Method Article
A Battery of Motor Tests in a Neonatal Mouse Model of Cerebral Palsy
In This Article
Summary
Presented is a concise battery of mouse neonatal motor tests. Using these tests, neonatal motor deficits can be demonstrated in a variety of neonatal motor disorders. By having a standardized set of tests, results from different studies can be compared, allowing for better and accurate reporting between groups.
Abstract
As the sheer number of transgenic mice strains grow and rodent models of pediatric disease increase, there is an expanding need for a comprehensive, standardized battery of neonatal mouse motor tests. These tests can validate injury or disease models, determine treatment efficacy and/or assess motor behaviors in new transgenic strains. This paper presents a series of neonatal motor tests to evaluate general motor function, including ambulation, hindlimb foot angle, surface righting, negative geotaxis, front- and hindlimb suspension, grasping reflex, four limb grip strength and cliff aversion. Mice between the ages of post-natal day 2 to 14 can be used. In addition, these tests can be used for a wide range of neurological and neuromuscular pathologies, including cerebral palsy, hypoxic-ischemic encephalopathy, traumatic brain injury, spinal cord injury, neurodegenerative diseases, and neuromuscular disorders. These tests can also be used to determine the effects of pharmacological agents, as well as other types of therapeutic interventions. In this paper, motor deficits were evaluated in a novel neonatal mouse model of cerebral palsy that combines hypoxia, ischemia and inflammation. Forty-eight hours after injury, five tests out of the nine showed significant motor deficits: ambulation, hindlimb angle, hindlimb suspension, four limb grip strength, and grasping reflex. These tests revealed weakness in the hindlimbs, as well as fine motor skills such as grasping, which are similar to the motor deficits seen in human cerebral palsy patients.
Introduction
Developing new models of pediatric injury or disease using rodents is often difficult due to the amazing ability of both rats and mice to rapidly recover from neurological injury. Therefore, in order to validate any new pediatric disease model, thoroughly examining the cellular and molecular changes must go hand-in-hand with behavioral outcomes. In many ways, functional behavioral recovery may be more important than underlying cellular changes in terms of therapeutic or translational relevance. As researchers learn more about injury in the adult and neonate, it is clear that their responses are very different and cannot be extrapolated between the two. For example, neonatal mice display different levels of nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3 and glial cell line-derived neurotrophic factor following spinal cord injury 1,2. Additionally, neonates have significant blood-brain barrier leakage after stroke 3, demonstrate cortical neuron rearrangement after peripheral nerve injury 4, and have a delayed or slowed astrogliosis following spinal cord injury and hypoxia-ischemia 5,6. Therefore, it is important that translational pediatric research use developmentally equivalent models and that those models are evaluated for both cellular/molecular changes and age-appropriate behavioral tests.
Cerebral Palsy (CP) is a motor disorder that affects 3:1000 live births annually (NIH). Children with CP exhibit a range of symptoms and co-morbid conditions, depending on the severity of the disease. Difficulty with movement and coordination are the most common signs, along with delays in reaching motor developmental milestones. Other signs include abnormal muscle tone (either increased or decreased), reduced fine motor skills, difficulty walking, excessive drooling and swallowing, and speech delays (NIH). The underlying cause of CP is believed to be a lack of oxygen and/or blood flow to the brain during the pre- or peripartum period, or up to one-year post-partum. In addition, inflammation is now believed to be a key component in the development of CP.
The majority of CP cases are associated with white matter damage around the ventricles, known as periventricular leukomalacia (PVL). This neurological hallmark suggests that the initial insult leading to CP occurs during the period of brain development when the oligodendrocytes are most vulnerable to insult. The period of rapid oligodendrocyte growth in a human, also the period when oligodendrocytes are the most susceptible to injury, is between 24 - 32 weeks gestation. In the rodent, the equivalent period is post-natal days 2 - 7 7, and is when CP is induced in this model.
The neonatal mouse model of CP that was used to conduct the tests outlined here combines hypoxia and ischemia with inflammation to create an injury that better mimics the neurodegeneration seen in human CP. This model addresses some of the major shortcomings observed in other animal models of CP, which lack distinct motor deficits that resembles human CP patients, as well as distinct white matter damage. Previous studies by a collaborator using the same model have demonstrated that the addition of inflammation enhances white matter damage, thus better emulating the PVL seen in children with CP 8. Building on the previous data, this paper presents a comprehensive battery of neonatal motor tests in order to evaluate changes in motor behavior as the animal ages.
Protocol
NOTE: All animal surgeries were performed in accordance with Temple University's ULAR department and IACUC policies and procedures. C57BL/6 dams and sires were purchased from Charles River Laboratories and were housed in breeding cages with a 12 hr light/dark cycle (light on 7:00 - 19:00) with free access to food and water. Breeding pairs produced litter sizes between 5 - 10 pups.
1. Cerebral Palsy Induction Surgery
- NOTE: Cerebral palsy was induced using post-natal day (PND) 6 mouse pups, as previously described8,9 (https://www.jove.com/video/1951/mouse-models-of-periventricular-leukomalacia).
- Place a pup in a glass bowl on ice with a laboratory wipe to protect the pup's skin. Check for appropriate anesthetic plane by foot pinch and lack of movement. Move the pup to a padded ice pack for surgery.
- Sterilize the skin of the pup using 70% ethanol. When dry, use a # 11 sterile surgical blade and make a 1 cm incision in the neck.
- Using a stereoscopic surgical microscope, isolate the right common carotid artery with a small hook and cauterize using a portable hand-held cauterizer. Visually confirm that the artery is occluded. Sham surgery includes visualization and isolation of the common carotid artery without cauterization.
- Realign the skin and close using suture glue (n-butyl cyanoacrylate).
- Place the pup on a 34 oC heating pad for 30 min to monitor for spontaneous breathing and normal movement.
- Return the pup(s) to dam for 30 min.
- Place the pups on a heating pad or other warming device set at 34 oC inside a hypoxia chamber set at 6% oxygen for 35 min. Oxygen is replaced by nitrogen. Closely monitor the chamber oxygen level and temperature for consistent injury results.
- Remove the pups from the hypoxia chamber and return them to the heating pad.
- Intraperitoneally inject lipopolysaccharide diluted in sterile saline at 1 µg/kg and return the pup to the dam. Sham injections are injections of saline only.
2. Neonatal Motor Tests
NOTE: On PND 8, 48 hr after CP induction, mouse pups are tested for neurobehavioral development. Pups are tested within a 4 hr block before noon in order to eliminate time of day differences in behavior. Pups are removed from the dam for no more than 15 min at a time to prevent rapid loss of body heat and hunger/separation issues. In addition, pups are allowed to rest in between tests so that maximal efforts will be elicited on each test. The basis of the neonatal motor tests is adapted using Fox's battery of tests 10,11 and Wahlsten's adaption of Fox's tests 12, as well as Treat-NMD and other behavior publications (as noted in the text for each test). Fox's battery of tests are appropriate for PND 2 - 21. Of Fox's tests, the battery present here includes: righting reflex, grasping reflex, negative geotaxis (called vertical screen test in Fox's battery) and four limb grip strength (modified from Fox and Wahlsten's screen climbing tests). Here, ambulation, front-limb strength, and hindlimb strength are also tested to distinguish reflexive motor behavior between sham and CP mouse pups. To eliminate improvements on testing due to learning, tests were limited to a maximum of 3 trials where noted. All other tests had only one trial per animal.
- Ambulation (Figure 1) (adapted from a rat protocol 13):
NOTE: Crawling is a behavior developed early in the mouse pup between PND 0 - 5, at which point mice begin to transition to walking, from 5 - 10 days old 14. At PND 8, the ambulation test takes advantage of this transitional time course. Ambulation can, however, be scored throughout the lifetime of a mouse and can be determined at any age. As there is no potential for learning, the ambulation test can be repeated as many times as needed through the course of the experiment.- Place mice in a clear enclosure where mice are visible from the top as well as the side. Use gentle prodding by touching the pup's tail to motivate the pup to walk.
- Score ambulation for 3 min using the following scale: 0 = no movement, 1 = crawling with asymmetric limb movement, 2 = slow crawling but symmetric limb movement, and 3 = fast crawling/walking.
NOTE: Here, symmetric limb movement is described where hindpaws meet frontpaws during each step, and each step smoothly transitions to the next step. A mouse displaying asymmetric limb movement has erratic paw placement and transitions from one step to the next are not smooth.
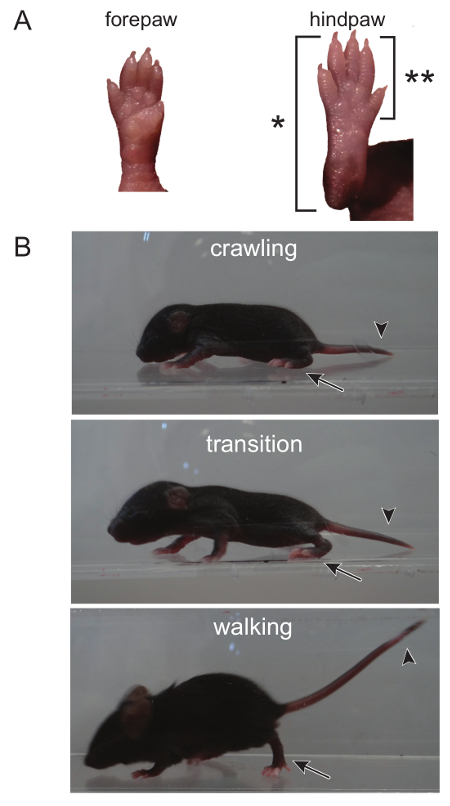
Figure 1. The Transition From Crawling to Walking can be Distinguished by Observing the Hindpaw, as well as the Head and Tail. (A) During crawling, the entire back paw, from the toes to the heel, touches the ground when ambulating, as denoted by (*). An adult walking pattern is seen when only the toes and front part of the hindpaw touch the ground (the heel is elevated, deonoted by [**]). (B) The head and tail of a crawling mouse is low to the ground. The head begins to rise during the transition from crawling to walking. The transition is complete when both the head and tail are elevated and only the front of the hindpaw touches the ground. Please click here to view a larger version of this figure.
- Hindlimb Foot Angle (Figure 2)
NOTE: There is an apparent developmental change in the hindlimb posture as the mouse matures from crawling to walking, where the hindlimbs are positioned under the body when walking and the angle between the hindlimbs is less than the angle seen in crawling. Even though the hindlimb foot angle changes over time, mouse pups of the same age with different injury or diseases can be compared. Similar to the ambulation test (3.1), there is no potential for learning. Thus, the hindlimb foot angle test can be repeated as many times as needed through the course of the experiment.- Either in a clear open field box or an enclosed area, mount a video camera from below or above, respectively, to record the pup as it moves around the field. Use gentle prodding by touching the pup's tail to motivate the pup to walk. Record for two mins.
- Using the video recordings, measure the foot angle of the pups by drawing a line from the end of the heel/shin to the tip of the longest (middle) toe. Only take the measurement when the pup is performing a full stride in a straight line and both feet are flat on the ground. Do not take measurements while the pup is stationary or while the pup is turning.
- Measure three to five sets of foot angles and calculate the average angle for each pup tested.
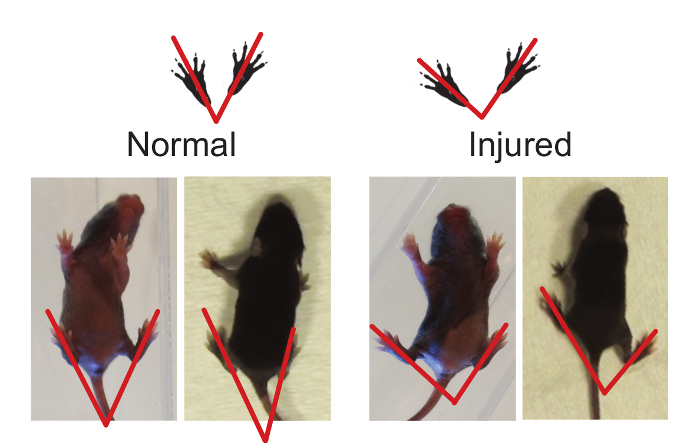
Figure 2. Hindlimb Foot Angle can be Used to Determine Gait Abnormalities. The foot angle can be measured by drawing a line from the mid-heel through the middle (longest) digit. Injured animals have a greater foot angle when compared to normal (see Representative Results, Foot Angle). Please click here to view a larger version of this figure.
- Surface Righting (Figure 3):
NOTE: The righting reflex is the motor ability for a mouse pup to be able to flip onto its feet from a supine position. The average age for the righting reflex to appear in rodents is PND 5 with a range from PND 1 - 10 15. As this test is a reflex, there is no learning component and it can be repeated throughout the experimentation period.- Place pups on their backs on a cotton sheet or bench pad and hold in position for 5 sec.
- Release the pups and record the time it takes the pup to return to prone position, as well as the direction of righting (left or right). A total of one min is given for each trial, if needed.
- Repeat for a total of three trials.
Figure 3. Surface Righting. This test requires trunk control and may test for postural imbalances. Human CP patients may have deficits in their core. Please click here to view a larger version of this figure.
- Negative Geotaxis (Figure 4)
NOTE: The average age for negative geotaxis reflex to appear in rodents is PND 7 with a range from PND 3 - 15 15. The negative geotaxis test assesses motor coordination in young mice. Mice are placed facing down a slope and, due to vestibular cues of gravity, pups turn to face up the slope. The response to stimulus, or taxis, is an innate behavior.- Place the pup with its head pointing downward on a 45o incline and hold it for 5 sec.
- Release the pup and record the time and direction the pup turns to face upward. Total testing time is 2 min.
- Repeat for a total of three trials. Mice that fall down the incline or fail to turn can be either re-tested, eliminated, or given a zero score.
NOTE: This decision is left to the examiner, as occasionally pups will roll down the incline due to sleepiness rather than weakness. Once the decision is made on how to score pups that fall down the incline, it should be noted in the methods and should be consistent throughout the testing of all subjects.
Figure 4. Negative Geotaxis. Motor and vestibular input is required for the mouse to recognize its orientation on a slope and turn around. Please click here to view a larger version of this figure.
- Front-limb Suspension (Figure 5) 16; adapted from 17,18 :
NOTE: Front-limb suspension tests the forelimb strength of pups, including arm and paw strength. This test is not recommended for pups younger than PND 10 15. Pups are allowed to grasp a wire strung across a stable object and hang onto the wire with both forepaws. The testing area is over a padded drop zone. The test can detect right/left side strength differences. Learning and the absence of negative reinforcement can lead to increased non-participation. Mice falling immediately when released or failure to grasp when placed on the wire are indicative of non-participation.- Hold the pups firmly by the body and enable them to grasp the wire with both forepaws.
- Release the pup. Using a timer or stopwatch, record the total time to fall, as well as paw weakness.
NOTE: Paw weakness is determined if a pup consistently falls from the wire with one paw before the other rather than releasing from the wire with both paws at the same time. - Repeat test for a total of three times.
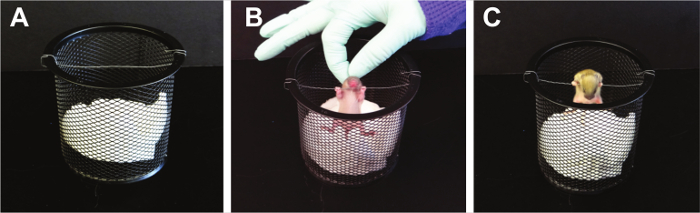
Figure 5. Front-Limb Suspension. This suspension test causes tension in the forelimbs until muscle fatigue. With this approach, baseline strength in the forelimbs are established. Please click here to view a larger version of this figure.
- Hindlimb Suspension (Figure 6):
NOTE: This suspension test determines hindlimb strength. It is a test designed specifically for neonates and was initially used on animals between PND 2 - 12 19,20, but can be adapted for mice up to PND 14. This test can detect right/left hindlimb strength differences as well as neuromuscular function. A standard 50 ml conical is used, padded with laboratory wipes. Similar to the front-limb suspension test, this test can be learned, especially since there is no negative consequence to falling. Thus, increased non-participation, as seen by mice falling as soon as released or failure to stay when placed on the edge of the tube, may be noted.- Using a 50 ml conical, place pup gently face down into the tube with its hind legs hung over the rim.
- Release the pup. Observe the hindlimb posture.
- Score posture according to the following criteria.
NOTE: Score of 4 indicates normal hindlimb separation with tail raised; score of 3 means weakness is apparent and hindlimbs are closer together but they seldom touch each other; score of 2 indicates hindlimbs are close to each other and often touching; score of 1 shows a weakness is apparent and the hindlimbs are almost always in a clasped position with the tail raised; a score of 0 indicates constant clasping of the hindlimbs with the tail lowered or failure to hold onto the tube for any period of time. - Count pulls if necessary. A pull is qualified when the pup attempts to lift its body using its hindlimbs while suspended on the side of the conical tube.
- Using a timer or stopwatch, record the latency to fall.
- Repeat the entire test in triplicate.
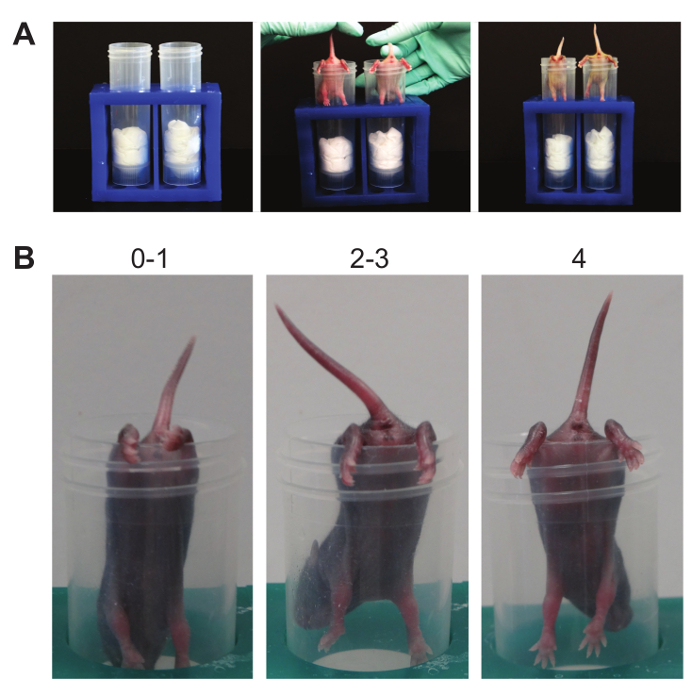
Figure 6. Hindlimb Suspension. (A). This suspension test causes tension in the hindlimbs until muscle fatigue. Baseline strength and posture in the hindlimbs are established. (B). Scoring. Note the numbers above the representative mice demonstrating the possible posture score. Please click here to view a larger version of this figure.
- Grip Strength (Figure 7):
NOTE: This test will examine the paw strength of all four paws at the same time. A 16 x 18 fiberglass screen wire is used. The average age for a rodent to be able to grasp a horizontal screen is PND 8 with a range from PND 5 - 15 15. Fox used the four limb horizontal screen test from PND 2 - 21 10. This test is modified from the standard horizontal screen test; here the screen is rotated slowly from a horizontal to vertical position, to challenge the grasping of all four limbs 21; adapted from Corti S 16. If the mouse holds on to the mesh screen when inverted to 180°, record the latency to fall. Also, note the body weight. A hanging impulse can be calculated as [weight (g) x latency to fall (sec)] reflecting the force needed to resist gravity.- Using a piece of wire mesh, place the pup on the screen. Allow the pup to adjust to this environment for approximately 5 secs.
- Invert the screen slowly to 180 degrees. Record the approximate angle of the screen when the pup falls off.
- Repeat for a total of three trials and average the trials.
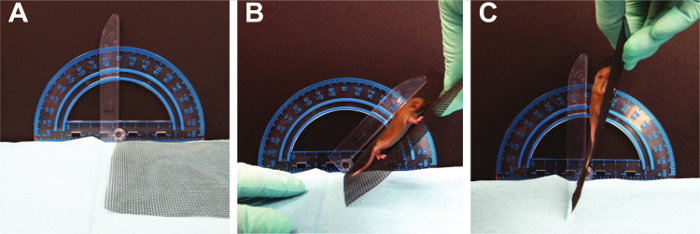
Figure 7. Grip Strength. Mice are required to sustain muscle tension in all four limbs as gravitational force increases. Please click here to view a larger version of this figure.
- Grasping Reflex (Figure 8)
NOTE: The grasping reflex usually appears in rodents at PND 7 with a range from PND 3 - 15 15. Each paw is tested individually, thus the test can reveal front- or hindlimb issues, as well as sidedness issues. As it is a reflex, this test can be repeated until the reflex appears. It is not prone to learning. As an important caveat, this test does not distinguish grasping strength, only ability, and must be tested prior to 15 days of age when juvenile mice begin to grasp due to fear response.- Hold the mouse by the scruff of its neck, similar to the way a mouse pup is carried by the dam. This hold causes the pup to become instinctively immobile and relaxed, allowing for ease of testing.
- Stroke each paw of the pup with the blunt, rounded side of a razor blade.
- Test each paw individually and record the presence or absence of grasping and score 1 point per paw with which the mouse grasps.
NOTE: The scoring for right paw preference is 100% for right paw preference, - - -100% for left paw preference, 50% for both paws grasping, and 0% for no paws grasping. The equation to determine these numbers is [(right paw - left paw)/(right paw + left paw + both paws)] x 100%.
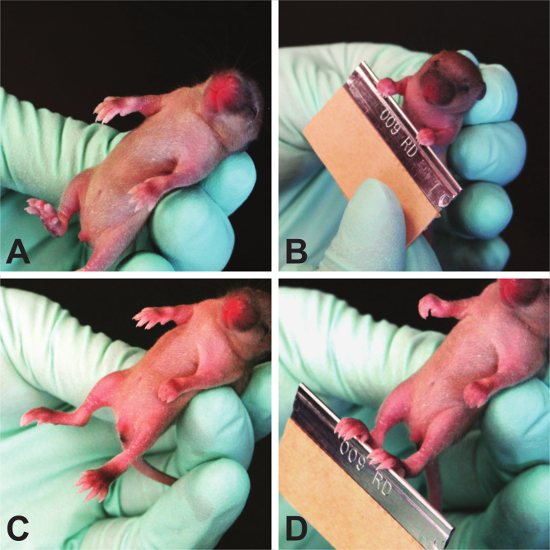
Figure 8. Grasping Reflex. Because neonatal mice do not have a strong fear response, this test strictly determines the plantar/palmar reflex. Please click here to view a larger version of this figure.
- Cliff Aversion (Figure 9):
NOTE: Cliff aversion tests labyrinth reflexes, as well as strength and coordination and can be used to test pups from PND 1-14 22. A pre-scented box (a box where a minimum of 5 mice have been allowed to freely roam) with a flat elevated ledge is used and the pup is placed with the digits only of their forepaws and their snout positioned over the edge. Scoring is performed by counting the total time it takes the pup to turn away from the cliff and move its paws and snout away from the edge. If no response is seen after 30 sec, the test is terminated. If the pup falls off the edge, a single additional trial can be performed.- Using a side view, place the pup on the edge of the pre-scented box, making sure that the forepaws, digits and snout are the only parts over the edge.
- Release pup and start timer.
- Once both the snout and paws have been removed from the edge, stop the timer and record time.
- Repeat test for a total of 3 trials. If the pup does not move away from cliff within 30 sec, no score is given.
NOTE: The determination whether the pup is a non-participator versus impaired is left up to the discretion of the examiner. The height of the cliff may be adjusted for the age of the pup to assure the pup’s safety. A smaller height can be used with a black “floor” to emulate a greater height.
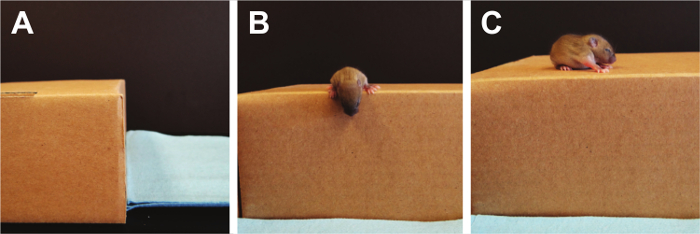
Figure 9. Cliff Aversion. Vestibular imbalances are measured using the cliff aversion test. Here, the pup's eyes are still closed so fear is not the driving factor to turn away from the cliff's edge. Please click here to view a larger version of this figure.
3. Statistical Significance
- Using a statistical software analyze the results. Express the data as mean ± standard error of the mean (SEM). Tests are parametric and thus, examine the data using t-test analyses.
NOTE: Experiments were not designed to test for gender differences. Differences are considered to be statistically significant when p < 0.05.
Results
Mice were tested from P7 (24 hr following surgery) to P13 (1 week following surgery), using different mice for each time-point so that learning a testing paradigm was not a confounding variable. P8 was selected as representative results, as mice showed the greatest deficits at this time point.
Transition from Crawling to Walking is Delayed in CP Neonatal Mice
Human CP patients have gait abnormalities, ranging from toe-walking to a scissored gait. As this CP model displays gait deficits similar to humans, ambulation was assessed. Mice were scored on gait symmetry and limb-paw movement during a straight walk. At 48 hr following surgery (PND 8), CP mice had less symmetric limb movement and a "crawling" gait as compared to their sham counterparts (average ambulation score: CP 1.083 ± 0.6337, n = 12 vs sham 1.639 ± 0.4859, n = 9; p < 0.05, Figure 10). By one week, both CP and sham mice have transitioned to walking (data not shown).
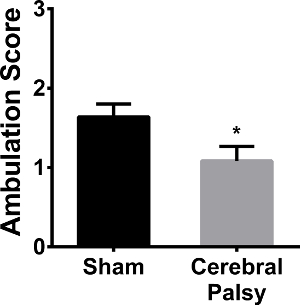
Figure 10. CP Mouse Pups do not Ambulate as well as Shams. Sham mice (black bar) have a mean score of 1.639 ± 0.4859 (n = 9), meaning their ambulatory development falls between asymmetric limb movement and slow crawling. CP mice (gray bar) receive an average score of 1.083 ± 0.6337 (n = 12), meaning their ambulation is less developed and tend to have asymmetric limb movement. Data are expressed as mean ± SEM; * is p < 0.05.Please click here to view a larger version of this figure.
Hindlimb Foot Angle is Increased in CP
In addition to ambulation, hindlimb foot-angle was assessed. Eight-day-old sham mouse pups walk with their hindpaws facing forward, compared to HIL mice, who have splayed hindpaws when walking in a straight line (Figure 2; average angle: CP 77.48 ± 9.848, n = 9, vs sham 54.54 ± 8.043, n = 11; p < 0.0001, Figure 11). This increased angle correlates with gait instability, in that the pups need to increase the angle of their rear paws in order to stabilize their gait and assist with balance and coordination.
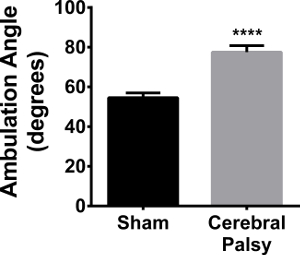
Figure 11. CP Mouse Pups Splay their Hindpaws When Walking. CP mice (black bars) have an average angle between their hindlimbs of 77.48 ± 8.043 (n = 11), while sham mice (gray bars) have an average angle of 54.54 ± 9.848 (n = 9). Data are expressed as mean ± SEM; **** is p < 0.0001. Please click here to view a larger version of this figure.
CP Mice do not Show Deficits when Surface Righting
The surface righting test was included as some CP patients have impaired trunk control (Heyrman et al., 2013). Additionally, the vestibular system is necessary to detect the need for righting and there are vestibular deficits in some CP patients23. CP mice do not show significant deficits when righting as compared to sham controls (data not shown).
CP Mice Perform the Same as Sham in Negative Geotaxis Testing
Negative geotaxis is used to test motor coordination in young pups. Mice are challenged by being place facing downhill on a sloped surface. Delay or failure to orient uphill could indicate deficits in coordination, balance, or vestibular input. CP mice show no deficits when challenged with negative geotaxis as compared to sham mice (data not shown). Additionally, CP mice did not show a preference to turn toward one side versus another when re-orienting.
Front-Limb Suspension Test is Appropriate for Mice Older than 10 Days
CP patients have decreased muscle tone and deficits in fine motor skills, such as grasping. To test weakness in this mouse model, we used a front-limb suspension test. Furthermore, this model uses unilateral ischemic injury and sided-ness could be determined using this suspension test. This test is better for mice older than 10 days 15. At 8 days old, two days following injury, there were no significant differences between CP and sham mice (data not shown).
Hindlimb Strength is Decreased in CP Mice
Human CP patients often need braces or assistive walking devices due to lack of motor control and strength. In order to compare the rodent CP model to humans, hindlimb strength was assessed using the hindlimb suspension test. When suspended from the side of a conical tube, CP mice showed hindlimb weakness, as demonstrated by a decrease in hanging score (hindlimb hanging score: CP 3.468 ± 0.5561, n = 13, vs sham 3.891 ± 0.1329, n = 13; p < 0.05, Figure 12). No difference was observed in hindlimb suspension time (data not shown). Thus, similar to human CP patients, CP mice demonstrate hindlimb (leg) weakness.
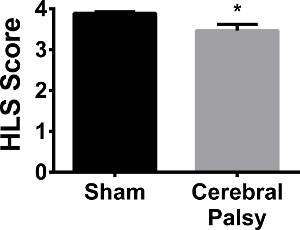
Figure 12. Sham Mice are Slightly but Significantly Stronger in their Hindlimbs than CP Mice. At an average hanging score of 3.891 ± 0.1329 (n = 13), sham mice (black bar) show more hindlimb separation, and therefore a stronger hindlimb stance, when hanging on the edge of a tube than CP mice (gray bar) with an average hanging score of 3.468 ± 0.5561 (n = 13). Data are expressed as mean ± SEM; * is p < 0.05. Please click here to view a larger version of this figure.
Grip Strength is Decreased Following CP Injury
Grasping with all four paws is important for a rodent in terms of climbing and running across uneven surfaces. Grip requires significant sustained strength, rather than dexterity or linear force, mainly in the digits and paws 24. Mice were required to hold their body weight on an inverted wire mesh screen. CP mice were not able maintain their grip and these mice fell at significantly lower angles (four limb average angle: CP 75.627 ± 24.48, n = 11, vs sham 96.57 ± 10.836, n = 9; p < 0.05, Figure 13). This data shows that there is a significant deficit in grip strength in CP mice.
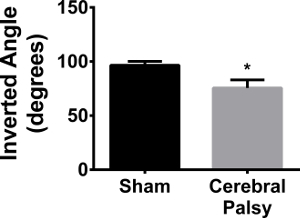
Figure 13. CP Mice have Weaker Grip than Shams. Sham mice (black bar) can grasp to an average inverted angle of 96.57 ± 10.836 (n = 9). CP mice (gray bar) can only reach an inverted angle of 75.627 ± 24.48 (n = 11). Data are expressed as mean ± SEM; * is p < 0.05. Please click here to view a larger version of this figure.
Grasping Reflex Deficits are Apparent in CP Mice
Along with gross motor deficits, fine motor movements are also impaired in CP patients 25,26. The grasping reflex in humans is present at birth and disappears around 5 - 6 months. However, changes in the grasping reflex, such as exaggerated velocity or strength of grasping, failure to grasp, or the reemergence of the grasping reflex after 6 months of age, all indicate damage to the nervous system. To compare grasping in the CP model, reflexive grasping deficits were determined.
At 48 hr after injury, CP mice demonstrate a decrease in grasping reflex (average paws grasped at 48 hr: CP 2.429 ± 0.9376, n = 14, vs sham 3.214 ± 0.8018, n = 14; p < 0.05, Figure 14A). There was a slight, but not significant increase in right paw preference in the forepaws (data not shown). There was a significant right paw preference in the hindpaws (CP 75.0 ± 42.74, n = 14, vs sham 17.86 ± 54.09, n = 14; p < 0.005, Figure 14B). One week after injury, CP mice show grasping deficits (average paws grasped at 1 week: CP 2.75 ± 1.035, n = 8, vs sham 3.80 ± 0.6325, n = 10; p < 0.05, Figure 14C), with no notable paw preference.
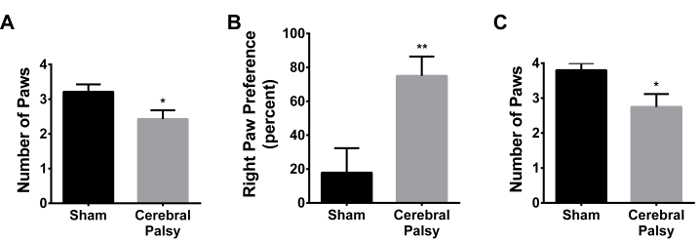
Figure 14. CP Mice have Grasping Deficits, in the Hindpaws, Contralateral to the Injured Brain Region. (A) 48 hr following injury (PND 8), CP mice (gray bar) grasp a stick with, on average, fewer paws than sham animals (black bar). (B) CP mice (gray bar) display a preference for grasping with the right hindpaw (contralateral to injury) as opposed to using the left hindpaw (ipsilateral to injury). Sham mice (black bar) do not display this right paw preference. Right paw preference is calculated as ([right paw - left paw]/[right paw + left paw + both paws] * 100). (C) One week following injury, CP mice (gray bar) still show grasping deficits as compared to shams (black bar). Data are expressed as mean ± SEM; * is p < 0.05, ** is p < 0.005. Please click here to view a larger version of this figure.
CP Mice turn away from the Edge During Cliff Aversion
The cliff aversion test relies on the inherent fear of the mice to turn away from a steep cliff and head towards safety. Although some CP patients have vestibular difficulties, as well as impaired motor control, the CP mice did not show any deficits on this test.
Discussion
Using animal models to study human diseases is only relevant if there is overlap between the cellular and molecular response between human and rodent and that the behavioral tests performed have direct relevance to human symptoms. One of the major issues with pediatric disease studies is that many researchers use adult rodents to create the model, as well as adult rodent behavioral evaluation, without considering the developmental differences that may be important for the disease process. Because of these issues, it is important that research on pediatric disease use not only the appropriate adjusted developmental time-points (e.g., human CNS development at 28 - 32 weeks is equivalent to a post-natal day 2 - 7 day rodent) 7, but also behavioral tests that will examine appropriate motor, sensory or reflexive developmental behaviors. Thus, as each new neonatal disease model is developed, it must be rigorously tested to assure that the cellular and behavioral responses will provide the most appropriate translatable data between rodent and human.
Cerebral palsy is a motor disorder, which persist into adulthood. One problem with many of the cerebral palsy models available today is the lack of repeatable, standardized motor testing that can correlate with the deficits seen in pediatric patients. In this new model, which combines hypoxia, ischemia and inflammation in a neonatal mouse, motor behavior was evaluated using a battery of tests specific for neonatal mice. In order to decrease the subjectivity and increase the quantitative reporting, several tests have been modified to include very specific, but easy to evaluate measures that can be standardized. In addition, front- and hindlimb evaluations can be performed separately, and left/right differences can be determined. This battery of tests is specific for neonatal mice up to two weeks of age.
This CP model demonstrates difficulty in walking (ambulation, hindlimb foot angle), as well limb-specific weakness (four limb suspension, hindlimb suspension), and deficits in developmental reflexes (grasping reflex). Although in this study only one timepoint was examined, these deficits can be tracked over time.
There are other batteries of tests that can be used on the neonate, such as the Fox's battery of tests or Heyser's Assessment of Developmental Milestones 15. However, these tests compare the neonate to the adult, whose responses may not be the same because the neonate is still developing. Fox's battery and Heyser's Assement tests rely on observational subjective information with dichotomous (yes or no) assessment, rather than objective data (angle, posture based on strength, etc). Because of the subjectiveness of these tests, many scientists have adapted, added, or removed criteria, thus making their results incomparable to others and limiting the usefulness of the data in terms of establishing a baseline deficit for a particular disease or disorder. By establishing one set of standardized motor tests that are qualitative and specifically designed to test neonates, results from individual research groups can be accurately and reliably reported and compared.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
We would like to thank everyone at Shriners Hospital Pediatric Research Center, in particular Dr. Mickey Seltzer, of whom without his support, this work would not have been funded. In addition, we would like to thank Isha Srivastava, who contributed to early data collection and Amy He, who helped with the figures. This study was funded by Shriners Hospitals for Children. No funding source played a role in experimental design or decision to submit the paper for publication.
Materials
| Name | Company | Catalog Number | Comments |
| C57BL/6 mice | Charles River Laboratories | STRAIN CODE: 027 | C57BL/6NCrl is the exact strain we use |
| Anesthesia Dish, PYREX™ Crystallizing Dish | Corning Life Sciences Glass | 3140125 | Capacity: 25.03 oz. (740ml); Dia. x H: 4.92 x 2.55 in. (125 x 65mm). However, any small round glass container will work. A 2 cup capacity pyrex food storage bowl with flat bottom will also work and is much cheaper (Pyrex model number: 6017399). |
| Covered lead ring | Fisher Scientific | S90139C | Lead ring for stablizing flasks in a water bath. It is used inside the anesthesia dish. |
| Scalpel Blade #11 | World Precision Instrucments, Inc. | 500240 | |
| Small Vessel Cauterizer | Fine Science Tools | 18000-00 | |
| Micro Hook | Fine Science Tools | 10064-14 | |
| Vetbond Suture Glue | 3M | 1469SB | n-butyl cyanoacrylate adhesive |
| Lipopolysaccharide | Sigma Life Science | L4391 | Lipopolysaccaride from E.coli 0111:B4, gamma irradiated |
| 12 x 12 inch opaque box | Acrylic Display Manufacturing: A division of Piasa Plastics | C4022 | Colored Acrylic 5-Sided Cube, 3/16" Colored Acrylic, 12"W x 12"D x 12"H; http://www.acrylicdisplaymfg.com/html/cubes_19.html |
| Camera/camcorder | JVC | GC-PX100BUS | Any camcorder that works well in low light and can be imported and edited. We use the JVC GC-PX100 Full HD Everio Camcorder. |
| Covidien Tendersorb™ Underpads | Kendall Healthcare Products Co | 7174 | |
| WypAll L40 | Kimberly-Clark Professional | 5600 | Any surface with moderate grip will do |
| Surface at 45 degree incline | We use a cardboard box. | ||
| Thin wire from a pipe cleaner | Creatology | M10314420 | Any pipe cleaner from any craft store will work. |
| 50mL conical tube | Falcon | 352070 | |
| Fiberglass Screen Wire | New York Wire www.lowes.com | 14436 | Any supplier can be used as long as their screen is 16 x 16 or 18 x 16 |
| Razor blade | Fisherbrand | 12-640 | A wooden stick applicator or wooden part of a cotton-tipped swab will also work. |
| OPTIX 24-in x 4-ft x 0.22-in Clear Acrylic Sheet to make Clear Acrylic Walkway | PLASKOLITE INC | 1AG2196A | Clear acrylic (1/8" thick) with sides and a top to limit exploration. We bought a sheet of acrylic from a local hardware store and had them cut it to size. (2) 2" x 2"; (3) 2" x 18"; (1) 2" x 15.5"; (1) 2" x 3". Using clear tape, tape all sides together, with the 15.5" piece on top. Tape the 3" piece to the end of the 15.5" piece to create a flap/entryway for the mice. Alternatively, part or all of the walkway can be glued together, and only taping on the top pieces. This design will allow for the walkway to be opened for easy cleaning. |
| Protractor | Westscott | ACM14371 |
References
- Nakamura, M., Bregman, B. S. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp Neurol. 169 (2), 407-415 (2001).
- Widenfalk, J., Lundströmer, K., Jubran, M., Brene, S., Olson, L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 21 (10), 3457-3475 (2001).
- Fernández-Lòpez, D., Faustino, J., et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci. 32 (28), 9588-9600 (2012).
- Cusick, C. G. Extensive cortical reorganization following sciatic nerve injury in adult rats versus restricted reorganization after neonatal injury: implications for spatial and temporal limits on somatosensory plasticity. Prog Brain Res. 108, 379-390 (1996).
- Barrett, C. P., Donati, E. J., Guth, L. Differences between adult and neonatal rats in their astroglial response to spinal injury. Exp Neurol. 84 (2), 374-385 (1984).
- Villapol, S., Gelot, A., Renolleau, S., Charriaut-Marlangue, C. Astrocyte Responses after Neonatal Ischemia: The Yin and the Yang. Neuroscientist. 14 (4), 339-344 (2008).
- Craig, A., Ling Luo, N., et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 181 (2), 231-240 (2003).
- Shen, Y., Liu, X. B., Pleasure, D. E., Deng, W. Axon-glia synapses are highly vulnerable to white matter injury in the developing brain. J Neurosci Res. 90 (1), 105-121 (2012).
- Shen, Y., Plane, J. M., Deng, W. Mouse models of periventricular leukomalacia. J Vis Exp. (39), (2010).
- Fox, W. M. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 13 (2), 234-241 (1965).
- Tremml, P., Lipp, H. P., Müller, U., Ricceri, L., Wolfer, D. P. Neurobehavioral development, adult openfield exploration and swimming navigation learning in mice with a modified beta-amyloid precursor protein gene. Behav Brain Res. 95 (1), 65-76 (1998).
- Wahlsten, D. A developmental time scale for postnatal changes in brain and behavior of B6D2F2 mice. Brain Res. 72 (2), 251-264 (1974).
- Balasubramaniam, J., Xue, M., Del Bigio, Long-term motor deficit following periventricular hemorrhage in neonatal rats: A potential model for human cerebral palsy. J Cerebr Blood F Met. , (2005).
- Williams, E., Scott, J. P. The Development of Social Behavior Patterns in the Mouse, in Relation to Natural Periods. Behaviour. 6 (1), 35-65 (1954).
- Heyser, C. J. Assessment of developmental milestones in rodents. Current protocols in neuroscience. Crawley, J. Q., et al. Chapter 8, (2004).
- Corti, S. Grip strength TREAT-NMD: Experimental protocols for SMA animal models. , http://www.treat-nmd.eu/research/preclinical/sma-sops/ (2014).
- Corti, S., Nizzardo, M., et al. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. J Clin Invest. 118 (10), 3316-3330 (2008).
- Grondard, C., Biondi, O., et al. Regular exercise prolongs survival in a type 2 spinal muscular atrophy model mouse. J Neurosci. 25 (33), 7615-7622 (2005).
- El-Khodor, B. F. Behavioral Phenotyping for Neonates. Experimental Protocols for SMA animal models. , (2011).
- El-Khodor, B. F., Edgar, N., et al. Identification of a battery of tests for drug candidate evaluation in the SMNDelta7 neonate model of spinal muscular atrophy. Exp Neurol. 212 (1), http://www.treat-nmd.eu/research/preclinical/sma-sops/ 29-43 (2008).
- Venerosi, A., Ricceri, L., Scattoni, M. L., Calamandrei, G. Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environ Health. 8 (12), (2009).
- Hill, J. M., Lim, M. A., Stone, M. M. Developmental milestones in the newborn mouse. Neuromethods 39: Neuropeptide Techniques. , Humana Press. New Jersey. (2008).
- Visual dependence influences postural responses to continuous visual perturbation in adults with spastic cerebral palsy. Yu, Y., Keshner, A. E., Tucker, C. A., Thompson, E. D., Lauer, R. T. Combined Sections Meeting of American Physical Therapy Association, Anaheim, CA, USA, , (2016).
- Carlson, G. The use of four limb hanging tests to monitor muscle strength and condition over time. Experimental Protocols for SMA animal models. , http://www.treat-nmd.eu/downloads/file/sops/dmd/MDX/DMD_M.2.1.005.pdf (2011).
- Gordon, A. M., Duff, S. V. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 41 (9), 586-591 (1999).
- Futagi, Y., Toribe, Y., Suzuki, Y. The grasp reflex and moro reflex in infants: hierarchy of primitive reflex responses. Int J Pediat. , (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved