A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Spatial Quantification of Drugs in Pulmonary Tuberculosis Lesions by Laser Capture Microdissection Liquid Chromatography Mass Spectrometry (LCM-LC/MS)
In This Article
Summary
Here, we describe a protocol using laser capture microdissection coupled with LC/MS analysis to spatially-quantify drug distributions within pulmonary tuberculosis granulomas. The approach has broad applicability to quantifying drug concentrations within tissues at high spatial detail.
Abstract
Tuberculosis is still a leading cause of morbidity and mortality worldwide. Improvements to existing drug regimens and the development of novel therapeutics are urgently required. The ability of dosed TB drugs to reach and sterilize bacteria within poorly-vascularized necrotic regions (caseum) of pulmonary granulomas is crucial for successful therapeutic intervention. Effective therapeutic regimens must therefore contain drugs with favorable caseum penetration properties. Current LC/MS methods for quantifying drug levels in biological tissues have limited spatial resolution capabilities, making it difficult to accurately determine absolute drug concentrations within small tissue compartments such as those found within necrotic granulomas. Here we present a protocol combining laser capture microdissection (LCM) of pathologically-distinct tissue regions with LC/MS quantification. This technique provides absolute quantification of drugs within granuloma caseum, surrounding cellular lesion and uninvolved lung tissue and, therefore, accurately determines whether bactericidal concentrations are being achieved. In addition to tuberculosis research, the technique has many potential applications for spatially-resolved quantification of drugs in diseased tissues.
Introduction
The ability to spatially resolve and quantify drug levels is a crucial requirement for determining whether anti-tuberculosis drugs reach bacterial subpopulations within pulmonary lesions at sterilizing concentrations1. Of particular importance is determining drug penetration into the necrotic core of the lesion (called caseum), which typically contains the highest number of bacilli and may be poorly accessible to drugs due to the absence of vascularization.
Traditional methods to assess lesion penetration, which involve homogenization of excised pulmonary lesions followed by solvent extraction and liquid chromatography mass spectrometry (LC/MS) analysis, are highly sensitive and selective for the drugs of interest. However, these methods offer poor spatial information, limited to the size of the original homogenized tissue. Mass spectrometry-based imaging approaches, such as matrix-assisted laser desorption ionization (MALDI)2,3, desorption electrospray ionization (DESI)4 or liquid-enhanced surface extraction5,6 offer highly spatially-resolved imaging capabilities, but direct quantification can be extremely challenging or impossible due to heterogeneous ion suppression effects and differing extraction efficiencies of analyte from the various cell or tissue types7. Additionally, most direct tissue MS imaging approaches are inherently less sensitive than LC/MS due to the lack of chromatographic separation of endogenous species competing for ionization and the lower solvent extraction efficiency of the drug from tissue.
Laser capture microdissection (LCM) combined with LC/MS analysis has been routinely applied to isolate and characterize distinct tissue regions for proteomic studies8,9 and recently utilized for drug quantification in dosed animal tissue10. Here we present an optimized protocol applying LCM combined with LC/MS (LCM-LC/MS) analysis to quantify anti-TB drugs within distinct granuloma compartments. In the laser capture microdissection process, a UV laser is focused through the microscope objective onto the tissue section, which cuts and isolates the desired tissue area by following a path defined by the user. For gravity-assisted LCM (the technique used for this research), the tissue section is mounted onto a thin polymer membrane slide (PET or PEN) and the tissue is captured in a collection tube cap sited below the slide. The drugs are extracted from the excised tissue and quantified using standard LC/MS approaches. The amount of tissue required to be collected is ultimately determined from the expected concentration of the drug present in the tissue and the sensitivity of the LC/MS method. For most analyses of drugs dosed at therapeutic levels and analyzed using a routine triple quadrupole mass spectrometer, 3 million µm2 (3 mm2) of tissue surface area is sufficient.
This protocol describes the powerful combination of spatial profiling and full quantification offered by LCM-LC/MS, providing absolute drug concentrations within all compartments of TB granulomas. The technique may also be applied to determining drug concentrations in many different diseased tissues providing vital drug discovery and development information.
Protocol
All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health with approval from the Institutional Animal Care and Use Committee of the NIAID (NIH), Bethesda, MD.
1. Animal Experiments and Tissue Collection
This section of the protocol describes animal procedures and sample collection under Biosafety Level 3 (BSL3) conditions. Detailed protocols of the Mycobacterium tuberculosis aerosol infection procedure and drug administration protocols in rabbits have been described previously11,12.
- Infect New Zealand White rabbits (male and female at 4 - 5 months old) with M. tuberculosis HN878 using a nose-only aerosol system, as previously described11.
- Administer the chosen drugs (Ethambutol in the example presented here) via the preferred route and euthanize the animals at 2, 6, and 24 h following administration. First, anesthetize the rabbit by intramuscular injection of Ketamine at 35 mg/kg and Xylazine at 5 mg/kg. Wait for ten minutes and confirm proper anesthetization by pinching the tail and gently touching the eye. If there is no reaction, euthanize by intravenous administration of pentobarbital and phenytoin (see Table of Materials) at 1 mL/4.5 kg in 2 mL sterile saline.
NOTE: These timepoints are optimal to cover the pharmacokinetic profile for Ethambutol and may require adjusting/optimizing for other study drugs. - Using forceps, scissors, and/or scalpel, remove the lungs from the chest cavity, resect lung biopsies containing large necrotic granulomas embedded in surrounding uninvolved lung tissue (as described previously3). Necrotic granulomas appear beige in color and typically protrude slightly from the surrounding red/pink-colored lung. To facilitate easy cryosectioning, ensure that biopsies are no larger than 2 x 1.5 x 1.5 cm.
- Using forceps, place the biopsy onto a pre-labeled cryomold tray with the desired cutting surface in direct contact with the base of the tray. After freezing, this will provide a flat surface from which cryosections will be cut.
- Freeze the biopsy in liquid nitrogen vapor. Fill a styrofoam container to a depth of 2 inches with liquid nitrogen and place a metal wire tube rack. The rack should protrude above the surface of the liquid nitrogen providing a flat surface on which the tissue trays are placed. Place the lid back on the styrofoam container and leave tissues for 10 minutes to fully freeze.
- Remove the tissue trays, quickly wrap in aluminium film and place in individually labeled resealable plastic bags and seal. Transfer to -80 °C freezer for storage.
NOTE: Steps 1.1 - 1.6 are performed in BSL3 conditions (including all animal work and handling of infected organs and tissues). Gamma irradiate the lung biopsies at 3 Megarads to enable handling outside of BSL3 containment. Laser capture microdissection on unsterilized tissue may be performed within the BLS3 facility if approved safety protocols are in place. However, the remainder of this protocol describes downstream processing in a BSL-2 facility.
2. Tissue Sectioning
- Set the cryostat to the desired cutting temperature. Transfer the gamma-irradiated lung biopsy from -80 °C storage to the cryostat and leave for 30 minutes to equilibrate the tissue temperature. Note: -20 to -22 °C is optimal for TB lesion biopsies.
- Using tweezers, fix the biopsy to the cryostat chuck using a small amount of optimal cutting temperature adhesive (OCT) to adhere the base of the tissue to the chuck. Orient the tissue so that the flat surface (that was in contact with the base of the cryomold) is the exposed surface for cutting. Ensure the OCT does not contaminate the tissue surface, as this may interfere with the subsequent mass spectrometry analysis.
- Cut three tissue sections at 25 µm thickness and mount onto PET membrane slides. Gently touch the membrane to the tissue section and remove. If too much pressure is applied, the thin membrane may tear.
- Avoid excessive handling of the slides prior to mounting as this will result in the PET membrane becoming charged and poor adhesion of the tissue sections. Ensure that the membrane slide is kept at room temperature to enable thaw-mounting and successful adhesion of the tissue to the membrane.
- Remove the slide from the cryostat and allow to air-dry for 3 minutes. If LCM-LC/MS/MS will not be performed immediately, seal the slide in a small airtight sealable bag and transfer to -80 °C storage until required for dissection.
- Cut an adjacent section at 10 - 12 µm and thaw-mount on a standard glass slide for Hematoxylin and Eosin (H&E) staining and reference. Additional sections can be cut at this time for other desired histochemistry stains (such as Ziehl-Niellsen for visualizing Mycobacterium tuberculosis (MTB)).
3. Microdissection
- Remove sealed bag containing the slide from -80 °C storage and allow to reach room temperature for 5 minutes.
NOTE: If the cold slide is immediately exposed to the laboratory atmosphere, the tissue will become coated with condensation, and the spatial integrity of the drug may be compromised. - Turn on the microscope and laser (laser requires 5 - 10 minutes warm up before cutting can commence). Load flat-cap 0.20 mL PCR tubes into the holder.
- Remove slide from the bag and take an optical image of the tissue section on the PET slide using a flatbed scanner.
- Place the slide into the slide holder (tissue side facing down) and assign separate collection tubes to specific granuloma regions of interest using the microscope software. Typically, these will be 'uninvolved lung,' 'cellular granuloma,' and 'caseum' (necrotic center), but may vary depending upon the specific pathology of the granuloma/biopsy.
- Focus on the tissue using the 5X microscope objective. This magnification should provide a good overview of the tissue containing both cellular and necrotic granuloma areas. In the software select the tube designated 'caseum' to move it into position under the tissue.
- Enter the desired dissection parameters. Typical settings for a 25 µm thick lung section are laser power 30, speed 15, and aperture 35 (arbitrary units). However, these will differ depending upon the microscope used and potential declining power due to the age of the laser.
- Select the 'free-draw' tool and, using either a mouse or touchscreen pen, outline the desired region for dissection. The surface area of the region will be displayed in the software. Keep selected regions under 500,000 µm2 (0.5 mm2) to facilitate easier dissection. Repeat the dissection until 3 million µm2 (3 mm2) have been collected in total in the tube cap.
- On occasion, the dissected region may remain stuck to the surrounding membrane (for example due to static attraction) and not fall into the collection cap. Remove these regions from the cumulative surface area total by selecting and removing manually within the software.
- Select the cap for 'cellular lesion' and collect 3 million µm2 of tissue using the same process as outlined in step 3.7.
- Select the cap for 'uninvolved lung' and collect 3 million µm2 of tissue using the same process as outlined in step 3.7. Note that uninvolved lung tissue contains many bronchioles and alveolar spaces. Pay careful attention to exclude these from the defined tissue regions for dissection.
- Remove the cap holder and carefully unclip, seal and label each tube. Protect the dissected tissues from surrounding air disturbances (such as from air flow disruption from an opening door). Analyze the dissected tissues immediately, or store at -80 °C and thaw prior to processing and LCMS analysis.
4. Extraction and LCMS Analysis
- Prepare extraction solution of 1:1 acetonitrile/methanol containing Ethambutol d-10 internal standard. When selecting an internal standard, use a stable labeled form of the analyte drug (such as deuterium-labeled EMB used in this demonstration) with sufficient mass shift to avoid isotope cross talk between the analyte drug and standard (usually a minimum of 4 daltons).
NOTE: Creating standards in homogenate of each respective tissue type is difficult because there is very limited control tissue with which to create homogenate standards. As an alternative to making standards from a spiked homogenate sample, a standard can be created by adding blank tissue and test compound together and extracting. A volume of control tissue in a homogenate that matches the target volume of the study sample tissue sections is combined directly with an amount of the test compound that would be present at a given concentration. - Calculate the targeted tissue volume based on the surface area and thickness of the tissue section and determine the necessary dilution factor for the homogenate using the volume of homogenate that will be added to the standard and QC samples. Calculations are illustrated below for a 3 million µm2 (3 mm2) target dissected area with a 25 µm thickness and 2 µL volume of homogenate.
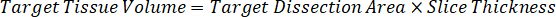
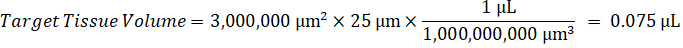
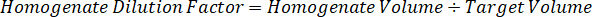

- Assuming a tissue density of 1 g/mL, prepare the homogenate stock by weighing 50 mg of control tissue and adding PBS buffer to dilute (using the 26.67 homogenate dilution factor calculated in step 4.2, the diluent is 1.283 mL). Homogenize by bead beating lung tissue and PBS buffer for 5 minutes at 1750 rpm on a bead homogenizer.
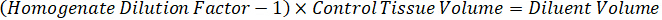
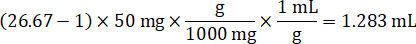
- Dilute 1 mg/mL drug stocks concentration in 1:1 acetonitrile/water to create standard curve spiking solutions. Determine spiking standard concentrations based on the spike volume and the target tissue volume. The illustrated example is for a 100 ng/mL standard using a 10 µL spike volume.
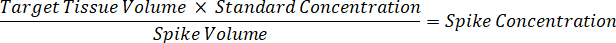
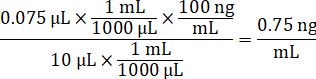
- Remove the tubes containing the microdissected tissues from -80 °C storage and allow to reach room temperature for 5 minutes.
- Add 10 µL of 1:1 acetonitrile/water solution and 2 µL of PBS buffer to the tubes containing microdissected tissue.
- For standard curve and quality control tubes, add 10 µL of spiking solution to 2 µL of control lung homogenate.
- Add 50 µL of extraction solution to each tube.
- Vortex each tube for 5 minutes, sonicate for 5 minutes and centrifuge at 5000 RPM for 5 minutes to form a pellet of film and tissue in each tube.
- Transfer 50 µL of supernatant to a 96-well deep-well plate and dilute with an additional 50 µL of deionized water in each well.
- Perform LC/MS/MS analysis using optimized instrument parameters for Ethambutol and Ethambutol-d10 internal standard (as previously described in detail12).
- Use a dilution factor to correct for the exact amount of tissue dissected for each sample.
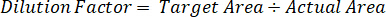
5. Method Validation
- Create a homogenate in control lung tissue by combining 1 part lung, 2 parts PBS, and 3 - 4 steel beads. Beat lung tissue and PBS buffer for 5 minutes at 1750 rpm using a bead homogenizer.
- Spike the homogenate by adding 10 µL of 1 mg/mL Ethambutol DMSO stock into 990 µL homogenate to create a final concentration of 10,000 ng/mL (10 mg/mL) and vortex for 1 minute.
- Create a frozen homogenate block by pouring the homogenate into a cryomold and rapidly freezing on dry ice for 5 minutes.
- Prepare 25 µm thick sections from the homogenate block as described in steps 2.1 - 2.5.
- Dissect the target tissue area as specified in steps 3.2 - 3.10.
- Add 10 µL 1:1 acetonitrile/water and 2 µL of PBS buffer to the tubes containing microdissected tissue.
- Add 50 µL of extraction solution to each tube. Follow steps 4.9 - 4.12 to create a standard curve and determine the drug concentration in the tissue homogenate block.
- Calculate extraction efficiency using the formula below:
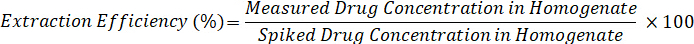
Results
An overview of the LCM-LC/MS approach is shown in Figure 1. After sterilizing the tissue by gamma-irradiation, all subsequent steps (from tissue sectioning onwards) take place outside of BSL3 conditions. Figure 2 shows the lesion biopsy sections before and after tissue isolation by LCM. Necrotic and cellular areas of TB lesions can be easily identified and isolated by visual inspection of optical images alone (without the require...
Discussion
Spatially-resolved quantification of drugs within pulmonary TB lesions is required to determine whether drug exposure reaches sterilizing concentrations to bacterial populations residing within the different lesion compartments. The LCM-LC/MS method described here enables absolute quantification of anti-TB drugs within all lesion compartments, including the bacteria-rich caseum, using only 1 - 3 tissue sections in total. Traditional tissue homogenization and LC/MS approaches for drug quantification in tissue often lack t...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Paul O'Brien, Marizel Mina and Isabella Freedman for animal experiments, Jacquie Gonzalez and Danielle Weiner from NIH/NIAID for help with gamma irradiation of rabbit tissues prior to laser capture microdissection and Jansy Sarathy for manuscript thoughts and advice. This work was supported by funding from the Bill and Melinda Gates Foundation (OPP1174780) and NIH shared instrumentation grant 1S10OD018072. We thank Eliseo A. Eugenin for providing access to the Leica LMD 6500 microscope and sharing expertise and advice. The purchase, and ongoing support of, the LMD 6500 was funded by The National Institute of Mental Health grant, MH096625, the National Institute of Neurological Disorders and Stroke, NS105584, PHRI funding (to E.A.E) and GSK contributions (to E.A.E).
Materials
| Name | Company | Catalog Number | Comments |
| New Zealand White rabbits | Covance | N/A | |
| HN878 Mycobacterium tuberculosis | BEI Resources | NR-13647 | |
| Ketathesia (Ketamine) 100 mg/mL C3N | Henry Schein Animal Health | 56344 | |
| Anased (Xylazine) 100 mg/mL | Henry Schein Animal Health | 33198 | |
| Euthasol (pentobarbital sodium and phenytoin sodium) Solution | Virbac | 710101 | |
| Acetonitrile (LC-MS grade) | Fisher | A955-212 | |
| Methanol (LC-MS grade) | Fisher | A456-212 | |
| Formic Acid (LC-MS grade) | Fisher | A117-50 | |
| Water (LC-MS grade) | Fisher | W6212 | |
| 0.2 mL flat-cap PCR tubes | Corning | 07-200-392 | |
| Steel frames, PET-membrane | Leica | 11505151 | |
| Premium Frosted Microscope Slides | Fisher | 12-544-2 | |
| 96 Deep well plate 2.0ML PP RB | Fisher | NC0363259 | |
| Zorbax SB-C8 column (4.6 by 50 mm; particle size, 3.5 μm) | Agilent | 820631-001D | |
| "Zipper” Seal Sample Bags | Fisher | 01-816-1B | |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| CM1850 cryostat | Leica | Discontinued | Leica CM1860 is the current model |
| Laser Microdissection System 6500 | Leica | Discontinued | Leica LMD 6 is the current model |
| Agilent 1260 Infinity II HPLC | Agilent | ||
| API 4000 QTRAP Mass Spectrometer | Sciex |
References
- Dartois, V. The path of anti-tuberculosis drugs: From blood to lesions to mycobacterial cells. Nat Rev Microbiol. 12 (3), 159-167 (2014).
- Prideaux, B., et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med. 21 (10), 1223-1227 (2015).
- Prideaux, B., et al. High-sensitivity MALDI-MRM-MS imaging of moxifloxacin distribution in tuberculosis-infected rabbit lungs and granulomatous lesions. Anal Chem. 83 (6), 2112-2118 (2011).
- Roscioli, K. M., et al. Desorption electrospray ionization (DESI) with atmospheric pressure ion mobility spectrometry for drug detection. Analyst. 139 (7), 1740-1750 (2014).
- Prideaux, B., et al. Mass spectrometry imaging of levofloxacin distribution in TB-infected pulmonary lesions by MALDI-MSI and continuous liquid microjunction surface sampling. Int J Mass Spectrom. 377, 699-708 (2015).
- Griffiths, R. L., Randall, E. C., Race, A. M., Bunch, J., Cooper, H. J. Raster-mode continuous-flow liquid microjunction mass spectrometry imaging of proteins in thin tissue sections. Anal Chem. 89 (11), 5683-5687 (2017).
- Prideaux, B., Stoeckli, M. Mass spectrometry imaging for drug distribution studies. J Proteomics. 75 (16), 4999-5013 (2012).
- Dilillo, M., et al. Mass spectrometry imaging, laser capture microdissection, and LC-MS/MS of the same tissue section. J Proteome Res. 16 (8), 2993-3001 (2017).
- Xu, B. J. Combining laser capture microdissection and proteomics: methodologies and clinical applications. Proteomics Clin Appl. 4 (2), 116-123 (2010).
- Cahill, J. F., Kertesz, V., Van Berkel, G. J. Laser dissection sampling modes for direct mass spectral analysis. Rapid Commun Mass Spectrom. 30 (5), 611-619 (2016).
- Subbian, S., et al. Chronic pulmonary cavitary tuberculosis in rabbits: A failed host immune response. Open Biol. 1 (4), 110016 (2011).
- Zimmerman, M., et al. Ethambutol partitioning in tuberculous pulmonary lesions explains its clinical efficacy. Antimicrob Agents Chemother. 61 (9), (2017).
- Kempker, R. R., et al. Cavitary penetration of levofloxacin among patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 59 (6), 3149-3155 (2015).
- Zhao, Y., et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother. , (2017).
- Pascal, J., et al. Mechanistic modeling identifies drug-uptake history as predictor of tumor drug resistance and nano-carrier-mediated response. ACS Nano. 7 (12), 11174-11182 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved