A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Surgical Placement of Catheters for Long-term Cardiovascular Exercise Testing in Swine
In This Article
Summary
Here we present a protocol to assess cardiopulmonary function in awake swine, at rest and during graded treadmill exercise. Chronic instrumentation allows for repeated hemodynamic measurements uninfluenced by cardiodepressive anesthetic agents.
Abstract
This protocol describes the surgical procedure to chronically instrument swine and the procedure to exercise swine on a motor-driven treadmill. Early cardiopulmonary dysfunction is difficult to diagnose, particularly in animal models, as cardiopulmonary function is often measured invasively, requiring anesthesia. As many anesthetic agents are cardiodepressive, subtle changes in cardiovascular function may be masked. In contrast, chronic instrumentation allows for measurement of cardiopulmonary function in the awake state, so that measurements can be obtained under quiet resting conditions, without the effects of anesthesia and acute surgical trauma. Furthermore, when animals are properly trained, measurements can also be obtained during graded treadmill exercise.
Flow probes are placed around the aorta or pulmonary artery for measurement of cardiac output and around the left anterior descending coronary artery for measurement of coronary blood flow. Fluid-filled catheters are implanted in the aorta, pulmonary artery, left atrium, left ventricle and right ventricle for pressure measurement and blood sampling. In addition, a 20 G catheter is positioned in the anterior interventricular vein to allow coronary venous blood sampling.
After a week of recovery, swine are placed on a motor-driven treadmill, the catheters are connected to pressure and flow meters, and swine are subjected to a five-stage progressive exercise protocol, with each stage lasting 3 min. Hemodynamic signals are continuously recorded and blood samples are taken during the last 30 sec of each exercise stage.
The major advantage of studying chronically instrumented animals is that it allows serial assessment of cardiopulmonary function, not only at rest but also during physical stress such as exercise. Moreover, cardiopulmonary function can be assessed repeatedly during disease development and during chronic treatment, thereby increasing statistical power and hence limiting the number of animals required for a study.
Introduction
Adequate cardiopulmonary function is essential to supply the body with oxygen and nutrients, particularly during conditions of increased metabolic demand such as during exercise1. The cardiopulmonary response to exercise is characterized by a number of adaptations in cardiac function, i.e., an increase in heart rate, contractility and stroke volume, and microvascular function, i.e., vasodilation in the vascular beds supplying exercising muscles as well as in the pulmonary vasculature, and vasoconstriction in the vascular beds supplying the gastrointestinal system as well as inactive muscles1. Impaired exercise capacity is an early hallmark of cardiopulmonary dysfunction, and cardiopulmonary exercise testing is used as an effective method to delineate between cardiac dysfunction, vascular dysfunction and/ or pulmonary dysfunction in patients with impaired exercise capacity2. Early cardiopulmonary dysfunction is difficult to diagnose, particularly in animal models, as cardiopulmonary function is often measured invasively, requiring anesthesia, with many anesthetic agents possessing cardiodepressive properties3.
Chronic instrumentation allows for measurement of cardiopulmonary function in the awake state, and when the animals are fully adjusted to the laboratory conditions measurements can be obtained under quiet resting conditions without the effects of anesthesia and acute surgical trauma. Furthermore, when the animals are appropriately trained, measurements can also be obtained during graded treadmill exercise4,5. More specifically, left and right ventricular function can be assessed and related to myocardial perfusion, while regulation of vasomotor tone in the coronary, systemic and pulmonary microcirculation can be determined. The use of fluid-filled catheters allows measurement of pressure as well as taking blood samples without imposing additional stress on the animals. Another advantage of studying chronically instrumented animals is that cardiopulmonary exercise testing can be repeated allowing the use of an animal as its own control, either during disease development or during chronic treatment, thereby increasing statistical power and hence limiting the number of animals required for a study.
Cardiopulmonary anatomy of swine closely resembles that of humans and it is possible to induce various forms of cardiopulmonary disease, such as diabetes 6, myocardial infarction 7, pulmonary hypertension 8,9 and pacing-induced heart failure10,11. Moreover, the size of swine allows chronic instrumentation, and repeated blood sampling of sufficient quantity to analyze not only blood gases, but also to perform neurohumoral measurements and/or to search for biomarkers of disease.
This protocol describes the surgery used to chronically instrument swine as well as the protocol for exercising the swine on a motor-driven treadmill.
Protocol
Procedures involving animal subjects have been approved by the Animal Care Committee at Erasmus Medical Center Rotterdam (NL). Swine with weights between 6 and 80 kg have been successfully instrumented using this protocol.
1. Adaptation of the Animals to Human Handling
- After arrival in the facility, house the animals solitarily but enable them to interact with each other.
- Accustomize swine to human handling and transportation from the animal facility to the experimental laboratory, by handling the animal at least once a day for one week.
- Train the animals appropriately for exercise experiments on a motor-driven treadmill by exercising them on the treadmill for a minimum of three times before surgery.
- Animals should be fasted O/N before surgery to prevent nausea, vomiting and thereby potential aspiration of stomach fluids.
2. Preparation for Surgery
- Sedation
- Prepare medication for sedation in a 10 ml syringe. Premedication consists of tiletamine/zolazepam (5 mg/kg), xylazine, (2.25 mg/kg) and atropine (1 mg).
- Inject the medication intramuscularly in the trapezius muscle with a 19 G 1.5'' needle to sedate the pig.
- Wait for approximately 10 min and check for muscle relaxation and unconsciousness to confirm appropriate and stable level of sedation.
- Place a 20 G peripheral safety catheter in an ear vein for subsequent intravenous administration of anesthesia and/or fluids.
- Intubation and Ventilation
- Place the animal on a table and/or trolley in supine position.
- Open the mouth of the animal with an oral spreader.
- In case of insufficient relaxation of the jaws or presence of swallowing reflexes, which hinder intubation, administer thiopental (10 mg/kg) intravenously via the ear vein catheter. Alternatively, the pig could be masked with isoflurane to induce sedation.
- Use a conventional laryngoscope with a light and a Miller blade to allow the laryngoscopist to directly view the larynx. If there is laryngospasm, apply 2% lidocaine to the cords and larynx to reduce the spasm and allow intubation.
- Insert an intubating stylet into the endotracheal tube to make the tube conform better to the upper airway anatomy and pass the tube through the mouth and between the vocal cords into the trachea.
- Inflate the balloon cuff with a 10 ml syringe to help secure it in place, to prevent leakage of respiratory gases, and to protect the airways from possible aspiration of stomach fluid.
- Connect the tube to a breathing filter (heat and moisture exchanger) and to the mechanical ventilator.
- Place the animal on its right side on the surgical table.
- To achieve pO2 levels of 100-120 mmHg, ventilate the animal with a mixture of oxygen and nitrogen (1:2 v/v), using the following ventilator settings: Pressure control mode: positive end-expiratory pressure (PEEP) 4 cmH2O; peak inspiratory pressure 16 - 18 cmH2O; breathing frequency depending on the size of the animal (for a 20 kg animal, decrease frequency with increasing body weight) this should result in a tidal volume of ~10 ml/kg, monitor ventilation with capnography.
- Monitor temperature using a rectal thermometer and maintain temperature between 37 - 39 ºC using a heat lamp or heat mat. Moreover, monitor heart rate with electrocardiography.
- Anesthesia
- Induce and maintain anesthesia preferably by adding 2.0% of isoflurane (v/v) to the ventilation gas-mixture or alternatively by intravenous administration of fentanyl (10 μg/kg/h) via the ear vein catheter.
- Check adequate depth of anesthesia by testing pain reflexes with a hind leg toe pinch before starting surgery. When necessary, add additional anesthesia or wait for a few minutes. Check pain reflexes regularly throughout the surgery.
- Fluids and Antibiotics
- Administer the first dose of amoxicillin (25 mg/kg) intravenously via the ear vein catheter.
- Connect a transfusion system to the ear vein catheter to enable slow infusion of glucose 10% (500 ml) during surgery.
- Sterilization of Surgical Site
- Shave and clean the skin of the animal over an area of approximately 25 cm width from the vertebral column all the way to the left axilla.
- Scrub the moisturized skin with povidone-iodine scrub (75 mg/ml) for approximately 5 min.
- Remove the povidone-iodine soap from the skin with wet sterile gauzes, before sterilizing the skin with povidone-iodine lotion (100 mg/ml).
- Cover the animal with sterile surgical drapes to reduce bacterial transfer and subsequent contamination of the surgical site.
3. Surgery
- Opening the Thorax (Thoracotomy)
- Make an incision in the skin, starting 1 cm caudal to the left inferior angle of the scapula down to the left axilla (Figure 1). Use diathermy to cauterize blood vessels in the skin to prevent excessive bleeding.
- Cut through the serratus muscle and pectoralis major muscle, using the cutting modality of the diathermy. Also use diathermy to cauterize blood vessels in the muscle layer to prevent excessive bleeding.
- Use blunt dissection to carefully divide the intercostal muscle of the fourth left intercostal space with a mosquito clamp. Now the costal surface of the left lung covered with visceral and parietal pleura should be exposed.
- To enter the pleural cavity, carefully pierce both layers of the pleura and tear them open.
- Use a thoracic retractor to separate the edges of the wound and the ribs and to forcefully drive tissues apart to obtain good exposure of the pleural cavity.
- Push away the left lung in the caudal direction and keep it in place with a wet gauze. Now the heart and great vessels should be clearly exposed.
- Placement of Catheters and Flow Probes (Figure 1)
- Use blunt dissection to remove ~2 cm2 of the surrounding connective tissue of the descending thoracic aorta.
- Perform a purse-string suture, consisting of three stitches, in the aortic wall with a non-absorbable USP3-0 braided silk suture (Ø0.2 mm).
- Penetrate the aortic vessel wall with a stainless steel 16 G needle in the middle of the purse-string suture.
- Insert the tip of the fluid-filled catheter (until the ring) into the aorta, pull the purse-string suture firmly together and tie the two strings of the suture.
- To keep the catheter in place, wind the suture 3 times around the catheter above the ring and again tie the two strings of the suture. Further secure the catheter with a new stitch approximately 1 cm cranial from the insertion place.
- Connect the fluid-filled catheter to the calibrated pressure transducer, which is connected to the computer, to monitor the mean arterial pressure during the surgery. Obtain an arterial blood gas to verify or adjust for correct ventilation settings.
- Open the pericardium with a crossed cut. Be aware to keep the phrenic nerve that runs over the pericardium intact.
- Identify the pulmonary artery and pull it slightly in the caudal direction with a Farabeuf retractor. Now the ascending aorta and aortic arch should be exposed. Monitor mean arterial pressure while retracting the pulmonary artery.
- Make a small cut (~1 cm) in the connective tissue between the ascending aorta and the pulmonary artery using Metzenbaum scissors, to be able to dissect either the ascending aorta or the pulmonary artery with a large curved mosquito clamp to place the flow probe.
- Place the rubber band of the flow probe around the vessel. To make this easier, place a suture through one end of the rubber band, place this suture around the vessel and pull it until the rubber band surrounds the vessel.
- Fix the flow probe measurement device on the rubber band. Connect the flow probe to the computer and check the cardiac output signal on the computer to confirm a correct placement of the flow probe.
- Place fluid-filled catheters in the pulmonary artery, right ventricle, left ventricle and left atrium at the same manner as described for the aortic fluid-filled catheter (3.2.2 - 3.2.5). Note that it is not necessary to remove connective tissue before performing a purse-string suture in these structures.
- Expose and dissect the proximal part of the left anterior descending coronary artery by first lifting the tissue with a forceps and making a small (2 - 3 mm) cut with Metzenbaum scissors, followed by carefully teasing the tissue away from the artery with a cotton swab. Ensure complete dissection of the coronary artery by passing a small straight angled mosquito clamp underneath.
- Make a stitch parallel to the anterior interventricular coronary vein with a suture, which is connected to the coronary venous catheter.
- Puncture the coronary vein with the 20 G needle of the coronary venous catheter and insert the cannula of the catheter intravenously.
- Remove the needle and secure the catheter with the already performed stitch (3.2.14). Further secure the catheter with a new stitch approximately 1cm from the place of initial puncture.
- Place the coronary flow probe around the previously dissected left anterior descending coronary artery. When the artery is constricted and is hardly visible, use lidocaine 10% spray to relax the vessel to get a better exposure of the vessel. Check the signal of the coronary flow on the computer to confirm a correct placement of the flow probe (Figure 2).
- Tunneling
- Tunnel the flow probes individually through the third left intercostal space beneath the muscle and above the rib by using a large curved mosquito clamp.
- Tunnel the fluid-filled catheters through either the third or the fifth left intercostal space by piercing the intercostal muscle. Clamp off the fluid-filled catheters and remove the three-way stopcock to minimize the piercing area and prevent leakage of the fluid-filled catheters during the tunneling.
- Fix the flow probes and the fluid-filled catheters with non-absorbable USP2-0 braided silk (Ø0.3 mm) by means of a purse string suture on the intercostal muscle. This suture also serves to prevent air leakage after re-instating negative intrathoracic pressure.
- Make three incisions in the skin approximately 2 cm sinister and parallel to the vertebral column, approximately 3 cm in length 3 cm apart of each other.
- Pierce a trochar beneath the left latissimus dorsi muscle from rostral incision site to the incisions on the back. Tunnel the flow probes and fluid catheters to the back within this trochar.
- Place the stopcocks on the fluid-filled catheters and remove the clamp. Withdraw blood to remove clots and air bubbles and fill the fluid-filled catheters with 1,000 IU/ml heparin. Coronary venous catheters should be filled with 5,000 IU/ml heparin.
- Closing the Thorax
- Make an incision with a length of approximately 1.5 cm, 8 cm caudal and parallel to the first incision.
- Lead the drain from the pleural cavity through the sixth intercostal muscles subcutaneously to this incision with a large curved mosquito clamp. Connect the drain to the suction device to remove any remaining fluid and reinstate negative pressure in the pleural cavity during the closing of the thorax.
- Relieve and inflate the lung with an end-inspiratory hold. Ensure adequate filling of the lung by visual monitoring.
- Close the thorax by pulling the ribs of the fourth intercostal space together at two separate sites with non-absorbable USP6 braided polyester (Ø0.8 mm).
- Close the serratus muscle and pectoralis major muscle with a running stitch and the skin with a running subcuticular suture using non-absorbable USP2-0 braided silk (Ø0.3 mm)
- Suture the incisions on the dorsal side with non-absorbable USP2-0 braided polyester (Ø0.3 mm) between the catheters. First tie a knot directly onto the skin to close the incision, then fixate the catheters to the suture with a knot 1 cm from the skin. For the flow probes, use an absorbable USP2-0 braided polyglactin (Ø0.3 mm) suture to prevent cutting of the suture in the flow probe wire (Figure 1).
- Carefully remove the drain while applying pressure on the cranial side of the incision to maintain negative pressure in the pleural cavity. Close the incision with a purse string suture using non-absorbable USP2-0 braided polyester (Ø0.3 mm) and seal the wound with petroleum jelly.
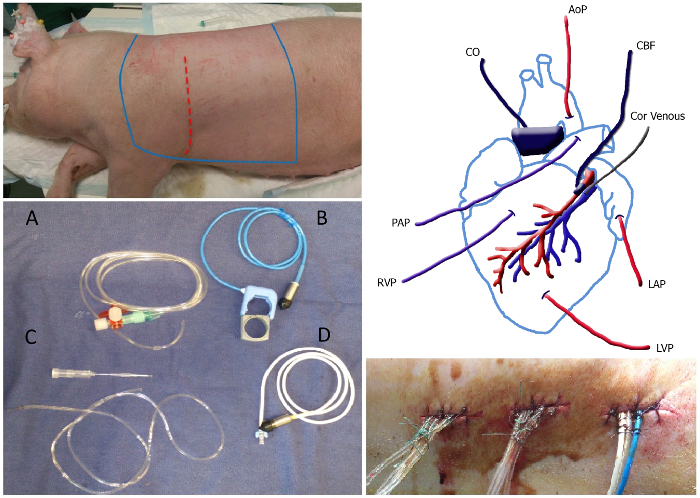
Figure 1. Overview of the Surgery. Top left panel: The sterile area of the animal, which should be shaved and sterilized lies between the bleu lines. The incision site is depicted as the red dotted line. Bottom left panel: Picture of catheters and flow probes: fluid-filled catheter (A), aorta/ pulmonary flow probe including rubber band (B), coronary venous catheter including 20 G needle (C) and the coronary flow probe (D). Top right panel: Schematic overview of placement of the catheters and flow probes. MAP, mean arterial pressure; Cor venous, coronary venous catheter; LAP, left atrial pressure; LVP left ventricular pressure; RVP, right ventricular pressure; PAP, pulmonary artery pressure; CO, cardiac output; CBF, coronary blood flow. Bottom right panel: Tunneled catheters exiting the back secured with a stitch and a knot at approximately 1 cm distance along the suture. Please click here to view a larger version of this figure.
- Termination of anesthesia and recovery from surgery
- Stop anesthesia when all incision sites are closed.
- Provide analgesia by administering buprenorphine (0.015 mg/kg) i.m. in the gracilis muscle.
- Stop the ventilation when the animal is breathing independently and disconnect the tracheal tube from the ventilator. Check regularly if the animal is breathing sufficiently.
- Place gauze pads between exteriorization sites of the catheters to absorb wound fluid.
- To protect the external segments of the catheters, give the animal an elastic vest and package the catheters between two pieces of artificial sheepskin.
- Deflate the balloon of the tracheal tube and extubate when the animal regains its swallowing reflex.
- Provide long-term analgesia by means of a Fentanyl slow-release patch (12 µg/hr for a 20 kg pig; adjust strength according to bodyweight). Place the patch on a thin part of the skin (such as the lower abdomen) to ensure adequate delivery of analgesia.
- House the animal separately for the entire post-operative period. Provide a heating lamp for the first week after surgery to keep the animal warm.
- Supply enough fluid i.v. if the animal is not drinking independently.
- Flush the fluid-filled catheters daily, by first withdrawing blood to remove clots, then refilling with saline and finally with heparinized saline (1,000 - 5,000 IU/ml) to prevent blood clot formation. Take care not to infuse any air bubbles while flushing the catheters.
- Administer amoxicillin (25 mg/kg) i.v. daily for 6 days after surgery to prevent post-surgical infections.
- Allow the animal to recover for one week before starting the treadmill experiments.
4. Treadmill Experiment (Figure 2)
- Flush the fluid-filled catheters as described (3.5.10) and attach the flushed catheters to the pressure transducers. Measure the rectal temperature to be able to obtain temperature corrected blood gas values.
- Flush the pressure transducers with saline to prevent damping of the signals due to air bubbles. Attach the pressure transducers to the elastic vest on the dorsal side.
- Connect the pressure transducers and flow probes to the amplifier. Start measuring in the computer program and calibrate the pressure transducers and flow probes with 0 mmHg being open to the air (and closed to animal) and 100 mmHg using a manometer.
- Switch the three-way stopcock in a way that the fluid catheters have an open connection with the pressure transducers. Note that the blood pressures can now be obtained. Check signals for shape and amplitude (Figure 2).
- If required, connect an extension line to either of the fluid catheters for sampling of mixed venous and arterial blood.
- Measure hemodynamics when the animal is lying as well as standing quietly on the treadmill. Average blood pressures are measured over a timeframe of 10 sec.
- Obtain arterial and mixed venous blood samples by first withdrawing 5 ml of blood using a 10 ml syringe so that 1 ml of pure blood can be obtained using a heparinized 1 ml syringe. For the coronary venous blood samples, a 2 ml syringe is used instead of the 10 ml syringe and withdrawal of 1 ml is sufficient to obtain pure blood.
- Keep the sealed 1 ml syringes on ice before processing the blood samples with a blood gas analyzer to determine the metabolic and ventilatory condition of the animal.
- Subject the swine to a five-stage exercise protocol on the treadmill, 3 min per speed, 1 - 5 km/hr (~85% of maximal heart rate). Obtain hemodynamics and blood gases after 1.5 - 2 min per speed on each speed as in the resting position.
- After the exercise protocol close the stopcocks and check if drift has occurred in the 0 mmHg calibration, make a note of this calibration. Remove the pressure transducers of the fluid-filled catheters and disconnect the flow probes.
- Flush the fluid-filled catheters with saline and heparin (1,000 - 5,000 IU/ml). Protect the catheters and flow probes by putting them beneath the elastic vest between two pieces of artificial sheepskin. The animal can now be returned to its cage.

Figure 2. Treadmill Experiment. Left panels: Instrumented swine on the treadmill. Fluid-filled catheters are connected to the pressure transducers, placed on the back of the swine. Top right panel: Overview of the total experimental set-up, including treadmill, amplifier and recording computer. Bottom right panel: Typical example of recorded hemodynamic data. From top to bottom; aortic pressure (AoP, blue) and left ventricular pressure (LVP, red); left atrial pressure (LAP,blue) and left ventricular pressure (red); pulmonary artery pressure (PAP, blue) and right ventricular pressure (RVP, red); aortic flow/cardiac output (AoF, blue); coronary blood flow (CBF, red). Please click here to view a larger version of this figure.
Results
Exercise up to 5 km/hr resulted in a doubling of cardiac output from 4.3 ± 0.3 to 8.5 ± 0.7 L/min which was principally accomplished by an increase in heart rate from 137 ± 7 to 256 ± 8 beats per min in combination with a small increase in stroke volume from 32 ± 2 to 36 ± 3 ml (Figure 3). The increase in stroke volume was facilitated by an increase in left ventricular contractility, as evidenced by an increase in the maximum of the first der...
Discussion
The present study describes the surgery for chronical instrumentation of swine as well as the protocol for exercising the instrumented swine on a motor-driven treadmill while measuring hemodynamics and taking blood samples for measurement of oxygen content in arterial, mixed venous and coronary venous blood.
Critical Steps within the Protocol
There are several critical steps within the protocol that start already during the intubation procedure. Thiopental (2.1.5) is a respiratory depres...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by Netherlands Heart Foundation grant 2000T038 (to D.J. Duncker) grant 2000T042 (to D. Merkus), European Commission FP7-Health-2010 grant MEDIA-261409 (to D.J. Duncker and D. Merkus), Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, the Dutch Federation for University Medical Centers, the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Sciences CVON- ARENA CVON 2011-11 (to D.J. Duncker), CVON-PHAEDRA CVON2012-08 ( to D. Merkus) and CVON-RECONNECT CVON 2014-11 (to D.J. Duncker and D. Merkus), Sophia Foundation (to D. de Wijs-Meijler, D. Merkus and I.K.M. Reiss).
Materials
| Name | Company | Catalog Number | Comments |
| 3-way stopcocks | B. Braun | 16496 | |
| Perfusor lines PVC (DEHP-free) 150 cm/2.6 ml | B. Braun | 8722960 | Used for fluid filled catheters |
| “python “ silicontubing | Rubber BV | 1757 ID 1 mm, OD 2 mm | Used for fluid filled catheters |
| Sodium Chloride 0.9% | Baxter | TKF7124 | |
| Glucose 10% | Baxter | WE0163 | |
| Suction device | |||
| Slim-Line electrosurgical pencil with 2 buttons | ERBE ELEKTROMEDIZIN GMBH | 20190-066 | |
| Servo Ventilator SV900C | Siemens-Elema AB | ||
| Laryngoscoop | Vererinary Technics Int. | 11.02.47 | |
| Sterile surgical gloves | |||
| tie-on surgical mask | 3M | 1818FS | |
| surgical hat | Klinidrape | 621301 | |
| Procedure pack | Molnlycke Health Care | 97027809 | Surgical drape, gauze pads, syringes, beaker etc |
| Droptears | Alcon | 288-28282-01 | |
| Betadine scrub 75 mg/ml Povidone-iodine | Meda Pharma BV | RVG08939 | |
| Betadine solution 100 mg/ml Povidone-iodine | Meda Pharma BV | RVG01331 | |
| Cuffed Endotracheal tube | Emdamed | size depends on animal size | |
| Breathing filter Hyrdo therm 3HME | Intersurgical | 1560000 | |
| Laryngoscope Handle+ Miller blade size 4 | Kawe Germany | ||
| Manual resuscitator- Combibag | Weinmann | 6515-12-313-5596 | |
| Perivascular flow probe 3PS | Transonic | For coronary artery; Size 2.5 - 4 mm depending on animal size | |
| Confidence flow probe | Transonic | For aorta/pulmonary artery, 16 - 20 mm; size depends on animal size | |
| Venflon-Venisystem 20 G x 32 mm | BD | 393224 | For coronary venous catheter |
| Blunt Needle 18 G | For coronary venous catheter | ||
| Tygon Tubing | Rubber BV | 2802 ID 0.8 mm (1/32’’), OD 2.4 mm (3/32’’) | For coronary venous catheter |
| Suction Handle 17 cm 6 6/8 " Coupland 18/8 martinit with tube connector | KLS Martin Group | 18-575-24 | |
| Scalple blade | |||
| Scalpel Handle 13.5 cm 5 3/8 " Stainless Steel solid | KLS Martin Group | 10-100-04 | |
| Vascular Forceps 20.2 cm 8 " De Bakey Stainless Stee | KLS Martin Group | 24-388-20 | ± 14 cm |
| Dressing Forceps 17 cm 6 6/8 " Cushing Stainless Steel | KLS Martin Group | 12-189-17 | ± 18 cm |
| halsted-musquito straight 12.5 cm - 5" | Rudolf Medical | RU-3100-13 | ± 12 cm |
| halsted-musquito curved 12.5 cm - 5" | Rudolf Medical | RU-3101-12 | ± 12 cm |
| Dissecting and Ligature Forceps 13 cm 5 1/8 " Gemini Stainless Steel | KLS Martin Group | 13-451-13 | ± 12 cm |
| Dissecting and Ligature Forceps 18.5 cm 7 2/8 " Schnidt Stainless Steel | KLS Martin Group | 13-363-18 | |
| Rib Retractor Finochietto, Baby Aluminium - | KLS Martin Group | 24-162-01 | |
| suture forceps Mayo-Hegar 3 mm 18 cm - 7" | Rudolf Medical | RU-6050-18 | |
| Metchenbaum blunt curved 14.5 cm - 5(3/4)" | Rudolf Medical | RU-1311-14M | |
| Retrector farabeuf 12 cm - 4 (3/4)" | Rudolf Medical | RU-4497-12 | |
| Towel forceps schrädel curved 9cm - 3,5" | Rudolf Medical | RU-3550-09 | |
| surgical scissors blunt 13 cm - 5" | Rudolf Medical | RU-1001-13 | |
| Gauzes Cutisoft 10 x 10 cm 4-ply | BSN Medical | 45846-00 | |
| Gauzes Cutisoft 5 x 5 cm 4-ply | BSN Medical | 45844-00 | |
| Flowmeter -CM2 / SF2 - 2gas (O2 and Air) | UNO BV | 180000008 | |
| Tec 7 Vaporizer | Datex-Ohmeda | ||
| Acederm wound spay | Ecuphar NV | ||
| Vaseline Album | Bufa | 165313 | |
| silkam 3-0 Natural silk, non-absorbable | B. Braun | F 1134043 | sutures for placement of catheters |
| silkam 2-0 Natural silk, non-absorbable | B. Braun | F 1134051 | sutures for muscular approximation |
| dagrofil 3-0 Polyester, non-absorbable | B. Braun | C 0842478 | sutures for fluid fille catheters after tunneling |
| Vicryl rapide 3-0, 1 x 45 cm FS2, V2930G | Daxtrio medische producten | 15560 | sutures for electrical catheters after tunneling |
| Vitafil 6 USP | SMI | 6080 | Ties |
| Syringes | 10 ml and 2.5 ml | ||
| Heparin LEO (heparin sodium) | LEO Pharma A/S | ||
| Zoletil | Virbac | tiletamine / zolazepam | |
| Sedazine | AST farma | 108855 | xylazine |
| Temgesic | RB Pharmaceuticals | 5429 | buprenorphine |
| Tensogrip | BSN Medical | 71522-00 | elastic vest |
References
- Laughlin, M. H., et al. Peripheral circulation. Compr Physiol. 2, 321-447 (2012).
- Datta, D., Normandin, E., ZuWallack, R. Cardiopulmonary exercise testing in the assessment of exertional dyspnea. Ann Thorac Med. 10, 77-86 (2015).
- Vatner, S. F., Braunwald, E. Cardiovascular control mechanisms in the conscious state. N Engl J Med. 293, 970-976 (1975).
- Duncker, D. J., Bache, R. J. Regulation of coronary blood flow during exercise. Physiol Rev. 88, 1009-1086 (2008).
- Tune, J. D., Gorman, M. W., Feigl, E. O. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 97 (1985), 404-415 (2004).
- van den Heuvel, M., et al. Coronary microvascular dysfunction in a porcine model of early atherosclerosis and diabetes. Am J Physiol Heart Circ Physiol. 302, H85-H94 (2012).
- Zhou, Z., et al. Pulmonary vasoconstrictor influence of endothelin in exercising swine depends critically on phosphodiesterase 5 activity. Am J Physiol Lung Cell Mol Physiol. 306, L442-L452 (2014).
- Pereda, D., et al. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res. 7, 494-506 (2014).
- Mercier, O., et al. Endothelin A receptor blockade improves regression of flow-induced pulmonary vasculopathy in piglets. J Thorac Cardiovasc Surg. 140, 677-683 (2010).
- Spinale, F. G., et al. Chronic supraventricular tachycardia causes ventricular dysfunction and subendocardial injury in swine. Am J Physiol. 259, H218-H229 (1990).
- Yarbrough, W. M., Spinale, F. G. Large animal models of congestive heart failure: a critical step in translating basic observations into clinical applications. J Nucl Cardiol. 10, 77-86 (2003).
- Duncker, D. J., Stubenitsky, R., Verdouw, P. D. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward beta-adrenergic control. Circ Res. 82, 1312-1322 (1998).
- Stubenitsky, R., Verdouw, P. D., Duncker, D. J. Autonomic control of cardiovascular performance and whole body O2 delivery and utilization in swine during treadmill exercise. Cardiovasc Res. 39, 459-474 (1998).
- Zhou, Z., et al. Phosphodiesterase-5 activity exerts a coronary vasoconstrictor influence in awake swine that is mediated in part via an increase in endothelin production. Am J Physiol Heart Circ Physiol. 306, H918-H927 (2014).
- Gross, D. R. . Animal Models in Cardiovascular Research. , (2009).
- Merkus, D., Duncker, D. J. Perspectives: Coronary microvascular dysfunction in post-infarct remodelled myocardium. Eur Heart J Suppl. 16, A74-A79 (2014).
- de Beer, V. J., de Graaff, H. J., Hoekstra, M., Duncker, D. J., Merkus, D. Integrated control of pulmonary vascular tone by endothelin and angiotensin II in exercising swine depends on gender. Am J Physiol Heart Circ Physiol. 298, H1976-H1985 (2010).
- Lautt, W. W. Resistance or conductance for expression of arterial vascular tone. Microvasc Res. 37, 230-236 (1989).
- Merkus, D., et al. Phosphodiesterase 5 inhibition-induced coronary vasodilation is reduced after myocardial infarction. Am J Physiol Heart Circ Physiol. 304, H1370-H1381 (2013).
- Heusch, G. The paradox of alpha-adrenergic coronary vasoconstriction revisited. J Mol Cell Card. 51, 16-23 (2011).
- Merkus, D., Houweling, B., van den Meiracker, A. H., Boomsma, F., Duncker, D. J. Contribution of endothelin to coronary vasomotor tone is abolished after myocardial infarction. Am J Physiol Heart Circ Physiol. 288, H871-H880 (2005).
- Haitsma, D. B., et al. Minimal impairment of myocardial blood flow responses to exercise in the remodeled left ventricle early after myocardial infarction, despite significant hemodynamic and neurohumoral alterations. Cardiovasc Res. 52, 417-428 (2001).
- Bender, S. B., van Houwelingen, M. J., Merkus, D., Duncker, D. J., Laughlin, M. H. Quantitative analysis of exercise-induced enhancement of early- and late-systolic retrograde coronary blood flow. J Appl Physiol. 108 (3), 507-514 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved