks: Synthesis, Stability, and Reversible Absorption of Guest Species. Angew. Chem. Int. Ed. 50, 11162-11166 (2011).
- Li, H. W., et al. Dehydriding and rehydriding processes of well-crystallized Mg(BH4)2 accompanying with formation of intermediate compounds. Acta Mater. 56 (6), 1342-1347 (2008).
- Schüth, F., Bogdanovic, B., Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Comm. , 2249-2258 (2004).
- Vitillo, J. G., Groppo, E., Bardají, E. G., Baricco, M., Bordiga, S. Fast carbon dioxide recycling by reaction with γ-Mg(BH4)2. Phys. Chem. Chem. Phys. 16, 22482-22486 (2014).
- Paskevicius, M., et al. In-Situ X-ray Diffraction Study of γ-Mg(BH4)2 Decomposition. J. Phys. Chem. C. 116, 15321-15240 (2012).
- Lemmon, E. W., Huber, M. L., McLinden, M. O. NIST standard reference database 23: reference fluid thermodynamic and transport properties. Standard Reference Data Program. , (2008).
- Moquin, P. H. L., Temelli, F. J. Kinetic modeling of hydrolysis of canola oil in supercritical media. J. Supercrit. Fluid. 45, 94-101 (2008).
- Myers, M. N., Giddings, J. C. Ultra-High-Pressure Gas Chromatography in Micro Columns to 2000 Atmospheres. Sep. Sci. 1 (6), 761-776 (1966).
- McLeary, E. E., Jansen, J. C., Kapteijn, F. Zeolite based films, membranes and membrane reactors: Progress and prospects. Microporous Mesoporous Mater. 90, 198-220 (2006).
- Richter, B., Ravnsbæk, D. B., Tumanov, N., Filinchuk, Y., Jensen, T. R. Manganese borohydride; synthesis and characterization. Dalton Trans. , (2015).
- Liang, S., Tilotta, D. C. Extraction of petroleum hydrocarbons from soil using supercritical argon. Anal. Chem. 70 (3), 616-622 (1998).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved