A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Extending the Lifespan of Soluble Lead Flow Batteries with a Sodium Acetate Additive
In This Article
Summary
A protocol for the construction of a soluble lead flow battery with an extended lifespan, in which sodium acetate is supplied in the methanesulfonic electrolyte as an additive, is presented.
Abstract
In this report, we present a method for the construction of a soluble lead flow battery (SLFB) with an extended cycle life. By supplying an adequate amount of sodium acetate (NaOAc) to the electrolyte, a cycle life extension of over 50% is demonstrated for SLFBs via long-term galvanostatic charge/discharge experiments. A higher quality of the PbO2 electrodeposit at the positive electrode is quantitatively validated for NaOAc-added electrolyte by throwing index (TI) measurements. Images acquired by scanning electron microscopy (SEM) also exhibit more integrated PbO2 surface morphology when the SLFB is operated with the NaOAc-added electrolyte. This work indicates that electrolyte modification can be a plausible route to economically enable SLFBs for large-scale energy storage.
Introduction
Renewable energy sources including solar and wind have been developed for decades, but their intermittent nature poses great challenges. For a future power grid with renewable energy sources incorporated, grid stabilization and load leveling are critical and can be achieved by integrating energy storage. Redox flow batteries (RFBs) are one of the promising options for grid-scale energy storage. Traditional RFBs contain ion-selective membranes separating anolyte and catholyte; for example, the all-vanadium RFB has shown to operate with high efficiency and a long cycle life1,2. However, their market share as energy storage is very limited in part due to the expensive comprising materials and ineffective ion-selective membranes. On the other hand, a single-flow soluble lead flow battery (SLFB) is presented by Plectcher et al.1,2,3,4,5. The SLFB is membrane-less because it has only one active species, Pb(II) ions. Pb(II) ions are electroplated at the positive electrode as PbO2 and the negative electrode as Pb simultaneously during charging, and convert back to Pb(II) during discharging. A SLFB thus needs one circulation pump and one electrolyte storage tank only, which in turn can potentially lead to reduced capital and operational cost compared to conventional RFBs. The published cycle life of SLFBs, however, is so far limited to less than 200 cycles under normal flow conditions6,7,8,9,10.
Factors leading to a short SLFB cycle life is preliminarily associated with deposition/dissolution of PbO2 at the positive electrode. During charge/discharge processes, the electrolyte acidity is found to increase over deep or repeated cycles11, and protons are suggested to induce the generation of a passivation layer of non-stoichiometric PbOx12,13. The shedding of PbO2 is another phenomenon related to SLFB degradation. Shed PbO2 particles are irreversible and can no longer be utilized. The coulombic efficiency (CE) of SLFBs consequentially declines because of imbalanced electrochemical reactions as well as accumulated electrodeposits at both electrodes. To extend cycle life of SLFBs, stabilizing the pH fluctuation and electrodeposit structure are critical. A recent paper demonstrates an enhanced performance and extended cycle life of SLFBs with addition of sodium acetate (NaOAc) in methanesulfonic electrolyte11.
Here, a detailed protocol for employing NaOAc as an additive to the methanesulfonic electrolyte in SLFBs is described. The SLFB performance is shown to be enhanced and the lifespan can be extended by over 50% in comparison to SLFBs without NaOAc additives. In addition, procedures for throwing index (TI) measurement are illustrated for the purpose of quantitative comparison of additive effects on electrodeposition. Finally, a scanning electron microscopy (SEM) sample preparation method for electrodeposit on SLFB electrodes is described and the additive impact on electrodeposit is manifested in acquired images.
Protocol
1. Construction of a SLFB Beaker Cell with a Sodium Acetate Additive
NOTE: This section describes the procedure to construct a SLFB beaker cell with an additive for long-term cycling experiment. The protocol includes the electrolyte preparation with and without additive, electrode pretreatment, cell assembly, and efficiency calculations.
- Preparation of lead methanesulfonate (1 L, 1 M as an example)
- In the fume hood, add 274.6 g of methanesulfonic acid (MSA, 70%) to a beaker stirring with a stir bar. Dissolve the MSA with 300 mL of deionized (DI) water.
- Prepare 223.2 g of lead (II) oxide (98%) and add in increments to the aforementioned beaker until the prepared lead oxide is completely dissolved.
- Filter through the Büchner funnel with 70 mm cellulose filter paper to separate any undissolved lead oxide.
- Repeat this procedure for 3 times. Add DI water to reach 1 L in total volume.
- Preparation of electrolyte without additive (300 mL)
- Add 20.595 g of MSA (70%) to a beaker. Add 150 mL of prepared 1 M lead methanesulfonate to the same beaker.
- Add DI water to reach 300 mL in total volume and stir the electrolyte until uniformly mixed, which results in a solution of 0.5 M lead methanesulfonate mixed with 0.5 M MSA.
- Preparation of electrolyte with sodium acetate (300 mL)
- Add 20.595 g of MSA (70%) to a beaker. Add 150 mL of prepared 1 M lead methanesulfonate to the same beaker.
- Add 1.23 g of NaOAc (98%) to the beaker as an additive agent.
- Add DI water to reach 300 mL in total volume and stir the electrolyte until uniformly mixed, which results in a solution of 0.5 M lead methanesulfonate, 0.5 M methanesulfonic acid, and 50 mM sodium acetate.
- Pretreatment of the positive and negative electrodes
- Repeatedly polish the positive (commercial carbon composite) and negative (nickel) electrodes with a sandpaper (aluminum oxide, P100) until no visible impurities are left and then rinse electrodes with DI water.
- Add 20.83 g of hydrogen chloride (35%) in 200 mL DI water and stir the solution until all of the hydrogen chloride is dissolved.
- Immerse the entire positive electrode in the prepared 1 M hydrogen chloride solution overnight to remove impurities at the electrode surface.
- Rinse the positive electrode thoroughly with DI water and dry the electrode with delicate task wipers. Tape one side of each electrode using polytetrafluoroethylene (PTFE) tape while exposing the other side of the electrodes.
- Prepare another solution with 3.03 g of potassium nitrate (99%) and 300 mL DI water, which results in a solution of 0.1 M potassium nitrate.
- Immerse the positive and negative electrodes in 0.1 M potassium nitrate with the exposed surface facing each electrode.
- Apply a potential of 1.80 V vs. Ag/AgCl to the positive electrode for 5 min. Subsequently, apply a potential of -1.0 V vs. Ag/AgCl to the positive electrode for 2 min.
- Assemble the SLFB beaker cell
- Attach the pretreated positive and negative electrodes to a home-made electrode positioning board for a fixed electrode distance. Place the positioning board together with electrodes in a beaker as schematically illustrated in Figure 1 and add electrolyte to the beaker until the designated level of immersion.
- Place a magnetic stirrer into the beaker, position the beaker on a hot plate and control the rotating rate of the stirrer. Connect the battery tester to the electrodes and cover the beaker cell with plastic wrap to prevent evaporation.
- Calculate the battery efficiency
- After galvanostatic charge and discharge, calculate the efficiency of the battery as the following:
Coulombic efficiency: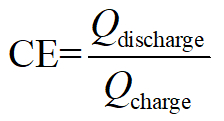
Voltage efficiency: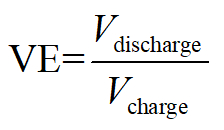
Energy efficiency:
Here, Q denotes coulombs of charged/discharged equivalent electrons, V the apply/output voltage, and E the total energy stored/consumed.
- After galvanostatic charge and discharge, calculate the efficiency of the battery as the following:
2. Throwing Index Measurement
NOTE: This section describes the procedure to measure throwing index (TI) of the electrodeposit at positive electrodes in SLFB cells. Reversing the role of positive and negative electrodes delivers the other set of TI results. Here, TI is investigated by using a home-made Haring-Blum cell as schematically depicted in Figure 2.
- Measurement
- Weigh and record two positive electrodes respectively before the experiments.
- Place the negative electrode at the center of a Haring-Blum cell and one positive electrode at a distance ratio of 1 from the negative electrode. Place the second positive electrode at another distance ratio from the negative electrode (take 6 as an example in Figure 2).
- Immerse the two positive electrodes and one negative electrode with the same immersed surface area (2 cm2 here) in the Haring-Blum cell with the electrolyte of interest.
- Apply a controlled current density (20 mA·cm-2 here) at the electrodes by using a battery tester. Carry out the galvanostatic charge for a certain duration (30 min here).
- After plating, rinse the two positive electrodes with DI water and dry them at room temperature overnight.
- Weigh and record two positive electrodes again respectively and calculate the metal distribution ratio (MDR) according to the equation listed below.
- Repeat the aforementioned experiments by placing the second positive electrode at various linear distance ratios (LR) to acquire the TI diagram (varied from 6 to 1 here).
- Calculation
- As an example, consider the anode as the electrode of interest, and determine each data on the TI diagram by the measured MDR versus LR, which are calculated as the following:
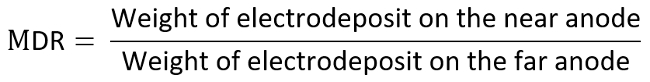
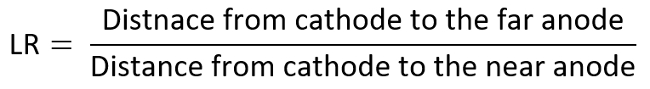
- As an example, consider the anode as the electrode of interest, and determine each data on the TI diagram by the measured MDR versus LR, which are calculated as the following:
3. SEM Sample Preparation
- Rinse the graphite electrode with DI water and dry at room temperature after electroplating.
- Slice graphite electrodes into the desired sample size by diamond saw with care. Cold mount the electrode sample and then mechanically polish it with 14, 8, and 3 μm silicon carbide sand papers, subsequently.
- Further polish the samples with 1 μm diamond suspension and 0.05 μm Al2O3. Deposit the cold-mounted sample with platinum and attach it with copper tapes to ensure conductivity for SEM observation.
Results
To extend cycle life of SLFBs, NaOAc is supplied as an electrolyte additive. Cycling performance of SLFBs with and without NaOAc additive are examined in parallel, and results are shown in Figure 3. For easier quantitative comparison of cycle life, we define the "death" of a SLFB as when its CE is lower than 80% under continuous galvanostatic charge/discharge. Figure 3a and 3b show that approximately 50% ...
Discussion
This paper describes an economical method to extend the cycle life of SLFBs: by employing NaOAc agent as an electrolyte additive. A batch of fresh graphite electrodes and nickel plates are preprocessed as aforementioned in Step 1 before long-term cycling experiments. Because inconsistency among commercial carbon electrodes may cause performance deviation of the SLFBs, the physical/chemical pretreatment in Step 1.4 is critical to remove surface residues. The second part of Step 1.4 is employing electrochemical methods to ...
Disclosures
We have nothing to disclose.
Acknowledgements
This work was supported by the Ministry of Science and Technology, R.O.C., under the funding number of NSC 102-2221-E-002-146-, MOST 103-2221-E-002-233-, and MOST 104-2628-E-002-016-MY3.
Materials
| Name | Company | Catalog Number | Comments |
| 70 mm cellulose filter paper | Advance | ||
| Autolab | Metrohm | PGSTA302N | |
| BT-Lab | BioLogic | BCS-810 | |
| commercial carbon composite electrode | Homy Tech,Taiwan | Density 1.75 g cm-3, and electrical conductivity 330 S cm-1 | |
| Diamond saw | Buehler | ||
| Hydrochloric Acid | SHOWA | 0812-0150-000-69SW | 35% |
| Lead (II) Oxide | SHOWA | 1209-0250-000-23SW | 98% |
| Lutropur MSA | BASF | 50707525 | 70% |
| nickel plate | Lien Hung Alloy Trading Co., LTD., Taiwan, | 99% | |
| Potassium Nitrate | Scharlab | 28703-95 | 99% |
| Scanning electron microscopy | JEOL | JSM-7800F | at accelerating voltage of 15 kV |
| Sodium Acetate | SHOWA | 1922-5250-000-23SW | 98% |
| water purification system | Barnstead MicroPure | 18.2 MΩ • cm |
References
- Soloveichik, G. L. Flow batteries: current status & trends. Chemical Reviews. 115 (20), 11533-11558 (2015).
- Ravikumar, M. K., Rathod, S., Jaiswal, N., Patil, S., Shukla, A. The renaissance in redox flow batteries. Journal of Solid State Electrochemistry. 21 (9), 2467-2488 (2017).
- Hazza, A., Pletcher, D., Wills, R. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead (II) Part I. Preliminary studies. Physical Chemistry Chemical Physics. 6 (8), 1773-1778 (2004).
- Pletcher, D., Wills, R. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead (II) Part II. Flow cell studies. Physical Chemistry Chemical Physics. 6 (8), 1779-1785 (2004).
- Pletcher, D., Wills, R. A novel flow battery-a lead acid battery based on an electrolyte with soluble lead (II). III. The influence of conditions on battery performance. Journal of Power Sources. 149, 96-102 (2005).
- Hazza, A., Pletcher, D., Wills, R. A novel flow battery-a lead acid battery based on an electrolyte with soluble lead (II). IV. The influence of additives. Journal of Power Sources. 149, 103-111 (2005).
- Pletcher, D., Zhou, H., Kear, G., Low, C. T. J., Walsh, F. C., Wills, R. G. A. A novel flow battery-A lead-acid battery based on an electrolyte with soluble lead (II). V. Studies of the lead negative electrode. Journal of Power Sources. 180 (1), 621-629 (2008).
- Pletcher, D., Zhou, H., Kear, G., Low, C. T. J., Walsh, F. C., Wills, R. G. A. A novel flow battery-A lead-acid battery based on an electrolyte with soluble lead (II). Part VI. Studies of the lead dioxide positive electrode. Journal of Power Sources. 180 (1), 630-634 (2008).
- Li, X., Pletcher, D., Walsh, F. C. A novel flow battery: a lead acid battery based on an electrolyte with soluble lead (II). Part VII. Further studies of the lead dioxide positive electrode. Electrochimica Acta. 54 (20), 4688-4695 (2009).
- Krishna, M., Fraser, E. J., Wills, R. G. A., Walsh, F. C. Developments in soluble lead flow batteries and remaining challenges: An illustrated review. Journal of Energy Storage. 15, 69-90 (2018).
- Lin, Y. -. T., Tan, H. -. L., Lee, C. -. Y., Chen, H. -. Y. Stabilizing the electrodeposit-electrolyte interphase in soluble lead flow batteries with ethanoate additive. Electrochimica Acta. 263, 60-67 (2018).
- Oury, A., Kirchev, A., Bultel, Y., Chainet, E. PbO2/Pb2+ cycling in methanesulfonic acid and mechanisms associated for soluble lead-acid flow battery applications. Electrochimica Acta. 71, 140-149 (2012).
- Oury, A., Kirchev, A., Bultel, Y. Potential response of lead dioxide/Lead (II) galvanostatic cycling in methanesulfonic acid: a morphologico-kinetics interpretation. Journal of The Electrochemical Society. 160 (1), A148-A154 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved