A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Exposure of the Pig CNS for Histological Analysis: A Manual for Decapitation, Skull Opening, and Brain Removal
In This Article
Summary
The goal of this paper and instructional video is to describe how to expose and remove the postmortem pig brain and pituitary gland in an intact state, suitable for subsequent macroscopic and histological analysis.
Abstract
Pigs have become increasingly popular in large-animal translational neuroscience research as an economically and ethically feasible substitute to non-human primates. The large brain size of the pig allows the use of conventional clinical brain imagers and the direct use and testing of neurosurgical procedures and equipment from the human clinic. Further macroscopic and histological analysis, however, requires postmortem exposure of the pig central nervous system (CNS) and subsequent brain removal. This is not an easy task, as the pig CNS is encapsulated by a thick, bony skull and spinal column. The goal of this paper and instructional video is to describe how to expose and remove the postmortem pig brain and the pituitary gland in an intact state, suitable for subsequent macroscopic and histological analysis.
Introduction
Translational neuroscience studies in pigs have become increasingly popular during the last two decades. The large size of the pig brain enables the use of conventional clinical brain imagers and the direct use and testing of neurosurgical procedures and equipment from the human clinic1,2,3,4,5,6,7,8. In the last 20 years, pigs, especially minipigs (e.g., Göttingen minipig), have been used to examine neuromodulatory treatment modalities, such as stem cell transplantation; viral vector transfection; and deep brain stimulation directed towards Parkinson disease, obesity, depression, and Alzheimer disease2,6,9,10,11,12,13,14,15,16,17. This has been followed by the development of stereotaxic and surgical approaches to manipulate the minipig CNS3,18,19,20,21. The instituted CNS changes have been evaluated in live animals using brain imaging (PET10,13,22,24 and MR23), cystometry11,12,25, gait analysis17, neurological evaluation9,17, and postmortem examination based on histology and stereological analysis14,15,17,26,27,31. However, postmortem analysis requires the exposure and removal of the pig brain, which is not an easy task, as a thick, bony skull and a fibrous dural covering surround the pig brain.
The goal of this paper and instructional video is to describe how the postmortem pig brain and pituitary may be exposed and removed in an intact state in 15-20 min using non-motorized surgical tools. The instructional video and photographic illustrations show male minipigs (age: 6 months, bodyweight: 20-25 kg) used for an anatomical study on the minipig pituitary gland.
Protocol
Animal anesthesia and euthanesia was performed in accordance with "Principles of laboratory animal care" (NIH publication No. 86-23, revised 1985) and approved by the Danish Council for Animal Research Ethics.
1. Instruments
- Collect the instruments presented in the video and listed in the Table of Materials.
2. Decapitation
NOTE: Anesthesia was induced by an intramuscular injection of 5 mL of midazolam (5 mg/mL) and 5 mL of ketamine (25 mg/mL). 5-10 min later, when the animal was deeply sedated, an ear vein was cannulated and a lethal overdose (100 mg/kg of bodyweight) of sodium pentobarbital (200 mg/mL) was given intravenously. To ensure that the animal was completely euthanized, the interdigital pain reflex was tested as shown by Ettrup et al. (2011)20. Complete euthanization was ensured as described in the ethics statement above and followed by a transcardial perfusion with 5 L of isotonic saline, as demonstrated by Ettrup et al. (2011)20. All the demonstrated procedures are performed postmortem, precluding the need for the welfare precautions necessary for long-term anesthesia and postprocedural survival.
- Decapitate the pig by a high circular cervical incision, using a surgical scalpel, just below the mandibular angle (Figure 1A).
- Still with the surgical scalpel, continue the incision anteriorly through the soft tissue of the neck, including the larynx and the esophagus, until the bony spinal column is reached, approximately at the level of the craniocervical junction.
- Advance the cut with a surgical scalpel from the anterior side of the craniocervical junction, above the anterior arc of atlas, and through the anterior atlantooccipital membrane, thereby exposing the spinal canal and the spinal cord (Figure 1B). Simultaneously ask an assistant to pull the pig body away from the pig head to ease the access between the skull base and the first cervical vertebra.
- Continue the surgical incision through the dural sac and the spinal cord (Figure 1B). Take special care to ensure that a complete transverse section of the spinal cord is achieved.
NOTE: Failure to perform the previous step may result in unwanted traction on the spinal cord and brain during the following steps of the decapitation process. - Forcefully extend the cranocervical junction at the section level (Figure 1C). At the same time, use the surgical scalpel to section the remaining atlantooccipital ligaments to release the articulation between the occipital condyles and the upper articulate process of atlas. Separate the pig head from the body.

Figure 1: Minipig decapitation. (A) Neck incision (arrow, mandibular angle). (B) Incision through the atlantooccipital ligaments and the dura-surrounded spinal cord (SC) at the craniocervical junction (C1, anterior arc of atlas; OC, occipital condyle). (C) The posterior part of the atlantooccipital articulation is released by a forceful extension (arrows) at the section level. Please click here to view a larger version of this figure.
3. Skull Opening
- Position the pig head on a table.
- Make a dorsal longitudinal incision with a surgical scalpel through the skin and the underlying soft tissue from the back of the snout, over the vertex of the head, and down through the posterior part of the occipital region.
- Expose the dorsal and posterior part of the skull by removing the soft tissue located lateral to the initial incision with a surgical scalpel.
- Release the temporal muscle bilaterally from the skull (Figure 2A) with a surgical scalpel. Make sure that the posterior occipital bone is cleaned of soft tissue.
- Use the posterior entrance of the foramen magnum to remove the occipital bone with a Kerrison bone punch and bone rongeurs and expose the dura-covered cerebellum (Figure 2B).
- Return to the exposed anterior side of the skull and select an entry point in the frontal bone, just in front of the eyes. At this point, use a bone chisel with a hammer to penetrate the skull and enter the frontal sinus (Figure 2C).
- Use the extent of the frontal sinus to further the dorsoposterior removal of the outer skull lamina with a bone rongeur or bone punch and expose the inner, thin bony skull lamina covering the cerebrum (Figure 2D).
- Gently open the inner bony skull lamina anteriorly with a hammer and a bone chisel to expose the dura-covered cerebrum (Figure 2E).
- Continue the bone removal laterally using a bone chisel and a bone rongeur through the temporal and parietal bone in order to release the final dorsoposterior part of the skull, located between the already exposed parts of the dura-covered cerebrum and cerebellum (Figure 2F).
NOTE: It is often possible, during the final step of this procedure, to use the chisel to break open the remaining posterior skull bone from one side, just like one opens a door.
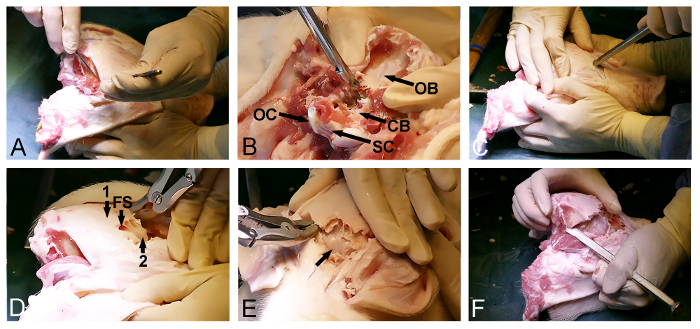
Figure 2: Minipig skull opening. (A) Exposure of the dorsoposterior skull surface, including the removal of the occipital and temporal muscles. (B) Removal of the occipital bone (CB, dura-covered cerebellum; OB, occipital bone; OC, occipital condyle; and SC, spinal cord). (C) A hammer and a bone chisel are used to penetrate the skull anteriorly and to enter the frontal sinus at the level of the eyes. (D) The extent of the frontal sinus (FS) is used to remove the outer thick skull bone (1), exposing an inner thin bone lamina (2) covering the cerebrum. (E) Removal of the thin bone lamina, exposing the dura-covered cerebrum (arrow). (F) Finally, a hammer and a bone chisel are used to laterally connect the anterior and the posterior skull openings. Please click here to view a larger version of this figure.
4. Brain Removal
- Use surgical forceps to lift the dura and create a gentle incision close to the venous superior sagittal sinus using a fine surgical scalpel (Figure 3A).
- Use micro-scissor or a dura-knife to further open the dura covering the dorsal surface of the brain.
NOTE: Special care must be taken when removing the dura corresponding to the cerebellar tentorium (Figure 3B), as preservation of this dural leaf will prevent subsequent brain removal. - Position the pig head vertically (Figure 3C).
- Use the bone chisel or a dissector to release the ventroanterior cerebrum by blunt dissection of the olfactory bulb from the dura-covered floor of the cranial cavity (Figure 3D).
- Use a fine surgical scalpel to section the exposed optic chiasm (Figure 3E). Expose and section the pituitary stalk and the oculomotor nerves.
- Release the ventral brainstem by sectioning the lower cranial nerves (Figure 3F) with a fine surgical scalpel. Ensure that the dural cerebellar tentorium was completely incised (Figure 3B), as this dural leaf will otherwise cut through the brainstem during the release process.
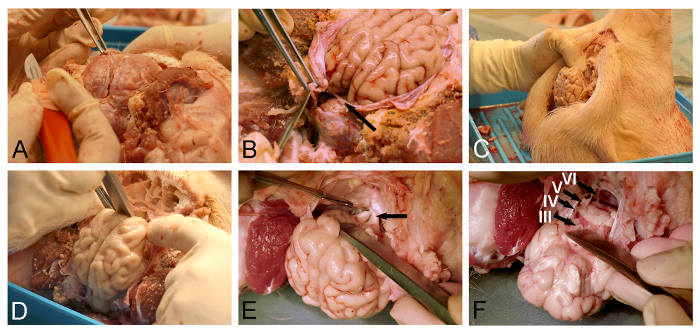
Figure 3: Minipig brain removal. (A) Dural opening with surgical forceps and a dura knife. (B) Care must be taken to completely incise the dural leaf (arrow), located between the cerebrum and the cerebellum. (C) The pig head is positioned vertically for better visualization of skull base structures and in order for gravity to assist in the intended displacement of the brain. (D) A dissector or a bone chisel is used to relieve the olfactory bulb by blunt section from the dura-covered skull base. (E) The dissection is continued in a posterior direction along the skull base for exposure and sectioning of the optic chiasm (arrow), infundibular stalk, and oculomotor nerves. (F) The brain release is completed with the section of the lower cranial nerves as they depart from the ventral surface of the brainstem (III, oculomotor nerve; IV, trochlear nerve; V, trigeminal nerve; and VI, abducens nerve). Please click here to view a larger version of this figure.
5. Pituitary Removal
- Identify the pituitary stalk and its surrounding dural leaf (the diapragma sellae) in the skull floor (Figure 4A).
- Incise the dural leaf lateral to the pituitary stalk using a fine surgical scalpel (Figure 4B).
- Use a dissector to release the pituitary and lift it out of the pituitary fossa (Figure 4C).

Figure 4: Minipig pituitary removal. (A) The pituitary fossa (*) is identified in the skull floor (1, olfactory bulb; 2, optic chiasm; and PF, posterior cranial fossa). (B) The dural covering (sellar diagphragm, (arrow)) is incised laterally. (C) The pituitary (arrow) is released with a dissector and lifted out of the pituitary fossa. Scale bar (A-C) = 10 mm. Please click here to view a larger version of this figure.
Results
To prevent the tissue material from drying out, it is recommended to store the removed brain and pituitary in a jar filled with fixative or isotonic saline immediately after macroscopic analysis has been performed. The tissue material may be stored in the fixative for years, whereas storage in isotonic saline, even in a refrigerator, will lead to tissue decay with time.
The removed pituitary may also be directly frozen by immers...
Discussion
Most experimental neuroscience studies are performed in small animal species, such as mice and rats, where access to the CNS is facilitated by a thin skull- and dural-thickness. However, in larger experimental animals like pigs1,4,8, sheep32, and non-human primates, the considerable thickness of these structures necessitates the use of robust instruments (table of materials) and proper entry points for sk...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge with gratitude the skillful assistance of Mrs. Trine W. Mikkelsen, Mrs. Lise M. Fitting, and the staff at Påskehøjgaard. The Danish Medical Research Council, the Lundbeck Foundation, and the Novo Nordisk Foundation financially supported the study.
Materials
| Name | Company | Catalog Number | Comments |
| Heavy Scalpel Handle #4 | FST (Fine Science Tools) | 10008-13 | Good for skin incision and soft tissue removal |
| Non-Sterile Scalpel Blades #23 | FST | 10023-00 | |
| Scalpel Handle #7 | FST | 10007-12 | Optimal for dural incision and precision work |
| Non-Sterile Scalpel Blades #11 | FST | 10011-00 | |
| Surgical Forceps | FST | 11024-18 | The tip of the surgical forceps ensure a firm grip |
| Kerrison Bone Punch | Aesculap Neurosurgery | FF713R | Must be robust, bite size 3-5 mm |
| Bone Rongeur | Aesculap Neurosurgery | MD615 | Must be robust, bite size 15 x 5 mm |
| Bone Rongeur | Aesculap Neurosurgery | FO551R | Must be robust, bite size 25 x 15 mm |
| Bone Chisel | Lawton | 67-0335 | The size of the chisel head should not exceed 20 mm |
| Mallet (Hammer) | Millarco | 5624108 | Weigth 300 g, length 30 cm, head hit area size 2 x 2 cm |
| Micro-Scissor | FST | 14002-14 | |
| Dissector | Aesculap Neurosurgery | OL165R | |
| Göttingen minipigs | Ellegaard Göttingen Minipigs A/S, Denmark | ||
| Euthanimal | pentobarbital | ||
| Ketamine | Pfizer | ||
| Midazolam | Hameln Pharmaceuticals |
References
- Lind, M. N., Moustgaard, A., Jelsing, J., Vajta, G., Cumming, P., Hansen, A. K. The use of pigs in neuroscience: Modeling brain disorders. Neurosci Biobehav Rev. 31, 728-751 (2007).
- Bjarkam, C. R., et al. Neuromodulation in a minipig model of Parkinson disease. British J Neurosurg. 22 (Suppl. 1), S9-S12 (2008).
- Bjarkam, C. R., Cancian, G., Glud, A. N., Ettrup, K. S., Østergaard, L., Sørensen, J. C. MRI-guided stereotaxic targeting in pigs based on a stereotaxic localizer box fitted with an isocentric frame and use of SurgiPlan computer-planning software. J Neurosci Methods. 183 (2), 119-126 (2009).
- Sauleau, P., Lapouble, E., Val-Laillet, D., Malbert, C. H. The pig model in brain imaging and neurosurgery. Animal. 3 (8), 1138-1151 (2009).
- Bjarkam, C. R., et al. Safety and function of a new clinical intracerebral microinjection instrument for stem cells and therapeutics examined in the Göttingen minipig. Stereotact Funct Neurosurg. 88 (1), 56-63 (2010).
- Fjord-Larsen, L., et al. Long-term delivery of nerve growth factor by encapsulated cell biodelivery in the minipig basal forebrain. Mol Therapy. 18 (12), 2164-2172 (2010).
- Sørensen, J. C., et al. Development of neuromodulation treatments in a large animal model - Do neurosurgeons dream of electric pigs?. Prog Brain Res. 194, 97-103 (2011).
- Dolezalova, D., et al. Pig models of neurodegenerative disorders: utilization in cell replacement-based preclinical safety and efficacy studies. J Comp Neurol. 522 (12), 2784-2801 (2014).
- Mikkelsen, M., Moller, A., Jensen, L. H., Pedersen, A., Harajehi, J. B., Pakkenberg, H. MPTP-induced Parkinsonism in minipigs: A behavioral, biochemical, and histological study. Neurotoxicol Teratol. 21, 169-175 (1999).
- Danielsen, E. H., et al. The DaNEX study of embryonic mesencephalic, dopaminergic tissue grafted to a minipig model of Parkinson's disease: Preliminary findings of effect of MPTP poisoning on striatal dopaminergic markers. Cell Transplant. 9 (2), 247-259 (2000).
- Dalmose, A., Bjarkam, C. R., Sørensen, J. C., Jørgensen, T. M., Djurhuus, J. C. Effects of high frequency deep brain stimulation on urine storage and voiding function in conscious minipigs. Neurourol Urodyn. 23 (3), 265-272 (2004).
- Dalmose, A., Bjarkam, C. R., Djurhuus, J. C. Stereotactic electrical stimulation of the pontine micturition center in the pig. Br J Urol. 95, 886-889 (2005).
- Andersen, F., Watanabe, H., Bjarkam, C. R., Danielsen, E. H., Cumming, P. Pig brain stereotaxic standard space: Mapping of cerebral blood flow normative values and effect of MPTP-lesioning. Brain Res Bull. 66 (1), 17-29 (2005).
- Glud, A. N., et al. Direct gene transfer in the minipig CNS using stereotaxic lentiviral microinjections. Acta Neurobiol Exp. 70 (3), 1-8 (2010).
- Glud, A. N., et al. Direct MRI-guided stereotaxic viral mediated gene transfer of alpha-synuclein in the minipig CNS. Acta Neurobiol Exp. 71 (4), 508-518 (2011).
- Ettrup, K. S., Sørensen, J. C., Rodell, A., Alstrup, A. K. O., Bjarkam, C. R. Hypothalamic deep brain stimulation influences autonomic and limbic circuitry involved in the regulation of aggression and cardiocerebrovascular control in the minipig. Stereotact Funct Neurosurg. 90 (5), 281-291 (2012).
- Nielsen, M. S., et al. Continuous MPTP intoxication in the minipig results in chronic parkinsonian deficits. Acta Neurobiol Exp. 76, 198-210 (2016).
- Bjarkam, C. R., et al. A MRI-compatible stereotaxic localizer box enables high-precision stereotaxic procedures in pigs. J Neurosci Methods. 139 (2), 293-298 (2004).
- Bjarkam, C. R., Jorgensen, R. L., Jensen, K. N., Sunde, N. A. A., Sørensen, J. C. H. Deep brain stimulation electrode anchoring using BioGlue®, a protective electrode covering, and a titanium microplate. J Neurosci Methods. 168, 151-155 (2008).
- Ettrup, K. S., et al. Basic Surgical Techniques in the Minipig: Intubation, Transurethral Bladder Catheterization, Femoral Vessel Catheterization, and Transcardial Perfusion. J Vis Exp. (52), e2652 (2011).
- Ettrup, K. S., Tornøe, J., Sørensen, J. C., Bjarkam, C. R. A surgical device for minimally invasive implantation of experimental deep brain stimulation leads in large research animals. J Neurosci Methods. 200 (1), 41-46 (2011).
- Danielsen, E. H., et al. Positron emission tomography of living brain in minipigs and domestic pigs. Scand J Lab Anim Sci Suppl. 25 (1), 127-135 (1998).
- Røhl, L., et al. Time evolution of cerebral perfusion and ADC measured by MRI in a porcine stroke model. J Magn Reson Imaging. 15 (2), 123-129 (2002).
- Cumming, P., Gillings, N. M., Jensen, S. B., Bjarkam, C. R., Gjedde, A. Kinetics of the uptake and distribution of the dopamine D2/3 agonist (R)-N-[1-11C]n-propylnorapomorphine in brain of healthy and MPTP-poisoned Gottingen miniature pigs. Nucl Med Biol. 30 (5), 547-553 (2003).
- Jensen, K. N., Deding, D., Sørensen, J. C., Bjarkam, C. R. Long-term implantation of deep brain stimulation electrodes in the pontine micturition centre of the minipig. Acta Neurochir. 151 (7), 785-794 (2009).
- Rosendal, F., et al. Does chronic low dose treatment with ciclosporin influence the brain? A histopathological study in pigs. Transplantation Proc. 37 (8), 3305-3308 (2005).
- Nielsen, M. S., Sørensen, J. C., Bjarkam, C. R. The substantia nigra pars compacta of the minipig: An anatomical and stereological study. Brain Struct Funct. (4-5), 481-488 (2009).
- Sørensen, J. C., Bjarkam, C. R., Simonsen, C. Z., Danielsen, E., Geneser, F. A. Oriented sectioning of irregular tissue blocks in relation to computerized scanning modalities. Results from the domestic pig brain. J Neurosci Methods. 104, 93-98 (2000).
- Bjarkam, C. R., Pedersen, M., Sørensen, J. C. New strategies for embedding, orientation and sectioning of small brain specimens enable direct correlation to MR-images, brain atlases, or use of unbiased stereology. J Neurosci Methods. 108, 153-159 (2001).
- Bjarkam, C. R., Sørensen, J. C., Geneser, F. A. Distribution and morphology of serotonin-immunoreactive axons in the hippocampal region of the New Zealand white rabbit. I. Area dentata and hippocampus proper. Hippocampus. 13 (1), 21-37 (2003).
- Bjarkam, C. R., Glud, A. N., Orlowski, D., Sørensen, J. C., Palomero-Gallagher, N. The telencephalon of the minipig, cytoarchitecture and cortical surface anatomy. Brain Struct Funct. , (2016).
- Boltze, J., Nitzsche, B., Geiger, K. D., Schoon, H. A. Histopathological investigation of different MCAO modalities and impact of autologous bone marrow mononuclear cell administration in an ovine stroke model. Transl Stroke Res. 2, 279-293 (2011).
- Jortner, B. S. The return of the dark neuron. A histological artifact complicating contemporary neurotoxicologic evaluation. Neurotoxicology. 27, 628-634 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved