A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Tachycardia-Induced Cardiomyopathy As a Chronic Heart Failure Model in Swine
In This Article
Summary
Here, we present a protocol to produce tachycardia-induced cardiomyopathy in swine. This model represents a potent way to study the hemodynamics of progressive chronic heart failure and the effects of applied treatment.
Abstract
A stable and reliable model of chronic heart failure is required for many experiments to understand hemodynamics or to test effects of new treatment methods. Here, we present such a model by tachycardia-induced cardiomyopathy, which can be produced by rapid cardiac pacing in swine.
A single pacing lead is introduced transvenously into fully anaesthetized healthy swine, to the apex of the right ventricle, and fixated. Its other end is then tunneled dorsally to the paravertebral region. There, it is connected to an in-house modified heart pacemaker unit that is then implanted in a subcutaneous pocket.
After 4 - 8 weeks of rapid ventricular pacing at rates of 200 - 240 beats/min, physical examination revealed signs of severe heart failure - tachypnea, spontaneous sinus tachycardia, and fatigue. Echocardiography and X-ray showed dilation of all heart chambers, effusions, and severe systolic dysfunction. These findings correspond well to decompensated dilated cardiomyopathy and are also preserved after the cessation of pacing.
This model of tachycardia-induced cardiomyopathy can be used for studying the pathophysiology of progressive chronic heart failure, especially hemodynamic changes caused by new treatment modalities like mechanical circulatory supports. This methodology is easy to perform and the results are robust and reproducible.
Introduction
The variety of new treatment methods for heart failure (HF), especially the growing worldwide use of mechanical circulatory supports and extracorporeal membrane oxygenation (ECMO) in clinical practice, is reflecting in preclinical experimental testing. The main focus has been on hemodynamic changes caused by the examined treatment modalities, namely on systemic blood pressure1, myocardial contractility, pressure and volume changes in heart chambers and heart work2,3, arterial blood flow in systemic and peripheral arteries, along with metabolic compensation4 - regional tissue saturation, pulmonary perfusion, and blood gas analysis. Other studies are directed on long-term effects of the circulatory support5, concomitant inflammation, or occurrence of hemolysis. All these types of study need a stable biomodel of congestive HF.
Most of the published experiments on left ventricular (LV) performance and hemodynamics of mechanical circulatory support have been performed on experimental models of acute HF2,6,7,8,9,10, or even on completely intact hearts. On the other hand, in clinical practice, mechanical circulatory supports are often being applied in a status of circulatory decompensation that develops on the grounds of previously present chronic heart disease. In such situations, the adaptation mechanisms are fully developed and can play important roles in inconsistency of outcomes observed according to the "acuteness or chronicity" of underlying cardiac disease11. Therefore, a stable model of chronic HF can offer new insights into pathophysiological mechanisms and hemodynamics. Although there are reasons why the use of chronic HF models is scarce - time consuming preparation, instability of heart rhythm, ethical questions, and mortality rate - their advantages are clear, as they offer presence of long-term neurohumoral activation, general systemic adaptation, functional changes of cardiomyocytes, and structural alterations of heart muscle and valves12,13.
In general, the availability and variety of animal models used for hemodynamic studies is wide and offers choice for many specific needs. For these experiments, mostly porcine, canine, ovine, or with smaller settings murine models, are being chosen and offer a good simulation of expected human bodily reactions14. Furthermore, forms of single organ experiments are becoming more frequent15. To reliably mimic the pathophysiology of HF, circulation is being artificially deteriorated. Damage to the heart can be caused by various methods, often by either ischemia, arrhythmia, pressure overload, or cardiotoxic effects of drugs, with any of these leading to hemodynamic deterioration of the model. To produce a true model of chronic HF, time has to be provided for developing the long-term adaptation of the whole organism. Such a reliable and stable model is represented well by tachycardia-induced cardiomyopathy (TIC), which can be produced by rapid cardiac pacing in experimental animals.
It has been shown that in predisposed hearts, long-lasting incessant tachyarrhythmias can lead to systolic dysfunction and dilation with decreased cardiac output. The condition referred to as TIC was first described in 191316, widely used in experiments since 196217, and is now a well-recognized disorder. Its origin can lie in various types of arrhythmias - both supraventricular and ventricular tachycardia can lead to progressive deterioration of systolic function, biventricular dilation, and progressive clinical signs of HF including ascites, edemas, lethargy, and ultimately cardiac decompensation leading to terminal HF and, if not treated, death.
Similar effects of circulatory suppression were observed by introduction of high rate cardiac pacing in animal models. In a porcine model, an atrial or ventricular heart rate over 200 beats/minute is potent enough to induce end-stage HF in a period of 3 - 5 weeks (progressive phase) with characteristics of TIC, though interindividual differences do exist18,19. These findings correspond well to decompensated cardiomyopathy and are, importantly, preserved also after the cessation of pacing (chronic phase)19,20,21,22,23.
Porcine, canine, or ovine TIC models were repeatedly prepared to study the pathophysiology of HF14, as changes to the LV mimic the characteristics of dilated cardiomyopathy24. The hemodynamic characteristics are well described - increased ventricular end-diastolic pressures, decreased cardiac output, increased systemic vascular resistance, and dilation of both ventricles. In contrast, wall hypertrophy is not observed consistently, and even wall thinning was described by some researchers25,26. With progression of ventricular dimensions, regurgitation on atrioventricular valves develops26.
In this publication, we present a protocol to produce a TIC by long-term fast cardiac pacing in swine. This biomodel represents potent means to study decompensated dilated cardiomyopathy, hemodynamics of progressive chronic HF with low cardiac output, and effects of applied treatment.
Protocol
This experimental protocol was reviewed and approved by the Institutional Animal Expert Committee at First Faculty of Medicine, Charles University, and was performed at the University experimental laboratory, Department of Physiology, First Faculty of Medicine, Charles University in Prague, Czech Republic, in accordance with Act No. 246/1992 Coll., on the protection of animals against cruelty. All animals were treated and cared for in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, published by National Academies Press, 2011. All procedures were performed according to standard veterinary conventions and at the completion of each study, the animal was sacrificed and a necropsy performed. Due to suitable anatomy, five healthy crossbred female swine (Sus scrofa domestica) up to 6 months of age were included in this experiment. Their mean body weight was 66 ± 20 kg at the day of data collection.
1. General Anesthesia
- After 1 day of fasting, initiate anesthesia by intramuscular administration of midazolam (0.3 mg/kg) and ketamine hydrochloride (15 - 20 mg/kg) to the gluteal region.
- Insert peripheral cannula into the marginal ear vein for intravenous drug applications.
- Administer intravenous boluses of propofol (2 mg/kg) and morphine (0.1 - 0.2 mg/kg).
- Provide animals with oxygen via a facial mask and advance orotracheal intubation with a cuffed endotracheal tube with a diameter of 6.5 - 7.5 mm.
- Continue the total intravenous anesthesia by combination of propofol (6 - 12 mg/kg/h), midazolam (0.1 - 0.2 mg/kg/h), and morphine (0.1 - 0.2 mg/kg/h), adjusting the doses according to individual responses - suppress spontaneous breaths, corneal reflexes, and motoric response. Protect the animal's eyes with ointment to prevent dryness.
- Operate the mechanical ventilation by a closed-loop automatic device set to adaptive support ventilation to maintain target end-tidal CO2 of 38 - 42 mmHg and adequate hemoglobin saturation of 95 - 99%. Monitor all vital functions, especially heart rate and body temperature.
- Attach the animal by securing its legs gently to the operation table in the supine position.
- Administer wide spectrum antibiotics - 1 g of cefazolin intravenously through the ear vein cannula.
2. Ventricular Lead Implantation
- Locate surgical sites and shave the skin properly using a razor at (1) the jugular region above the sternocleidomastoid muscle and (2) the unilateral paravertebral region on the back side of the animal's neck.
- Using the ultrasound vascular probe, visualize the external jugular vein and mark its location on the skin. Locate the carotid artery as well to prevent its injury.
- After the wide skin disinfection using povidone iodine, cover with a sterile surgical drape with the hole over the marked jugular region.
- Prepare all necessary tools for pacemaker implantation and keep them sterile. It is crucial to maintain a sterile environment throughout the procedure.
- Cut the skin parallel above the external jugular vein, form a shallow subcutaneous pocket in soft tissue not more than 10 mm deep. Do not expose any large vessels.
- From the bottom of the preformed pocket, insert a sheath into the external jugular vein, using the standard Seldinger technique. First, insert a soft-tip guidewire through a 12G puncture needle, and then over the guidewire introduce a 7-French plastic tear away introducer sheath with a dilator.
- Under fluoroscopic guidance, introduce a 58 cm pacing lead through this sheath and position its tip to the apex of the right ventricle. Then, remove the sheath and fixate the active tip of the electrode to the myocardium by screwing out its helix.
- Test the pacing parameters - the lead sensed signal from ventricular electrocardiogram and impedance must be stable, the pacing threshold should be below an amplitude of 1 V with 0.4 ms of pulse duration.
- Pull a rubber sleeve on the pacing lead and fix both together to the bottom of the preformed jugular subcutaneous pocket by two non-absorbable suture braided thread stitches. Importantly, enough length of the pacing lead must be inserted, considering the future possible growth of the animal.
3. Subcutaneous Lead Tunneling
- Turn the animal over on its side and disinfect the previously shaved skin region lateral to the backbone, then cover with a sterile surgical drape with a hole. Make sure the jugular subcutaneous pocket and the lead remain sterile.
- Cut the skin lateral to the backbone and form a deep, spacious, subcutaneous pocket. Use dull preparation and stop any possible bleeding.
- Take a soft rubber extension tube from a sterile infusion set and cut off both its ends. Using a tunneling tool, preform a direct subcutaneous tunnel connecting the jugular and dorsal subcutaneous pockets with this extension tube.
- Connect the tube's free end to the ventricular lead by pulling it onto its IS-1 connector and draw the lead through the preformed tunnel into the dorsal subcutaneous pocket by pulling the tube dorsally. It may be useful to secure the connection with a silk tie.
- Remove both the tunneling tool and the extension tube, exposing the ventricular lead from the dorsal subcutaneous pocket.
4. Pacemaker Implantation
- Set up the implantable dual-chamber heart pacemaker unit with the "Y" connecting part. The "Y" connection allows a convergent connection of both pacemaker outputs to be joined and connected together to the single pacing lead (Figure 1 and Figure 2). This setting will later provide a wide range of pacing frequencies.
- After connecting the pacing lead, fasten all the IS-1 connection screws in the pacemaker header unit and the "Y" lead connection.
- Hide the whole pacing system in the deep dorsal pocket. There must be enough space to comfortably accommodate the pacemaker unit and any redundant lead.
- Check the final pacing parameters. Make sure that cardiac ventricular pacing is possible from both pacemaker outputs.
- Flush with povidone iodine and close both subcutaneous pockets. Use absorbable braided thread to suture fibrous tissue layers and non-absorbable suture for skin adaptation.
5. Postoperative Care
- Observe the animal carefully until it regains sufficient consciousness.
- Continue in a wide spectrum intravenous antibiotic regimen until the wounds are healed - cefazolin 1 g every 12 h. Administer analgesics in appropriate dosing, e.g., morphine 0.2 mg/kg every 6 - 12 h for 3 days by subcutaneous injection. If necessary, make dose adjustments to adequately prevent pain.
- Place the animal in a comfortable, calm facility at room temperature. Allow free access to water and suitable alimentation.
- Dress the wounds with sterile scrubs regularly to preserve clean healing.
- To provide rest after the surgical procedure, keep the pacemaker inhibited by native heart rhythm for at least 3 days.
- Remove the non-absorbable skin sutures when fully healed, approximately 10 - 14 days after the procedure.
6. Pacing Protocol
- Start the pacing protocol after an adequate resting period. Initially, increase the paced ventricular heart rate to 200 beats/min by setting the dual-chamber pacemaker to mode D00, 100 beats/min, and concomitantly adjusting the AV delay to 300 ms (to exactly match the pace to pace interval, see Table 1). Select the unipolar pacing in both outputs.
- Increase stepwise the paced heart rate to 220 beats/min after 1 week and to 240 beats/min after 2 weeks (Figure 3). Keep continuous pacing at this frequency unless it is not hemodynamically tolerated. If the HF progresses too quickly, reduce the paced heart rate before increasing it again after another week.
- Use auscultation of heart beat, ECG, and pacemaker interrogation daily to verify the heart rate and constant pacing parameters, including battery life.
7. Heart Failure Induction and Monitoring
- Ensure regular care by a specialized veterinarian and monitor the animal's general health status. Clinical observations of increasing native heart and respiratory rates, evaluation of peripheral pulse oximetry, and reduction in spontaneous physical activity or appetite provide information about HF progression.
- Use the advantage of wireless transcutaneous pacemaker interrogation and, if possible, continuous ECG recording - frequent non-sustained ventricular tachycardias (VT) are a sign of severe HF progression.
- Use echocardiographic assessments to reveal the structural and functional heart changes. Pay attention to find an optimal image window according to porcine anatomy and heart dilation - for a typical 4 chamber view, place the transducer to the right just below the xiphoid and angle it to point to the neck or left shoulder. For short-axis views, use intercostal windows. Reduction of ventricular ejection fraction in native heart rhythm and atrioventricular regurgitations should be noticeable after a few weeks.
NOTE: Significant interindividual differences of high rate ventricular pacing tolerance exist. Therefore, frequent monitoring and individually adapted adjustment of the pacing protocol are necessary.

Figure 1: Heart pacing unit schematic. The dual-chamber pacemaker (1), a "Y" shaped adapter (2) conducting convergently both pacemaker outputs together to a single pacing lead (3). The tip of the lead is fixated into the apical part of the RV cavity (4). This setting provides a wide range of high pacing frequencies. Please click here to view a larger version of this figure.

Figure 2: Heart pacing unit. X-ray (A) and photography (B) of the dual-chamber pacemaker (1), a "Y" shaped adapter (2), and the ventricular pacing lead (3). Please click here to view a larger version of this figure.
| Desired HR | Set pacemaker rate | Pace to pace interval |
| beats/min | beats/min | ms |
| 200 | 100 | 300 |
| 220 | 110 | 270 |
| 240 | 120 | 250 |
| 250 | 125 | 240 |
Table 1: Pacemaker parameters. To allow high rate cardiac pacing with the implanted in-house-modified dual-chamber pacemaker unit, the table shows the desired paced heart rate (HR) and matching pace to pace interval values. The pacemaker must be set to D00 operation mode at a rate of half of the desired HR, and the AV delay set to the corresponding pace to pace interval in milliseconds.
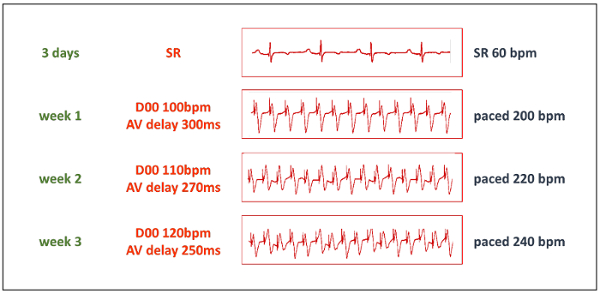
Figure 3: Pacing protocol. The progressive phase of the TIC induction starts after a resting period of 3 days. Then, the pacemaker is set to D00 mode with a pacing frequency of 50% of the desired paced frequency, and AV delay is set to the matching pace to pace interval (see Table 1). Thanks to the "Y" shaped adapter, both pacemaker outputs are conducted to a single pacing lead. bpm = beats/minute. Please click here to view a larger version of this figure.
Results
Testing the model: After signs of decompensated chronic HF became prominent, anesthesia and artificial ventilation were administered again following the principles described above, but dosing was adjusted due to low cardiac output27. Due to possible cardiodepressive effects of anesthetics, careful intensive monitoring of vital functions is necessary.
The animal was attached in the supine ...
Discussion
Chronic HF is a major health problem that contributes greatly to morbidity and mortality. The pathogenesis and progression of HF in humans is complex, so an appropriate animal model is critical to investigate the underlying mechanisms and to test novel therapeutics that aim to interfere with native severe disease progression. To study its pathogenesis, large animal models are being used for experimental testing.
In general, surgical models of chronic HF closely mimic this disease. When compare...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by Charles University research grants GA UK No. 538216 and GA UK No. 1114213.
Materials
| Name | Company | Catalog Number | Comments |
| Medication | |||
| midazolam | Roche | Dormicum | anesthetic |
| ketamine hydrochloride | Richter Gedeon | Calypsol | anesthetic |
| propofol | B.Braun | Propofol | anesthetic |
| cefazolin | Medochemie | Azepo | antibiotic |
| Silver Aluminium Aerosol | Henry Schein | 9003273 | tincture |
| povidone iodine | Egis Praha | Betadine | disinfection |
| morphine | Biotika Bohemia | Morphin 1% inj | analgetic |
| Tools | |||
| Metzenbaum scissors, lancet with #22 blade, DeBakey forceps, needle driver | basic surgical equipment | ||
| cauterizer | |||
| 2-0 Vicryl | Ethicon | V323H | absorbable braided suture |
| 2-0 Perma-Hand Silk | Ethicon | A185H | silk tie suture |
| 2-0 Prolene | Ethicon | 8433H | non-absorbable suture |
| Diagnostic devices | |||
| ESP C-arm | GE Healthcare | ESP | X-ray fluoro C-arm |
| Acuson x300 | Siemens Healthcare | ultrasound system | |
| Acuson P5-1 | Siemens Healthcare | echocardiographic probe | |
| Acuson VF10-5 | Siemens Healthcare | sonographic vascular probe | |
| 3PSB, 4PSB and 6PSB | Transonic Systems | perivascular flow probes | |
| TS420 | Transonic Systems | perivascular flow module | |
| TruWave | Edwards Lifesciences | T001660A | fluid-filled pressure transducer |
| 7.0F VSL Pigtail | Transonic Systems | pressure sensor catheter | |
| INVOS 5100C Cerebral/Somatic Oximeter | Somanetics/Medtronic | near infrared spectroscopy | |
| CCO Combo Catheter | Edwards Lifesciences | 744F75 | Swan-Ganz pulmonary artery catheter |
| Vigillace II | Edwards Lifesciences | VIG2E | cardiac output monitor |
| 7.0F VSL Pigtail | Transonic Systems | pressure-volume catheter | |
| ADV500 | Transonic Systems | pressure-volume system | |
| LabChart and PowerLab | ADInstruments | data acquisition and analysis system | |
| Prism 6 | GraphPad | statistical analysis software | |
| Pacing devices | |||
| ICS 3000 | Biotronic | 349528 | pacemaker programmer |
| ERA 3000 | Biotronic | 128828 | external pacemaker |
| Effecta DR | Biotronic | 371199 | dual-chamber pacemaker |
| Tendril STS | St. Jude Medical | 2088TC/58 | ventricular pacing lead |
| Lead permanent adapter | Osypka | Article 53422 | convergent "Y" connecting part |
| Lead permanent adapter | Osypka | Article 53904 | convergent "Y" connecting part |
| Tear-Away Introducer 7F | B.Braun | 5210593 | tear away introducer sheath |
| Split Cath Tunneler | medComp | AST-L | tunneling tool |
| infusion line | MPH Medical Devices | 2200045 | connecting line |
References
- Ostadal, P., et al. Direct comparison of percutaneous circulatory support systems in specific hemodynamic conditions in a porcine model. Circ Arrhythm Electrophysiol. 5 (6), 1202-1206 (2012).
- Ostadal, P., et al. Increasing venoarterial extracorporeal membrane oxygenation flow negatively affects left ventricular performance in a porcine model of cardiogenic shock. J Transl Med. 13, 266 (2015).
- Shen, I., et al. Left ventricular dysfunction during extracorporeal membrane oxygenation in a hypoxemic swine model. Ann Thorac Surg. 71 (3), 868-871 (2001).
- Hala, P., et al. Regional tissue oximetry reflects changes in arterial flow in porcine chronic heart failure treated with venoarterial extracorporeal membrane oxygenation. Physiol Res. 65 (Supplementum 5), S621-S631 (2016).
- Church, J. T., et al. Normothermic Ex-Vivo Heart Perfusion: Effects of Live Animal Blood and Plasma Cross-Circulation. ASAIO J. , (2017).
- Bavaria, J. E., et al. Changes in left ventricular systolic wall stress during biventricular circulatory assistance. Ann Thorac Surg. 45 (5), 526-532 (1988).
- Shen, I., et al. Effect of extracorporeal membrane oxygenation on left ventricular function of swine. Ann Thorac Surg. 71 (3), 862-867 (2001).
- Ostadal, P., et al. Novel porcine model of acute severe cardiogenic shock developed by upper-body hypoxia. Physiol Res. 65 (4), 711-715 (2016).
- Ostadal, P., et al. Noninvasive assessment of hemodynamic variables using near-infrared spectroscopy in patients experiencing cardiogenic shock and individuals undergoing venoarterial extracorporeal membrane oxygenation. J Crit Care. 29 (4), e611-e695 (2014).
- Mlcek, M., et al. Hemodynamic and metabolic parameters during prolonged cardiac arrest and reperfusion by extracorporeal circulation. Physiol Res. 61 (Suppl 2), S57-S65 (2012).
- Tarzia, V., et al. Extracorporeal life support in cardiogenic shock: Impact of acute versus chronic etiology on outcome. J Thorac Cardiovasc Surg. 150 (2), 333-340 (2015).
- Howard, R. J., Stopps, T. P., Moe, G. W., Gotlieb, A., Armstrong, P. W. Recovery from heart failure: structural and functional analysis in a canine model. Can J Physiol Pharmacol. 66 (12), 1505-1512 (1988).
- Moe, G. W., Armstrong, P. Pacing-induced heart failure: a model to study the mechanism of disease progression and novel therapy in heart failure. Cardiovasc Res. 42 (3), 591-599 (1999).
- Power, J. M., Tonkin, A. M. Large animal models of heart failure. Aust N Z J Med. 29 (3), 395-402 (1999).
- Trahanas, J. M., et al. Achieving 12 Hour Normothermic Ex Situ Heart Perfusion: An Experience of 40 Porcine Hearts. ASAIO J. 62 (4), 470-476 (2016).
- Gossage, A. M., Braxton Hicks, J. A. On auricular fibrillation. Quarterly Journal of Medicine. 6, 435-440 (1913).
- Whipple, G. H., Sheffield, L. T., Woodman, E. G., Theophilis, C., Friedman, S. Reversible congestive heart failure due to chronic rapid stimulation of the normal heart. Proceedings of the New England Cardiovascular Society. 20 (1), 39-40 (1962).
- Spinale, F. G., Grine, R. C., Tempel, G. E., Crawford, F. A., Zile, M. R. Alterations in the myocardial capillary vasculature accompany tachycardia-induced cardiomyopathy. Basic Res Cardiol. 87 (1), 65-79 (1992).
- Shinbane, J. S., et al. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 29 (4), 709-715 (1997).
- Moe, G. W., Stopps, T. P., Howard, R. J., Armstrong, P. W. Early recovery from heart failure: insights into the pathogenesis of experimental chronic pacing-induced heart failure. J Lab Clin Med. 112 (4), 426-432 (1988).
- Takagaki, M., et al. Induction and maintenance of an experimental model of severe cardiomyopathy with a novel protocol of rapid ventricular pacing. J Thorac Cardiovasc Surg. 123 (3), 544-549 (2002).
- Tomita, M., Spinale, F. G., Crawford, F. A., Zile, M. R. Changes in left ventricular volume, mass, and function during the development and regression of supraventricular tachycardia-induced cardiomyopathy. Disparity between recovery of systolic versus diastolic function. Circulation. 83 (2), 635-644 (1991).
- Schmitto, J. D., et al. Large animal models of chronic heart failure (CHF). J Surg Res. 166 (1), 131-137 (2011).
- Spinale, F. G., et al. Chronic supraventricular tachycardia causes ventricular dysfunction and subendocardial injury in swine. Am J Physiol. 259 (1 Pt 2), H218-H229 (1990).
- Chow, E., Woodard, J. C., Farrar, D. J. Rapid ventricular pacing in pigs: an experimental model of congestive heart failure. Am J Physiol. 258 (5 Pt 2), H1603-H1605 (1990).
- Howard, R. J., Moe, G. W., Armstrong, P. W. Sequential echocardiographic-Doppler assessment of left ventricular remodelling and mitral regurgitation during evolving experimental heart failure. Cardiovasc Res. 25 (6), 468-474 (1991).
- Roberts, F., Freshwater-Turner, D. Pharmacokinetics and anaesthesia. Contin Educ Anaesth Crit Care Pain. 7 (1), 25-29 (2007).
- Carter, B. S., Farrell, C., Owen, C. Microsurgical clip obliteration of middle cerebral aneurysm using intraoperative flow assessment. J Vis Exp. (31), (2009).
- Wolf, M., Ferrari, M., Quaresima, V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Opt. 12 (6), 062104 (2007).
- Mateu Campos, M. L., et al. Techniques available for hemodynamic monitoring. Advantages and limitations. Med Intensiva. 36 (6), 434-444 (2012).
- Baan, J., et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 70 (5), 812-823 (1984).
- Ellenbroek, G. H., et al. Primary Outcome Assessment in a Pig Model of Acute Myocardial Infarction. J Vis Exp. (116), (2016).
- Townsend, D. Measuring Pressure Volume Loops in the Mouse. J Vis Exp. (111), (2016).
- van Hout, G. P., et al. Admittance-based pressure-volume loops versus gold standard cardiac magnetic resonance imaging in a porcine model of myocardial infarction. Physiol Rep. 2 (4), e00287 (2014).
- Kass, D. A., et al. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 76 (6), 1422-1436 (1987).
- Glower, D. D., et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 71 (5), 994-1009 (1985).
- Hendrick, D. A., Smith, A. C., Kratz, J. M., Crawford, F. A., Spinale, F. G. The pig as a model of tachycardia and dilated cardiomyopathy. Lab Anim Sci. 40 (5), 495-501 (1990).
- Wyler, F., et al. The Gottinger minipig as a laboratory animal. 5. Communication: cardiac output, its regional distribution and organ blood flow (author's transl). Res Exp Med (Berl). 175 (1), 31-36 (1979).
- Cruz, F. E., et al. Reversibility of tachycardia-induced cardiomyopathy after cure of incessant supraventricular tachycardia. J Am Coll Cardiol. 16 (3), 739-744 (1990).
- Umana, E., Solares, C. A., Alpert, M. A. Tachycardia-induced cardiomyopathy. Am J Med. 114 (1), 51-55 (2003).
- Dixon, J. A., Spinale, F. G. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2 (3), 262-271 (2009).
- Xanthos, T., et al. Baseline hemodynamics in anesthetized landrace-large white swine: reference values for research in cardiac arrest and cardiopulmonary resuscitation models. J Am Assoc Lab Anim Sci. 46 (5), 21-25 (2007).
- Little, W. C. Diastolic dysfunction beyond distensibility: adverse effects of ventricular dilatation. Circulation. 112 (19), 2888-2890 (2005).
- Montgomery, C., Hamilton, N., Ianuzzo, C. D. Effects of different rates of cardiac pacing on rat myocardial energy status. Mol Cell Biochem. 102 (2), 95-100 (1991).
- Qin, F., Shite, J., Mao, W., Liang, C. S. Selegiline attenuates cardiac oxidative stress and apoptosis in heart failure: association with improvement of cardiac function. Eur J Pharmacol. 461 (2-3), 149-158 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved