A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Method for Evaluating the Reinforcing Properties of Ethanol in Rats without Water Deprivation, Saccharin Fading or Extended Access Training
In This Article
Summary
This protocol describes a novel and efficient method to quickly initiate operant responding for ethanol in rats that, contrary to standard methods, does not require water deprivation or saccharin/sucrose fading to initiate responding.
Abstract
Operant oral self-administration methods are commonly used to study the reinforcing properties of ethanol in animals. However, the standard methods require saccharin/sucrose fading, water deprivation and/or extended training to initiate operant responding in rats. This paper describes a novel and efficient method to quickly initiate operant responding for ethanol that is convenient for experimenters and does not require water deprivation or saccharin/sucrose fading, thus eliminating the potential confound of using sweeteners in ethanol operant self-administration studies. With this method, Wistar rats typically acquire and maintain self-administration of a 20% ethanol solution in less than two weeks of training. Furthermore, blood ethanol concentrations and rewards are positively correlated for a 30 min self-administration session. Moreover, naltrexone, an FDA-approved medication for alcohol dependence that has been shown to suppress ethanol self-administration in rodents, dose-dependently decreases alcohol intake and motivation to consume alcohol for rats self-administering 20% ethanol, thus validating the use of this new method to study the reinforcing properties of alcohol in rats.
Introduction
The development of animal models to study the reinforcing effects of drugs has proven an important tool to study human drug addiction. More specifically, operant self-administration is a widely used behavioral model that is one of the most effective means for assessing the positive reinforcing effects of an orally consumed ethanol solution. An early issue with developing such a model was the primary aversive taste of high concentrations of ethanol for most rodents, a phenomenon that is also shared in humans with little or no experience with alcohol1. A standard protocol to overcome this barrier requires water deprivation and/or saccharin or sucrose fading for the acquisition and maintenance of self-administration. However, these two approaches are not advantageous. They require long periods of training to simply initiate responding for ethanol and obtain a relative success rate of acquisition. The use of sweeteners also introduces a potential bias in the interpretation of the self-administration data. These limitations don't apply to the following protocol.
Briefly, Samson and colleagues2 have shown that dissolving ethanol in a sweet solution of 20% sucrose and then fading out the sweetness over 4 weeks of training is required to initiate responding for 10% ethanol in water. Furthermore, reliable ethanol intake is usually achieved in 6 to 8 weeks1-3. This approach is highly problematic. First, it requires extended periods of training before investigators can begin to measure ethanol self-administration. In contrast, intravenous self-administration of cocaine or heroin requires 0 - 1 days of pre-drug training on a food-delivering lever in food restricted animals, and stable responding for drug is often achieved in 10 - 12 days4,5. Another limitation of this method is the fact that saccharin and sucrose are highly rewarding to rats and elicit brain activation patterns similar to drugs of abuse, thus introducing the potential for confounds in ethanol self-administration studies6-9. Finally, rats acquiring self-administration of an ethanol solution using this method show variability in acquisition and response rate1,10, with a substantial proportion of rats consistently excluded from experiments due to unsuccessful acquisition and/or insufficient response rate.
By contrast, with this protocol, we present a simple yet efficient method for acquisition and maintenance of oral self-administration of a 20% ethanol in water solution that does not necessitate water deprivation, sucrose/saccharin fading or extended access training. A recent investigation found that self-administration for oral ethanol displays an inverted U shaped dose response curve with highest ethanol intake during self-administration at a 20% ethanol concentration, thus providing a rationale for selecting 20% ethanol solution in our experimental design11.
Access restricted. Please log in or start a trial to view this content.
Protocol
All procedures are conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals.
1. Animal Care and Housing
- Upon arrival in the colony, house male Wistar rats weighing 200 - 225 g at arrival in pairs in a temperature (21 °C) and humidity-controlled environment with a reversed 12 h light-dark cycle.
Note: Depending on the rationale of the experiments, rats may be single-housed. - Allow the rats to acclimate to the vivarium and the light cycle for at least one week before starting an experiment and handle them daily. Weigh the animals once weekly.
- Give rats free access to chow and tap water for the duration of the experiment (water deprivation is not necessary to initiate responding12). Conduct all behavioral testing during the dark phase of the light-dark cycle in a dark testing room.
Note: Although we routinely use young adult male Wistar rats (postnatal days 62 - 65), this protocol can theoretically be suitable for investigating other strains of rats, as well as sex and/or age differences.
2. Operant Training
- Conduct all behavioral training and testing in identical operant chambers measuring 30.5 × 29.2 × 24.1 cm and containing grid floors and waste pans lined with bedding (changed in between animals to reduce odor cues), housed in sound-attenuating cubicles equipped with exhaust fans for ventilation.
Note: Rats are trained by operant-conditioning from day 1 of training (a response on a lever is necessary to obtain a reinforcer) between + 1 h and + 8 h of the dark cycle. There is no pre-exposure to alcohol, nor food or fluid shaping before alcohol training. - Equip each operant chamber with stimulus lights, syringe pumps and two retractable levers positioned laterally to a liquid cup receptacle. Interface chambers and control by a computer using the appropriate software.
Note: Most vendors will provide self-administration packages containing all the necessary components as well as software and interfaces necessary to control the chambers. - Write an operant-conditioning program that initiates the fixed-ratio (FR) schedule of 20% ethanol reinforcement (an operant schedule in which a response is reinforced only after a specified number of responses) according to the manufacturer's instructions. For example, in a fixed-ratio 1, rats have to produce one response to obtain a reinforcer (see the Supplemental Code Files section for an example of a FR1 program). Execute the following commands.
- Extend two levers to mark the onset of the session and to signal alcohol availability.
Note: One lever press on the lever associated with ethanol (active) is rewarded by the delivery of a volume of 100 μL of 20% ethanol in water in the adjacent drinking well and initiates a concomitant 5-s time-out period signaled by the illumination of the cue-light above the lever. The cue-light is introduced on day 1 of the training. - During the time-out period, record responding although it has no scheduled consequences. Record responses on the other lever (inactive) although they never have behavioral consequences. Note: The inactive lever serves as a control to assess non-specific behavior.
- Record the number of responses on the active lever (response rates) and the number of 20% ethanol rewards earned.
- After 30 min have elapsed, retract the two levers to signal the end of the session and to signal the end of alcohol availability.
- Save and archive all data.
- Extend two levers to mark the onset of the session and to signal alcohol availability.
- Before starting the training session, check the proper functioning of all devices in each operant chamber (retractable levers, fluid dispenser).
- Prepare the 20% v/v ethanol solution from 190 Proof (95%) ethanol diluted in tap water.
Note: Ethanol solution can be kept at room temperature. Depending on the manufacturer, stock ethanol solutions may be up to 99.98%. Also note that, while in most published manuscripts, ethanol concentrations are usually presented as volume/volume, some may present them as weight/volume. - Fill the syringe with the 20% ethanol solution and make sure that there are no leaks or air bubbles in the infusion lines. Manually push a small volume of ethanol through the infusion lines to make sure that ethanol will be correctly delivered into the receptacle from the first reinforced response. Dry the receptacle with a paper towel and make sure that it is empty before starting the session.
- Load the software that controls the FR schedule of 20% ethanol reinforcement
- Train operant- and drug-naive rats under a FR1 schedule to self-administer 20% ethanol without water deprivation during 30 min sessions.
- Transport the rats from the vivarium to the testing room using a transport cage.
- Assign each rat to one chamber. Take each individual rat from the transport cage and put it in the assigned self-administration chamber.
- Try to keep the conditions of the experiment consistent each day. Therefore, always test rats in the same self-administration chamber at roughly the same time every day.
- Start the software that initiates the FR1 schedule of 20% ethanol reinforcement.
- At the end of the session, remove the rats from the self-administration chambers and return them to the vivarium.
- In order to manually confirm that the alcohol solution is consumed by the animals, use a paper towel and check if the receptacle is dried. Alternatively, use a 1 or 2 mL syringe and collect the fluid remaining in the receptacle to estimate the volume that is not consumed.
Note: For a more precise measurement (but also more expensive), it is possible to equip each chamber with a lickometer system connected to the liquid cup receptacle that allows a precise count of the licks produced by the rats during the self-administration session. - Clean the walls and grid floor of each chamber with a surface disinfectant.
- After use, maintain the infusion lines by cleaning them with a 70% ethanol solution. When not in use, insert the cap to avoid mold and dust.
- After each session, collect and analyze the data generated. More particularly, calculate the mean number of active lever presses, the mean number of alcohol rewards obtained as well as the mean number of inactive lever presses.
Note: In most papers, alcohol consumption is also presented as ethanol intake in g/kg (bodyweight). Ethanol intake can be calculated using the following formula:

With volume consumed (mL) - number of rewards earned ± unitary volume/reward and density of ethanol at room temp - 0.789 g/mL.
For example, if a rat weighing 400 g earned 20 rewards of 20% v/v ethanol solution:
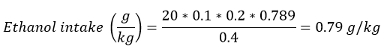
- Conduct sessions 5/6 days a week until stabilization of performance (defined as a minimum of 15 sessions and no change greater than 15% in the total number of rewards earned during the last 3 sessions).
Note: Once rats have reached a stable baseline on FR1, the operant conditioning program can be modified according to the manufacturer's instructions to increase the number of responses necessary to obtain an alcohol reward. The fixed-ratio can for example be increased to FR2 or FR3 in order to minimize accidental reinforcement. - Measure blood ethanol concentrations:
- To confirm that the rewards obtained in a self-administration session are consumed by the rats, measure the blood ethanol concentrations following self-administration. Once rats have reached a stable baseline, collect blood from the lateral tail vein immediately following the session. Insert a 23 G needle into the blood vessel and collect 50 µL blood samples using a capillary tube.
- Transfer the 50 µL blood samples to 10 mL headspace vials and add 50 µL of 1:1,000 isopropyl alcohol as internal standard. Prepare a standard curve 10 to 300 mg/dL from ethanol standards. Process samples with a headspace sampler with a chromatography column13 according to the manufacturer settings.
- Progressive ratio
- Use progressive ratio (PR) schedules to assess the motivation of the animals to consume a reward14. PR is an operant schedule in which the number of responses to obtain a reinforcer is increased gradually (in other terms, rats have to work harder for each successive reward).
Note: Contrary to FR sessions, a PR session is not timed and will only be terminated once 30 min has elapsed without a completed ratio.- Place rats in their assigned operant boxes and allow them to respond for 20% ethanol under a PR schedule of reinforcement: Keep all experimental conditions identical to those used in the FR schedule (see step 2.3), except increase the response requirement or cost within-session (i.e., the number of lever presses required on the active lever to receive a single ethanol reward) according to the following formula: 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 28, 32… Note: An example of the PR program is provided in the Supplemental Code Files section.
- Terminate the PR session once 30 min has elapsed without a reward.
Note: The breakpoint is defined as the last completed response requirement during the PR session.
- Use progressive ratio (PR) schedules to assess the motivation of the animals to consume a reward14. PR is an operant schedule in which the number of responses to obtain a reinforcer is increased gradually (in other terms, rats have to work harder for each successive reward).
3. Testing the Predictive Validity of an Operant Model of Alcohol Self-administration
Note: Once rats have acquired a stable self-administration baseline (see step 2), it is possible to assess the predictive validity of the model by testing the efficacy of naltrexone, a currently FDA-approved medication for alcohol dependence in reducing 20% ethanol self-administration. We recommend doing this testing on the highest FR achieved during training (FR2), when responses are reliably high. Once predictive validity is established, the model can be used to evaluate new drug candidates.
- Before test day, give rats subcutaneous saline injections 30 min prior to self-administration sessions to habituate them to the injection during a minimum of two consecutive self-administration sessions or until responding for ethanol is not affected by the saline injections (defined as no change greater than 15% in the total number of rewards earned during the last 2 sessions).
- Dissolve naltrexone in saline and adjust the pH to reach neutrality to facilitate the injections to the animals. Inject the drug at a volume of 1.0 mL/kg 30 min before a session, using the subcutaneous route of administration. Note: The literature provides good evidence for a dose choice between the ranges of 0.1 - 1mg/kg, so 0.1, 0.3 and 1 mg/kg are used in this protocol.
Note: Research indicates use of both subcutaneous and intraperitoneal routes of administration for naltrexone, though their potency may differ with subcutaneous injection being 30-fold more potent that intraperitoneal injections15. For this reason, we recommend using the subcutaneous route as the use of this route for the injection of naltrexone has been validated and replicated by various laboratories in a range of concentration between 0.1 and 1 mg/kg12,15,16. - During test day, inject rats in a balanced/random order in a between-subjects design across one of the four naltrexone dosing cycles (0, 0.1, 0.3 and 1 mg/kg) 30 min before the self-administration session.
- Between each dosing cycle, allow rats to washout the drug with a minimum of two consecutive self-administration sessions or until responding for ethanol is back to baseline. Note: As a result, at the end of the test, all rats have been injected with each of the four doses.
- After this phase, select the most efficient dose (1 mg/kg) and test the effect of naltrexone on the motivation of the animals to consume alcohol using a progressive ratio schedule (see step 2.12).
Access restricted. Please log in or start a trial to view this content.
Results
Figure 1 shows the representative self-administration behavior of operant- and drug-naive Wistar rats (eight different cohorts amounting to a total of 239 rats) trained on a FR1 schedule to self-administer 20% ethanol without water deprivation or saccharin/sucrose fading during 30-minute sessions. With this protocol, rats initiate lever pressing to obtain an ethanol reward very quickly, already obtaining more than 10 rewards during the first sessions (Figure 1A
Access restricted. Please log in or start a trial to view this content.
Discussion
With this protocol, we are presenting a new method to acquire and maintain stable oral self-administration of 20% ethanol in rats that, contrary to classic models of ethanol self-administration, does not require the use of water deprivation, extended access training, or saccharin/sucrose fading12. Furthermore, naltrexone, a currently FDA-approved medication for alcohol dependence, successfully decreases alcohol self-administration and the motivation to consume alcohol of Wistar rats trained with this protocol....
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Swedish Research Council.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Extra Tall MDF Sound Attenuating Cubicle, Interior: 22"W x 22"H x 16"D | Med Associates Inc. | ENV-018MD | |
| Extra Tall Modular Test Chamber with modified Top, Waste Pan and Photobeam | Med Associates Inc. | ENV-007CT-PH | |
| Stainless Steel Grid Floor for Rat or Small Primate | Med Associates Inc. | ENV-005 | |
| Retractable Lever | Med Associates Inc. | ENV-112CM | 2 by SA chambers |
| Stimulus Light, 1" White Lens, Mounted on Modular Panel | Med Associates Inc. | ENV-221M | 2 by SA chambers |
| Dual Cup Liquid Receptacle with 18 ga Stainless Steel Pipes | Med Associates Inc. | ENV-200R3AM | |
| Single Speed Syringe Pump, 3.33 rpm | Med Associates Inc. | PHM-100 | |
| Liquid Delivery Kit | Med Associates Inc. | PHM-122-18 | |
| SmartCtrl 8 Input / 16 Output Package | Med Associates Inc. | DIG-716P2 | |
| MED-PC software | Med Associates Inc. | SOF-735 | |
| http://www.mednr.com/ | Med Associates Inc. | A website that is open-source and has been created to offer researchers a place to exchange MEDState Notation code | |
| Kendall Monoject 20 cc Syringes, Regular Luer Tip | VWR International | MJ8881-520657 | |
| Ethanol, Pure, 190 Proof (95%), USP, KOPTEC | Decon Labs | 2801 | |
| 0.9% Sodium Chloride Injection, USP | Hospira | 0409-4888-50 | |
| Naltrexone hydrochloride | Sigma Aldrich | N3136-1G | |
| 23 G BD PrecisionGlide Needles | BD | 305145 | |
| Minivette POCT 50 µL, K3EDTA | Sarstedt | 17.2113.150 | For capillary blood collection |
References
- Koob, G. F., et al. Animal models of motivation for drinking in rodents with a focus on opioid receptor neuropharmacology. Recent developments in alcoholism : an official publication. of the American Medical Society on Alcoholism, the Research Society on Alcoholism, and the National Council on Alcoholism. 16, 263-281 (2003).
- Samson, H. H. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism, clinical and experimental research. 10, 436-442 (1986).
- Weiss, F., Mitchiner, M., Bloom, F. E., Koob, G. F. Free-choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology. , 178-186 (1990).
- Koya, E., et al. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 56, 177-185 (2009).
- Karlsson, R. M., Kircher, D. M., Shaham, Y., O'Donnell, P. Exaggerated cue-induced reinstatement of cocaine seeking but not incubation of cocaine craving in a developmental rat model of schizophrenia. Psychopharmacology. , 45-51 (2013).
- Augier, E., Vouillac, C., Ahmed, S. H. Diazepam promotes choice of abstinence in cocaine self-administering rats. Addiction biology. 17, 378-391 (2012).
- Cantin, L., et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PloS one. 5, (2010).
- Lenoir, M., Serre, F., Cantin, L., Ahmed, S. H. Intense sweetness surpasses cocaine reward. PloS one. 2, (2007).
- Spangler, R., et al. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain research. Molecular brain research. , 134-142 (2004).
- Rassnick, S., Pulvirenti, L., Koob, G. F. SDZ-205,152, a novel dopamine receptor agonist, reduces oral ethanol self-administration in rats. Alcohol. 10, 127-132 (1993).
- Carnicella, S., Yowell, Q. V., Ron, D. Regulation of operant oral ethanol self-administration: a dose-response curve study in rats. Alcoholism, clinical and experimental research. 35, 116-125 (2011).
- Augier, E., et al. Wistar rats acquire and maintain self-administration of 20 % ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology. , (2014).
- Macchia, T., et al. Ethanol in biological fluids: headspace GC measurement. Journal of analytical toxicology. 19, 241-246 (1995).
- Hodos, W. Progressive ratio as a measure of reward strength. Science. 134, 943-944 (1961).
- Williams, K. L., Broadbridge, C. L. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol. 43, 119-126 (2009).
- Czachowski, C. L., Delory, M. J. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology. , 335-348 (2009).
- Stromberg, M. F., Volpicelli, J. R., O'Brien, C. P. Effects of naltrexone administered repeatedly across 30 or 60 days on ethanol consumption using a limited access procedure in the rat. Alcoholism, clinical and experimental research. 22, 2186-2191 (1998).
- Stromberg, M. F., Casale, M., Volpicelli, L., Volpicelli, J. R., O'Brien, C. P. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 15, 281-289 (1998).
- Gonzales, R. A., Weiss, F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 18, 10663-10671 (1998).
- Biggs, T. A., Myers, R. D. Naltrexone and amperozide modify chocolate and saccharin drinking in high alcohol-preferring P rats. Pharmacology, biochemistry, and behavior. 60, 407-413 (1998).
- Beczkowska, I. W., Bowen, W. D., Bodnar, R. J. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain research. 589, 291-301 (1992).
- Cooper, S. J. Effects of opiate agonists and antagonists on fluid intake and saccharin choice in the rat. Neuropharmacology. 22, 323-328 (1983).
- Samson, H. H., Pfeffer, A. O., Tolliver, G. A. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcoholism, clinical and experimental research. 12, 591-598 (1988).
- Koob, G. F., Weiss, F. Pharmacology of drug self-administration. Alcohol. 7, 193-197 (1990).
- Schank, J. R., et al. The Role of the Neurokinin-1 Receptor in Stress-Induced Reinstatement of Alcohol and Cocaine Seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. , (2013).
- Avena, N. M., Bocarsly, M. E., Rada, P., Kim, A., Hoebel, B. G. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiology. 94, 309-315 (2008).
- Avena, N. M. The study of food addiction using animal models of binge eating. Appetite. 55, 734-737 (2010).
- Morgan, D., Sizemore, G. M. Animal models of addiction: fat and sugar. Current pharmaceutical design. 17, 1168-1172 (2011).
- Lenoir, M., Cantin, L., Vanhille, N., Serre, F., Ahmed, S. H. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 38, 1209-1220 (2013).
- Augier, E., et al. The mGluR2 Positive Allosteric Modulator, AZD8529, and Cue-Induced Relapse to Alcohol Seeking in Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41, 2932-2940 (2016).
- Bice, P. J., Kiefer, S. W. Taste reactivity in alcohol preferring and nonpreferring rats. Alcoholism, clinical and experimental research. 14, 721-727 (1990).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved