A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Intraluminal Drug Delivery to the Mouse Arteriovenous Fistula Endothelium
In This Article
Summary
After puncturing the aorta through the inferior vena cava (IVC) to create an aorto-caval fistula in the mouse, solution containing a drug is infused into the IVC via the same needle, followed by incubation. This method enables more robust drug delivery to the venous endothelium compared to the external route.
Abstract
Delivery of therapeutic agents to enhance arteriovenous fistula (AVF) maturation can be administered either via intraluminal or external routes. The simple murine AVF model was combined with intraluminal administration of drug solution to the venous endothelium at the same time as fistula creation. Technical aspects of this model are discussed. Under general anesthesia, an abdominal incision is made and the aorta and inferior vena cava (IVC) are exposed. The infra-renal aorta and IVC are dissected for clamping. After proximal and distal clamping, the puncture site is exposed and a 25 G needle is used to puncture both walls of the aorta and into the IVC. Immediately after the puncture, a reporter gene-expressing viral vector was infused in the IVC via the same needle, followed by 15 min of incubation. The intraluminal administration method enabled more robust viral gene delivery to the venous endothelium compared to administration by the external route. This novel method of delivery will facilitate studies that explore the role of the endothelium in AVF maturation and enable intraluminal drug delivery at the time of surgical operation.
Introduction
The murine aortovenous fistula (AVF) puncture model between the aorta and the inferior vena cava (IVC) is now an established technique.1 In this model, both walls of the infra-renal aorta are punctured with a 25 G needle, exiting into the adjoining infra-renal vena cava; the anterior aortic entrance hole is repaired with simple compression, and does not require suture repair. Serial follow-up examination by high-resolution Doppler ultrasound and histological analysis shows the AVF to have a maturation phase and then a failing phase, recapitulating the known pathophysiology of human AVF.2
To explore mechanisms that modulate AVF maturation, improved methods for delivery of therapeutic agents to the maturing AVF endothelium are needed. Delivery of therapeutic agents to vessels can be either via endovascular delivery to the lumen, or via external delivery to the adventitia. One example of external delivery is the commonly used adventitial application of Pluronic gel. This copolymer is thermo-reversible and transformed from liquid to solid gel when warmed to body temperature. Prior studies have shown sustained drug delivery is achieved when drug mixed in pluronic gel is applied topically in vivo.3,4 Adventitial application of viral vectors or siRNA with Pluronic gel has been reported to be effective as a perivascular delivery system.5,6 We have also reported that treatment of explanted human saphenous veins with adventitial stimulation by peptides resulted in phosphorylation of endothelial receptor proteins.7
On the other hand, investigators have also used intraluminal delivery of both viral and nonviral vectors in canine8-10 and rabbit11,12 models of vein grafts. In these reports, gene transfer was performed ex vivo after vein harvest. Eslami et al. reported endovascular viral gene delivery to carotid veins in situ without creating a bypass.13 Gloverman et al. reported intraluminal and adventitial delivery of naked DNA in rat femoral artery-superficial epigastric vein fistulae.14 The Mayo group reported adventitial drug delivery in mouse carotid artery-jugular vein fistulae.15,16 However, these previously reported models required a sutured anastomosis to create the AVF. In this report, intraluminal drug delivery with simultaneous AVF creation in mice is described, using a suture-less model of AVF creation. By using this modified murine AVF model a simple method for intraluminal drug delivery to the venous limb of the fistula can be performed.
Protocol
Approval by the appropriate Institutional Animal Care and Use Committee is obtained.
1. Anesthesia and Pre-operative Procedures
- Anesthetize male C57Bl/6 ice, aged 8 weeks, with vaporized 3% isoflurane and 0.8 L/min oxygen administered into an acrylic induction chamber.
- Confirm adequate anesthesia by lack of reaction to toe pinch. Position the mouse supine on the operation table and position a silicone mask to deliver vaporized 2 - 3% isoflurane by continuous inhalation.
- Remove ventral hair from the neck to lower abdomen using a chemical depilatory cream.
- Perform Doppler ultrasound examination prior to AVF surgery to record baseline characteristics of arterial and venous flow and vessel diameter at the areas of interest.1,2
- Attach a 1 ml syringe to a 25 G needle and load the syringe with the desired drug. Bend the needle to a 60 degree angle approximately 4 mm from the needle tip. Grasp the 25 G needle with a curved needle holder.
2. Operative Procedures
- Prepare the incision site with a topical antiseptic and apply a surgical drape. Use sterile gloves and instruments to maintain aseptic technique throughout the surgery.
- Make a midline abdominal incision with a scalpel extending from the level of the lower liver edge to just above the pubis.
- Insert a retractor and eviscerate all bowels from the abdominal cavity toward the right side. Wrap the bowels in gauze soaked with saline. Dissect the membrane connecting the retro-peritoneum and lower colon to obtain full view of the aorta and IVC.
- Dissect the infra-renal aorta and IVC from surrounding tissues, preparing for proximal and distal clamping.
- Place a single microsurgery clip across both the proximal aorta and the proximal IVC at the level just below the left renal vein. Place a second microsurgery clip across both the distal aorta and the distal IVC.
- Grasp the connective tissue surrounding the aorta and rotate medially so that the dorsal surface of the aorta is slightly exposed for the arterial puncture, as described previously.1
- Quickly expose the puncture site. The puncture site will be at the caudal aspect of the vessels, approximately three quarters of the distance from the left renal vein to the aortic bifurcation. Keeping the aorta in a rotated position with the left hand, dissect the left lateral margin of the aorta so that there is ample exposure to allow puncture with the right hand. Be careful not to dissect between the aorta and the IVC.
- Maintain the aorta in a rotated position and puncture the aorta through into the IVC using a 25 G needle with an attached 1 ml syringe containing drug solution. (Figure 1A)
- Infuse the drug solution (100 - 200 µl) using the left hand. The needle can be seen through the dilated and thin IVC wall when the transparent drug solution displaces the venous blood out of the IVC (Figure 1B,C). Remain still and maintain the needle in position for 15 min.
- Remove the distal microsurgery clip to de-clamp only the distal aorta and the distal IVC.
- Remove the needle and then cover the puncture site of the aorta by pulling up adjacent retro-peritoneal tissue.
- Remove the proximal microsurgery clip to de-clamp the proximal aorta and the proximal IVC. Upon de-clamping, arterial blood is observed flowing into the IVC instead of dark venous blood flow. Keep covering the puncture hole for 1 min.
- After confirmation of hemostasis by observation for 30 sec without compression, return the bowels into their natural position and close the abdomen with a running suture according to your approved animal protocol.
3. Post-operative Procedures
- After closure of the abdomen, discontinue anesthesia. Apply post-operative care including analgesia and wound care in accordance with instructions recommended by the Institutional Animal Care and Use Committee. For analgesia we use buprenorphine at 0.1 mg/kg intrasmuscularly every 12 hr for 24 hr following the surgical procedures.
- On the first day after operation, perform Doppler ultrasound to confirm patency of the AVF. In addition, measure other vessel and flow characteristics serially and compare for changes from pre-operative baseline values.1,2
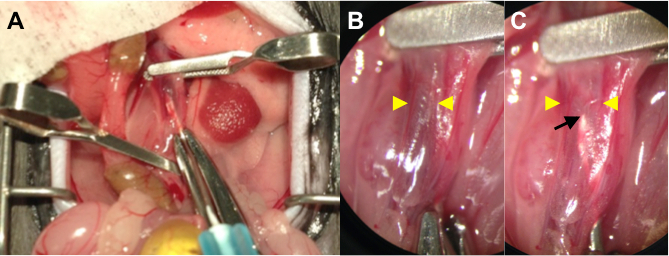
Figure 1. (A) Operative Photo Showing Intraluminal Delivery during AVF Surgery. Clamp the proximal and distal aorta, as well as the IVC by applying microsurgery clips. Puncture the aorta through into the IVC using a 25 G needle with attached syringe containing drug solution. (B) A Higher Power Picture (4X magnification) of the Punctured IVC before Infusion. The needle tip is obscured by dark colored venous blood. Yellow arrowheads denote the wall-to-wall diameter of the IVC. (C) A Higher Power Picture (4X magnification) of the Punctured IVC after Infusion. The needle tip (black arrow) can be seen through the gently distended and thin IVC wall (yellow arrowheads) as the transparent drug solution displaces the venous blood.
Results
In a series of 33 mice, survival on the first post-operative day was 97.0%; AVF patency, as determined by ultrasound, was 84.9%.
Gene transduction efficiency of this endovascular delivery route with the traditional external route was compared. For intraluminal delivery (ILD), immediately after the puncture, 200 µl of Adenovirus-GFP (Ad-GFP) vector solution (1 × 109 PFU/ml) was infused into the IVC via t...
Discussion
This modification of the murine AVF model incorporates intraluminal drug delivery to the venous endothelium at the time of AVF creation. An AVF was created by puncturing the infra-renal aorta with a 25 G needle and extending the puncture through the opposite aortic wall into the IVC, followed by injection of drug solution through the same needle. The solution is maintained intra-caval, i.e., in the venous limb of AVF, until de-clamping. What distinguishes this model from other murine AVF models17-19
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program Merit Review Award I01-BX002336, the National Institute of Health grant R56-HL095498, as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
Materials
| Name | Company | Catalog Number | Comments |
| Pluronic F-127 | Sigma-Aldrich | P2443-250G | Used as 30% solution in d-water |
| GFP antibody | NOVUS BIOLOGICALS INC | NB100-1770 | |
| Ad-CMV-GFP | VECTOR BIOLABS | 1060 | |
| 0.9% Sodium Chloride Irrigation, USP | Baxter | 2F7122 | |
| BD PrecisionGlide Needle 25 G x 5/8 | BD | 305122 | |
| BD 1 ml Syringe Tuberculin Slip Tip | BD | 309659 | |
| Scalpel | Surgical Design Inc | 22079707 | |
| 6-0 ETHILON P-1 11 mm 3/8c Reverse Cutting | ETHICON INC | 697G | |
| Vevo 770 ultrasound machine | Visualsonics | 20 - 60 Mhz scan head; RMV-704 | |
| Vascular clamp | Roboz Surgical Instrument Co. | RS-5424 | |
| Clamp applying forceps | Roboz Surgical Instrument Co. | RS-5410 |
References
- Yamamoto, K., Li, X., Shu, C., Miyata, T., Dardik, A. Technical Aspects of the Mouse Aortocaval Fistula. J. Vis. Exp. (77), e50449 (2013).
- Yamamoto, K., et al. The mouse aortocaval fistula recapitulates human arteriovenous fistula maturation. Am. J. Physiol.: Heart Circ. Physiol. 305 (12), H1718-H1725 (2013).
- Escobar-Chávez, J. J., Lòpez-Cervantes, M., Naïk, A., Kalia, Y. N., Quintanar-Guerrero, D., Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 9 (3), 339-358 (2006).
- Almeida, H., Amaral, M. H., Lobão, P., Lobo, J. M. S. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 15 (4), 592-605 (2012).
- Karper, J. C., et al. Toll-like receptor 4 is involved in human and mouse vein graft remodeling, and local gene silencing reduces vein graft disease in hypercholesterolemic APOE*3Leiden mice. Arterioscler., Thromb., Vasc. Biol. 31 (5), 1033-1040 (2011).
- Redmond, E. M., Hamm, K., Cullen, J. P., Hatch, E., Cahill, P. A., Morrow, D. Inhibition of patched-1 prevents injury-induced neointimal hyperplasia. Arterioscler., Thromb., Vasc. Biol. 33 (8), 1960-1964 (2013).
- Wong, D. J., et al. Ephrin type-B receptor 4 activation reduces neointimal hyperplasia in human saphenous vein in vitro. J. Vasc. Surg. , (2014).
- Matsumoto, T., et al. Hemagglutinating virus of Japan-liposome-mediated gene transfer of endothelial cell nitric oxide synthase inhibits intimal hyperplasia of canine vein grafts under conditions of poor runoff. J. Vasc. Surg. 27 (1), 135-144 (1998).
- Petrofski, J. A., et al. Gene delivery to aortocoronary saphenous vein grafts in a large animal model of intimal hyperplasia. J. Thorac. Cardiovasc. Surg. 127 (1), 27-33 (2004).
- Hata, J. A., et al. Modulation of phosphatidylinositol 3-kinase signaling reduces intimal hyperplasia in aortocoronary saphenous vein grafts. J. Thorac. Cardiovasc. Surg. 129 (6), 1405-1413 (2005).
- Ohta, S., et al. Intraluminal gene transfer of endothelial cell-nitric oxide synthase suppresses intimal hyperplasia of vein grafts in cholesterol-fed rabbit: a limited biological effect as a result of the loss of medial smooth muscle cells. Surg. 131 (6), 644-653 (2002).
- Baldwin, Z. K., et al. Modulation of vascular remodeling induced by a brief intraluminal exposure to the recombinant R7020 strain of Herpes simplex-1. J. Vasc. Surg. 41 (1), 115-121 (2005).
- Eslami, M. H., et al. Gene delivery to in situ veins: differential effects of adenovirus and adeno-associated viral vectors. J. Vasc. Surg. 31 (6), 1149-1159 (2000).
- Globerman, A. S., et al. Efficient transgene expression from naked DNA delivered into an arterio-venous fistula model for kidney dialysis. J. Gene Med. 13 (11), 611-621 (2011).
- Yang, B., et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int. 85 (2), 289-306 (2014).
- Brahmbhatt, A., et al. The role of Iex-1 in the pathogenesis of venous neointimal hyperplasia associated with hemodialysis arteriovenous fistula. PLoS ONE. 9 (7), e102542 (2014).
- Karram, T., et al. Induction of cardiac hypertrophy by a controlled reproducible sutureless aortocaval shunt in the mouse. J. Investig. Surg. 18 (6), 325-334 (2005).
- Perry, G. J., et al. Genetic variation in angiotensin-converting enzyme does not prevent development of cardiac hypertrophy or upregulation of angiotensin II in response to aortocaval fistula. Circ. 103 (7), 1012-1016 (2001).
- Guzman, R. J., Krystkowiak, A., Zarins, C. K. Early and sustained medial cell activation after aortocaval fistula creation in mice. J. Surg. Res. 108 (1), 112-121 (2002).
- Alexander, J. H., et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 294 (19), 2446-2454 (2005).
- Khaleel, M. S., et al. High-pressure distention of the saphenous vein during preparation results in increased markers of inflammation: a potential mechanism for graft failure. Ann. thorac. surg. 93 (2), 552-558 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved