A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Sulfate Separation by Selective Crystallization with a Bis-iminoguanidinium Ligand
In This Article
Summary
A protocol for in situ aqueous synthesis of a bis(iminoguanidinium) ligand and its utilization in selective separation of sulfate is presented.
Abstract
A simple and effective method for selective sulfate separation from aqueous solutions by crystallization with a bis-guanidinium ligand, 1,4-benzene-bis(iminoguanidinium) (BBIG), is demonstrated. The ligand is synthesized as the chloride salt (BBIG-Cl) by in situ imine condensation of terephthalaldehyde with aminoguanidinium chloride in water, followed by crystallization as the sulfate salt (BBIG-SO4). Alternatively, BBIG-Cl is synthesized ex situ in larger scale from ethanol. The sulfate separation ability of the BBIG ligand is demonstrated by selective and quantitative crystallization of sulfate from seawater. The ligand can be recycled by neutralization of BBIG-SO4 with aqueous NaOH and crystallization of the neutral bis-iminoguanidine, which can be converted back into BBIG-Cl with aqueous HCl and reused in another separation cycle. Finally, 35S-labeled sulfate and β liquid scintillation counting are employed for monitoring the sulfate concentration in solution. Overall, this protocol will instruct the user in the necessary skills to synthesize a ligand, employ it in the selective crystallization of sulfate from aqueous solutions, and quantify the separation efficiency.
Introduction
Selective separation of hydrophilic oxoanions (e.g., sulfate, chromate, phosphate) from competitive aqueous solutions represents a fundamental challenge with relevance to environmental remediation, energy production, and human health.1,2 Sulfate in particular is difficult to extract from water due to its intrinsic reluctance to shed its hydration sphere and migrate into less polar environments.3 Making aqueous sulfate extraction more efficient typically requires complex receptors that are difficult and tedious to synthesize and purify, often involving toxic reagents and solvents.4,5
Selective crystallization offers a simple yet effective alternative to sulfate separation from water.6-9 Though some metal cations such as Ba2+, Pb2+, or Ra2+ form very insoluble sulfate salts, their use in sulfate separation is not always practical due to their high toxicity and sometimes-low selectivity. Employing organic ligands as sulfate precipitants takes advantage of the structural diversity and amenability to design characteristic to organic molecules. An ideal organic ligand for aqueous sulfate crystallization should be soluble in water, yet form an insoluble sulfate salt or complex in a relatively short time and in the presence of high concentrations of competing ions. Additionally, it should be easy to synthesize and recycle. One such a ligand, 1,4-benzene-bis(iminoguanidinium) (BBIG), self-assembled in situ from two commercially available precursors, terephthalaldehyde and aminoguanidinium chloride, was recently found to be extremely effective in aqueous sulfate separation.10 The ligand is water-soluble in the chloride form, and selectively crystallizes with sulfate into an extremely insoluble salt that can be easily removed from solution by simple filtration. The BBIG ligand can then be recovered by deprotonation with aqueous NaOH and crystallization of the neutral bis-iminoguanidine, which can be converted back into the chloride form with aqueous HCl, and reused in another separation cycle. The efficacy of this ligand in removing sulfate from water is so great that monitoring the remaining sulfate concentration in solution is no longer a trivial task, requiring a more advanced technique that allows accurate measurement of trace amounts of the anion. For this purpose, radiolabeled 35S sulfate tracer in conjunction with β liquid scintillation counting was employed, a technique commonly utilized in liquid-liquid extractive separations, and recently demonstrated to be effective in monitoring sulfate crystallization.8
This protocol demonstrates the one-pot in situ synthesis of the BBIG ligand and its crystallization as the sulfate salt from aqueous solutions. The ex situ synthesis of the ligand11 is also presented as a convenient method for the production of larger amounts of BBIG-Cl, which can be stored in the crystalline form until ready to use. Sulfate removal from seawater using the previously prepared BBIG-Cl ligand is then demonstrated. Finally, the use of 35S-labeled sulfate and β liquid scintillation counting for measuring the sulfate concentration in seawater is demonstrated. This protocol is intended to provide a tutorial for those broadly interested in exploring the use of selective crystallization for aqueous anion separation.
Protocol
1. Synthesis of 1,4-Benzene-bis(iminoguanidinium) Chloride (BBIG-Cl)
- In Situ Synthesis of the 1,4-Benzene-bis(iminoguanidinium) Chloride Ligand (BBIG-Cl) and Its Crystallization with Sulfate
- Add 0.067 g of terephthalaldehyde and 2.2 ml of a 0.5 M aqueous solution of aminoguanidinium chloride to 10 ml of deionized water in a 25 ml round bottom flask equipped with a magnetic stir bar.
- Stir the solution magnetically for four hours at 20 °C. This will yield a slightly yellow solution of BBIG-Cl.
- Add 0.5 ml of a 1 M aqueous solution of sodium sulfate. This will result in the instant precipitation of BBIG-SO4 as a crystalline white solid.
- Filter the solid using vacuum filtration to recover BBIG-SO4. Wash the solid on the filter paper five times with 5 ml aliquots of water in order to obtain the pure sulfate salt.
- Check the phase purity of the crystalline BBIG-SO4 obtained by powder X-ray diffraction12. Compare with the pattern shown in Figure 1.
- Ex Situ Synthesis of 1,4-Benzene-bis(iminoguanidinium) Chloride11
- Add 4 g of terephthalaldehyde and 7.26 g of aminoguanidinium chloride to 20 ml of ethanol in a 50 ml round bottom flask equipped with a magnetic stir bar.
- Heat the solution to 60 °C using a hotplate, and stir with a magnetic stir bar for 2 hr. Cool the solution to 20 °C and let it sit for 3 hr, then collect the solid by vacuum filtration through a filter-paper equipped Büchner funnel.
- Suspend the obtained solid in 20 ml of ethanol and heat on a hotplate until boiling. If the solid does not go completely into solution at this point, add small aliquots (1 ml) of ethanol, allowing each time the solution to reach boiling temperature, until all solid is dissolved.
- Allow the flask to cool to room temperature, then place in a 0 °C freezer overnight. Collect the solid by filtering through a filter-paper equipped Büchner funnel using vacuum filtration.
- Confirm the identity and purity of BBIG-Cl by 1H NMR spectroscopy13. Compare with the spectrum shown in Figure 2.
2. Sulfate Separation from Seawater
- Sulfate crystallization as BBIG-SO4
NOTE: The amount of BBIG-Cl necessary to remove the sulfate depends on the exact amount of sulfate in the seawater. It was found that using 1.5 equivalents of BBIG-Cl relative to sulfate results in 99% removal of sulfate. The seawater used in this protocol has a concentration of 30 mM sulfate, as determined by titration with BaCl2.- Filter the seawater with a 0.22 μm syringe filter or filtration membrane with small pore size to remove suspended particulates and bio organisms.
- Make a 30 mM solution of BBIG-Cl using deionized water and solid BBIG-Cl prepared as described in the previous section.
- Add the BBIG-Cl solution to the seawater in a 1.5:1 (v/v) proportion.
- Stir the mixture for a few hours to ensure quantitative (> 99%) removal of sulfate.
- Collect the solid by filtering through a filter-paper equipped Büchner funnel using vacuum filtration. Wash the solid on the filter paper five times with 5 ml aliquots of water.
- Dry the isolated solid under vacuum and weigh it to determine the yield.
- Ligand Recovery
- Add 53.1 mg of BBIG-SO4 to a 2 ml solution of NaOH (10%) in a 20 ml scintillation vial equipped with a magnetic stir bar.
- Stir the mixture for two hours at 20 °C. A slightly yellow precipitate will form.
- Filter the solid through a filter-paper equipped Büchner funnel using vacuum filtration. Wash the solid on the filter paper with 0.2 ml of water, and dry under vacuum.
- Characterize the recovered solid by NMR13 to confirm its identity as the bis(guanidine) free base. Compare with the NMR spectrum shown in Figure 3.
- Determination of the Amount of Sulfate Removed from Seawater by β Liquid Scintillation Counting
CAUTION: This technique involves the use of radioisotopes, which pose a different class of hazards than what is normally encountered in most labs. Special radiation protection equipment is usually required when handling the radionuclides. Thus, it is essential that the procedure is followed carefully and that a safety officer is consulted for advice and guidance.- Calculate the volume of the stock solution of the sulfur-35 radioisotope (5 mCi/ml) used to ensure there is more than 5 million counts per minute (cpm) per milliliter of seawater solution, using the following equations (cpm and curies (Ci) are both units of measure for radioactivity):
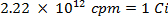
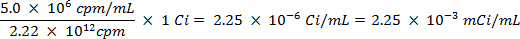
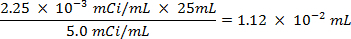
- Spike 25 ml of the seawater with 0.0112 ml of 5.0 mCi/ml solution of 35S radiolabeled sodium sulfate solution.
- Prepare 0, 15, 30, 33, 45, and 60 mM solutions of BBIG-Cl in deionized water and combine 0.750 ml of these solutions with an equal volume of 35S-radiolabeled sulfate spiked seawater in a 2 ml centrifuge tube.
- Stir the mixture via a rotating wheel or vortex in an incubator/air-box maintained at a constant temperature of 25 ± 0.2 °C for 24 hr.
- Centrifuge the solutions at 1,500 x g for 10 min at 25 °C.
- After centrifugation, remove 1.2 ml of each solution using a syringe, then filter it through a 0.22 μm syringe filter to remove the suspended precipitate. Pipette 1.0 ml from each of these solutions into 20 ml of scintillation cocktail in polypropylene scintillation vials. The solution containing no BBIG-Cl (the control solution) should be diluted ten-fold with deionized water prior to addition to the scintillation cocktail.
- Place the scintillation vials containing the samples and the scintillation cocktail on a liquid scintillation counter and let it sit for 1 hr prior to counting to allow the samples to dark-adapt.
NOTE: Prior to counting the samples, calibrate the instrument and allow each sample to count for 30 min. Count additional vials containing only scintillation cocktail in order to allow for a background correction that is used when determining the concentrations of sulfate in solution. - Determine the amount of sulfate removed, using the following equations:
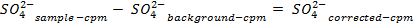
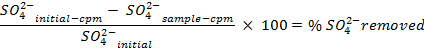
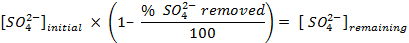
- Calculate the volume of the stock solution of the sulfur-35 radioisotope (5 mCi/ml) used to ensure there is more than 5 million counts per minute (cpm) per milliliter of seawater solution, using the following equations (cpm and curies (Ci) are both units of measure for radioactivity):
Results
The powder X-ray diffraction pattern of BBIG-SO4 (Figure 1) allows for unambiguous confirmation of the identity of the crystallized solid. In comparing the obtained pattern versus the reference one, peak intensity matters less than peak positioning. All strong peaks shown in the reference should be present in the obtained sample. The appearance of strong peaks in the sample that are absent in the reference pattern indicates the presence of impurities.
Discussion
This technique is rather tolerant to many deviations from the written procedure, which makes it quite robust. There are however two critical steps that must be followed. First, the BBIG-Cl ligand needs to be as pure as possible. Impurities will not only affect the crystallization and the solubility of the resulting sulfate salt, but will also make it difficult to calculate the amount required for quantitative sulfate removal from solution. Second, all steps in the β liquid scintillation counting section need to be f...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division. We thank the University of North Carolina Wilmington for providing the seawater.
Materials
| Name | Company | Catalog Number | Comments |
| Terephthalaldehyde | Sigma | T2207 | |
| Aminoguanidinium Chloride | Sigma | #396494 | |
| Sodium Sulfate | Sigma | #239313 | |
| Barium Chloride | Sigma | #342920 | Highly Toxic |
| Ethanol | Any | Reagent Grade (190 proof) | |
| Sodium Hydroxide | EMD | SX0590-1 | |
| Hydrochloric Acid | Sigma | #258148 | |
| Filter Paper | Any | - | Any qualitative or analytical filter paper will work |
| Syringe Filter (0.22 μm) | Any | - | Nylon filter |
| 35S Labeled Sulfate | Perkin Elmer | NEX041005MC | |
| Ultima Gold Scintillation Cocktail | Perkin Elmer | #6013329 | |
| Polypropylene Vials | Any | - | |
| Disposable Syringe (2-3 ml) | Any | - | Any disposable plastic syringe works |
References
- Langton, M. L., Serpell, C. J., Beer, P. D. Anion Recognition in Water: Recent Advances from Supramolecular and Macromolecular Perspective. Angew. Chem. Int. Ed. 55, 1974-1987 (2016).
- Busschaert, N., Caltagirone, C., Van Rossom, W., Gale, P. A. Applications of Supramolecular Anion Recognition. Chem. Rev. 115, 8038-8155 (2015).
- Moyer, B. A., Custelcean, R., Hay, B. P., Sessler, J. L., Bowman-James, K., Day, V. W., Sung-Ok, K. A Case for Molecular Recognition in Nuclear Separations: Sulfate Separation from Nuclear Wastes. Inorg. Chem. 52, 3473-3490 (2013).
- Kim, S. K., Lee, J., Williams, N. J., Lynch, V. M., Hay, B. P., Moyer, B. A., Sessler, J. L. Bipyrrole-Strapped Calix[4]pyrroles: Strong Anion Receptors That Extract the Sulfate Anion. J. Am. Chem. Soc. 136, 15079-15085 (2014).
- Jia, C., Wu, B., Li, S., Huang, X., Zhao, Q., Li, Q., Yang, X. Highly Efficient Extraction of Sulfate Ions with a Tripodal Hexaurea Receptor. Angew. Chem. Int. Ed. 50, 486-490 (2011).
- Rajbanshi, A., Moyer, B. A., Custelcean, R. Sulfate Separation from Aqueous Alkaline Solutions by Selective Crystallization of Alkali Metal Coordination Capsules. Cryst. Growth Des. 11, 2702-2706 (2011).
- Custelcean, R. Urea-Functionalized Crystalline Capsules for Recognition and Separation of Tetrahedral Oxoanions. Chem. Commun. 49, 2173-2182 (2013).
- Custelcean, R., Sloop, F. V., Rajbanshi, A., Wan, S., Moyer, B. A. Sodium Sulfate Separation from Aqueous Alkaline Solutions via Crystalline Urea-Functionalized Capsules: Thermodynamics and Kinetics of Crystallization. Cryst. Growth Des. 15, 517-522 (2015).
- Custelcean, R., Williams, N. J., Seipp, C. A. Aqueous Sulfate Separation by Crystallization of Sulfate-Water Clusters. Angew. Chem. Int. Ed. 54, 10525-10529 (2015).
- Custelcean, R., Williams, N. J., Seipp, C. A., Ivanov, A. S., Bryantsev, V. S. Aqueous Sulfate Separation by Sequestration of [(SO4)(H2O)4]4- Clusters within Highly Insoluble Imine-Linked Bis-Guanidinium Crystals. Chem. Eur. J. 22, 1997-2003 (2016).
- Khownium, K., Wood, S. J., Miller, K. A., Balakrishna, R., Nguyen, T. B., Kimbrell, M. R., Georg, G. I., David, S. A. Novel Endotoxin-Sequestering Compounds with Terephthaldehyde-bis-guanylhydrazone Scaffolds. Bioorg. Med. Chem. Lett. 16, 1305-1308 (2006).
- Pecharsky, V. K., Zavalij, P. Y. . Fundamentals of Powder Diffraction and Structural Characterization of Materials. , (2005).
- Goldenberg, D. P. . Principles of NMR Spectroscopy: An Illustrated Guide. , (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved