A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Simultaneous Electrical and Mechanical Stimulation to Enhance Cells' Cardiomyogenic Potential
In This Article
Summary
Here we present a protocol for training a cell population using electrical and mechanical stimuli emulating cardiac physiology. This electromechanical stimulation enhances the cardiomyogenic potential of the treated cells and is a promising strategy for further cell therapy, disease modeling, and drug screening.
Abstract
Cardiovascular diseases are the leading cause of death in developed countries. Consequently, the demand for effective cardiac cell therapies has motivated researchers in the stem cell and bioengineering fields to develop in vitro high-fidelity human myocardium for both basic research and clinical applications. However, the immature phenotype of cardiac cells is a limitation on obtaining tissues that functionally mimic the adult myocardium, which is mainly characterized by mechanical and electrical signals. Thus, the purpose of this protocol is to prepare and mature the target cell population through electromechanical stimulation, recapitulating physiological parameters. Cardiac tissue engineering is evolving toward more biological approaches, and strategies based on biophysical stimuli, thus, are gaining momentum. The device developed for this purpose is unique and allows individual or simultaneous electrical and mechanical stimulation, carefully characterized and validated. In addition, although the methodology has been optimized for this stimulator and a specific cell population, it can easily be adapted to other devices and cell lines. The results here offer evidence of the increased cardiac commitment of the cell population after electromechanical stimulation. Electromechanically stimulated cells show an increased expression of main cardiac markers, including early, structural, and calcium-regulating genes. This cell conditioning could be useful for further regenerative cell therapy, disease modeling, and high-throughput drug screening.
Introduction
Heart function is based on the coupling of electrical excitation and mechanical contraction. Briefly, cardiomyocyte intercellular junctions permit electrical signal propagation to produce almost synchronous contractions of the heart that pump blood systemically and through the pulmonary system. Cardiac cells, thus, undergo both electrical and mechanical forces that regulate gene expression and cellular function. Accordingly, many groups have attempted to develop culture platforms that mimic the cardiac physiological environment to understand the role of mechanical and electrical stimulation on cardiac development, function, and maturation. In vitro electrical and mechanical stimulations individually have been applied extensively in cardiac tissue engineering to enhance functional properties, increase cell maturation, or improve cell-cell coupling and calcium handling1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21. Nevertheless, synchronous electromechanical conditioning remains unexploited because of the challenge of developing a stimulator and protocol, and because of the mandatory optimization22.
Preliminary work addressed electromechanical stimulation as a combination of electrical stimulation and media perfusion; however, the flow does not involve the strain-based deformation typical of ventricular filling23,24,25. Later, more physiological approaches combined electrical stimuli with physical deformation or stretch to mimic the isovolumetric contraction26,27,28,29,30,31. Feng et al. described the first demonstration of electromechanical stimulation in 2005, reporting enhanced cardiomyocyte size and contractile properties26. Wang et al. pretreated mesenchymal stem cells with 5-azacytidine and applied simultaneous electrical and mechanical conditioning, improving recellularization, cell viability, cardiac differentiation, and tissue remodeling27. Since those publications, more groups have reported on electromechanical stimulation of cell monolayers or engineered tissues (e.g., Black28, Vunjak-Novakovic29,31, and our group30) with the first conditioned cells tested in vivo30. Briefly, Morgan and Black tested several combinations of electrical and mechanical stimuli, reporting that the timing between stimulations was crucial because delayed combined electromechanical stimulation yielded the best results28. Next, Godier-Furnémont and collaborators optimized an electromechanical stimulation protocol for engineered heart muscle constructs from neonatal rat heart cells and achieved, for the first time, a positive force-frequency relationship29. Afterward, our group reported that electromechanically preconditioned cells increased the expression of main cardiac markers in vitro and broad beneficial effects in vivo, such as improved cardiac function or increased vessel density in the infarct border region30. The most recent publication demonstrated that cardiac tissues from stem-cell-derived cardiomyocytes subjected to electromechanical conditioning reached a maturation level closer to human adult cardiac structure and function31. Additionally, alternative three-dimensional stimulation platforms comprise electroactive scaffolds that provide electrical, mechanical, and topographical cues to the cells attached32. Moreover, mechanical deformation (cell monolayer stretching and compression) can also be induced with stretchable electrodes mimicking normal physiological conditions, as well as extreme conditions33.
Therefore, the rationale is that in vitro electromechanical stimuli based on physiological conditions could enhance the cardiomyogenic potential of a cell. Indeed, this stimulation could benefit further integrations of therapeutic cells into the myocardium in a clinical scenario or increase tissue maturation for drug-screening applications.
In addition, we isolated and characterized a population of human adipose tissue-derived progenitor cells of cardiac origin (cardiac ATDPCs)34. These cells are located in the epicardial fat. These cells display beneficial histopathological and functional effects in the treatment of myocardial infarction and also maintain cardiac and endothelial differentiation potential.30,35. We hypothesized that these benefits would increase after biophysical stimulation.
Consequently, we developed a device and a stimulation regime for the cell population of interest and investigated the effects. This electromechanical protocol is a new strategy to induce active cell stretching in a sterile manner and noninvasively compared to previous publications36, in combination with electric field stimulation. The technique reported here explains in detail the device and method used for the electrical, mechanical, and electromechanical stimulation of cells.
This device can provide both electrical and mechanical stimulation, independently or simultaneously. The stimulation is performed with a noninvasive and aseptic novel approach that includes presterilized cell support, electrodes placed inside a standard culture plate, and a platform that induces the mechanical and electrical forces (Figure 1).
The platform can hold up to six culture plates and consists of a sandwich structure of laser-cut poly(methyl methacrylate) and printed circuit-board pieces. The platform prototype relies on a combination of a monophasic programmable computer-controlled electrical stimulator, a printed circuit board for the robust connection of the electrodes, and six 10 mm x 10 mm x 5 mm nickel-plated neodymium-fixed magnets placed near one side of the culture plates. There is also an aluminum bar with six driving magnets (same model) placed in front of the other side of the culture plates and moved with a linear servomotor. The motor is driven by a motor controller, operated through an RS-232 port by commercial software (see the Table of Materials). Through the user interface and programmable stimulator, it is possible to program the electrical intensity, the pulse duration and frequency, the frequency of mechanical stimulation, its duty cycle, the number of pulses, the pulse amplitude (magnet excursion), and the slope.
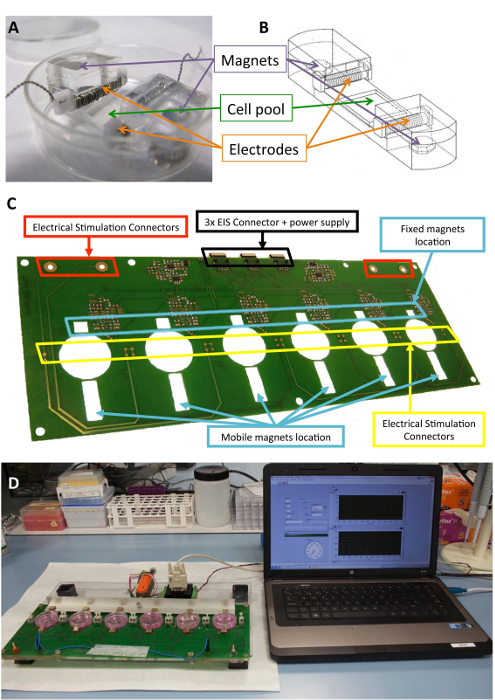
Figure 1: Electromechanical stimulator. (A) PDMS construct used for the cell conditioning. (B) Drawing of the PDMS construct, including electrodes and magnets. (C) Detail of the printed circuit board (platform) used to perform the electromechanical conditioning. This panel has been modified from Llucià-Valldeperas et al.30. (D) Picture of the electromechanical stimulation platform and user interface (computer). Please click here to view a larger version of this figure.
Both the stimulator and the method for electromechanical conditioning are fully described in two international patents, WO-2013185818-A137 and WO-2017125159-A138.
The biocompatible silicone constructs designed to provide structural support to cells, electrodes, and magnets have been described previously10,21. Briefly, they consist of polydimethylsiloxane (PDMS), molded and cured at room temperature, with a Young's modulus of 1.3 MPa, close to physiological levels. The construct contains a cell culture pool in a flexible area (10 mm x 10 mm x 2 mm), two inner transverse slots to hold the electrodes, and two embedded 6 mm x 2 mm x 4 mm nickel-plated neodymium magnets. The electrodes are built with 0.2 mm platinum wire twisted around a 2 mm x 3 mm x 12 mm polytetrafluoroethylene (PTFE) core bar (21 cm per electrode, approximately 23 turns) and placed at opposite sides of the flexible area to create an electric field for inducing electrical stimulation. Mechanical stretching is achieved through magnetic attraction between magnets embedded in the support and external magnets placed next to the culture plate and on the moving aluminum arm. In this way, the cell support can be extended without breaking the sterile barrier. This approach is suitable for a cell monolayer but could be adapted to three-dimensional constructs, as well.
In addition, a regular pattern could be imprinted where the cells are seeded, using a ruled diffraction grating (1,250 grooves/mm). The direct visualization of cells cultured on the PDMS construct under brightfield and fluorescent microscopes is possible because of its transparency and 0.5 mm thickness. In the current case, the PDMS culture pool has a vertical surface pattern, perpendicular to the stretching force, to align the cells perpendicularly to the electric field, which minimizes the electric field gradient across the cell.
Figure 1 shows a detailed description of the construct and device used for the stimulation. The PDMS construct and characteristics are optimized for cell stretching (Figure 1A,B). The stimulator is developed and validated for the effective application of the desired electrical and mechanical stimulation to cells attached to the PDMS construct. This process includes ensuring good connectivity and user operability through the software interface (Figure 1C,D).
The procedure for cell stimulation using this custom-made device is described in the protocol section.
Protocol
This study uses human cardiac ATDPCs from patient samples. Their use has been approved by the local ethics committee, and all patients gave informed consent. The study protocol conforms to the principles outlined in the Declaration of Helsinki.
1. Preparations
- Autoclave two tweezers, 12 platinum PTFE electrodes for electrical stimulation, and some paper towels, at 121 °C for 20 min.
- Sterilize 12 PDMS custom-made constructs (Figure 1A).
- Wash each construct with 5 mL of sterile, distilled water with magnetic agitation at room temperature for 15 min.
- Wash 1x with 5 mL of 70% ethanol with magnetic agitation at room temperature for 5 min.
- Wash 5x with 5 mL of sterile, distilled water with magnetic agitation at room temperature for 10 min per wash, to remove alcoholic residues.
- Dry the constructs on sterile paper towels inside the flow cabinet overnight.
- Store them in sterile 50 mL centrifuge tubes until use.
2. Cell Seeding (Day -1)
- Before cell seeding, transfer the cleaned PDMS constructs to sterile plates and expose them to ultraviolet light for 5 min to ensure complete sterilization.
- Transfer each construct to a 35 mm cell culture plate, or 6-well plate, for immediate cell seeding.
- Trypsinize a confluent T75 flask of cardiac ATDPCs.
- Wash the T75 flask with 5 mL of 1x phosphate-buffered saline (PBS).
- Add 1 mL of 0.05% trypsin-EDTA and incubate at 37 °C for 5 min to detach the cells.
- Add 5 mL of complete medium to inactivate the trypsin-EDTA.
- Collect all cells in a 15 mL tube and wash the flask 2x with 5 mL of PBS to collect any remaining cells.
- Centrifuge at 230 x g for 5 min at 22 °C, remove the supernatant, and resuspend the cells in 2 mL of complete medium to count them with the hemocytometer chamber.
- Seed 200 µL of cardiac ATDPCs (2.5 x 105 cells/mL) into the cell pool of the 12 PDMS constructs (Figure 1A,B) to have ~80% of the seeding surface covered by cells the day after, and incubate at 37 °C and 5% CO2.
NOTE: After 2–4 h, the cells should be attached. Cell inoculum is prepared according to the cell size and growth. For smaller cells, the seeding density should be increased. - Gently add 2 mL of prewarmed complete medium (α-MEM supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin-streptomycin) per plate.
- Incubate the constructs at culture conditions (usually 37 °C and 5% CO2) overnight.
3. Electromechanical Stimulation Setup (Day 0)
- Before starting the procedure, take six constructs for electromechanical stimulation and six as nonstimulated controls. With fewer than six plates per stimulation, use empty constructs with the same medium volume to ensure proper electric field stimulation.
- Clean the stimulation unit with 70% ethanol and place it in the flow cabinet.
- Bring the sterile electrodes and tweezers inside the flow cabinet.
- Remove 90% of the media from the culture plate to easily manipulate the electrodes and constructs. First, place the PDMS constructs in the right position to ensure magnetic attraction (PDMS displacement within the culture plate toward the magnet) between both fixed and mobile magnets. Next, connect the platinum wire to the electrode connectors and the PTFE part in its designated space in the PDMS construct.
- Add 2.5 mL of fresh prewarmed complete medium to each construct.
NOTE: Maintain the sterility throughout the procedure and operate on one construct at a time. Maintain the rest of the constructs in the incubator at 37 °C and 5% CO2 until use. - Once all PDMS constructs are placed and electrically connected to the platform, bring the platform back into the incubator at 37 °C and 5% CO2.
- Connect the electrical and mechanical source.
- Configure the stimulation program. Specify electrical and mechanical stimulation regimes through the user interfaces of the electrical stimulator and the application, which controls the mechanical stimulation. Set the synchronism as follows.
- Switch on the electrical stimulator. Wait for the main menu to appear in the display.
- Then, select Option 2: Edit sequence + Enter.
- Edit the sequence Menu as follows.
- Use the Mode tab to select either voltage or current. Select current by clicking + and press Enter.
- For the Amplitude tab, select 1 (mA) with +/- and press Enter.
- For the Period (T), select 1000 (ms) with +/- and press Enter.
- Set the Pulse duration (Tw) to 2 (ms) with +/- and press Enter.
- For the Trigger mode tab, select External by software and press Enter.
- Back in the main menu, select Option 4: Generate sequence and press Enter.
NOTE: The electrical stimulator rests in the standby until it receives a trigger command from the mechanical stimulator application through the serial port.
- Perform the following steps in the mechanical stimulation section of the control application panel (Figure 2C).
- Write 1,000 (ms) in the Pulse Period text control.
- Write 500 (ms) in the ON time (Tw) text control to set the mechanical pulse duration.
- Write 2,000 (AU) in the Excursion text control to deliver a 10% construct elongation. This is the number of steps in the linear control motor.
NOTE: The stimulation protocol applied here consists of alternating-current 2 ms monophasic square-wave pulses of 50 mV/cm at 1 Hz and 10% stretching for 7 days. The rise and fall times of the mechanical pulse are set at 100 ms, to roughly imitate the shape of the hearth pressure pulse. Also, the repetition mode is set to Continuous and there is a counter displaying the number of pulses.
- Switch on the electrical stimulator. Wait for the main menu to appear in the display.
- Change the media 2x a week (Monday and Thursday afternoon). First, remove the old media; next, add the warm media on the sides of the PDMS support, never directly on the cell pool.
NOTE: If the cells have a high growth rate, the media should be changed 3x a week (e.g., Monday, Wednesday, and Friday). It is necessary to disconnect and reconnect all cables, but there is no need to remove the culture plates and electrodes from their place. - Collect the samples after the experimentation is performed.
4. Sample Collection at the End of the Experimentation (Day 7)
- For RNA analyses
- Wash the construct 2x with 3 mL of 1x PBS for 5 min at room temperature.
- Add 3 mL of 0.05% trypsin-EDTA to each plate (enough to cover the whole construct) and wait for 5 min at 37 °C.
- After the cells are detached, add 2 mL of complete medium to inactivate the trypsin-EDTA.
- Collect all cells in a 15 mL tube and wash the construct 2x with 3 mL of PBS to collect any remaining cells.
- Centrifuge at 230 x g for 5 min at 22 °C.
- Remove the supernatant and resuspend the pellet in 1 mL of PBS.
- Transfer the cell solution to a 1.5 mL tube and centrifuge at 230 x g for 5 min.
- Remove the supernatant and store the pellet at -80 °C in 700 µL of lysis reagent for further RNA isolation.
- Isolate RNA using a commercial kit, following the manufacturer's instructions.
- Reverse-transcribe the isolated RNA using the kit and random hexamers, according to the manufacturer's protocol.
- For small RNA concentrations, preamplify and, then, dilute 1:5 with RNase-free water before subsequent real-time reverse transcription polymerase chain reaction (RT-PCR) is performed. Continue with the standard protocol for real-time RT-PCR and check main cardiac markers.
NOTE: Typical cardiac markers comprise early and late markers from different categories, such as cardiac transcription factors (myocyte-specific enhancer factor 2A [MEF2A], GATA-binding protein 4 [GATA-4]) and structural (cardiac troponin I [cTnI], cardiac troponin T [cTnT], α-actinin) and calcium regulation (Connexin43 [Cx43], sarco-/endoplasmic reticulum Ca2+-ATPase [SERCA2])30. Protein isolation can also be performed if needed. Simultaneous RNA and protein isolation can be performed with the same sample, using commercially available reagents and kits (Table of Materials) if the sample quantity is less.
- For immunostainings
NOTE: This is performed directly on the cells attached to the cell pool of the PDMS construct. Therefore, we recommend placing, every time, 1 cm x 1 cm of paraffin film on the top of the cell pool, apart from the plate lid, to minimize the evaporation of the incubation solutions.- Wash the construct 2x with 3 mL of PBS for 5 min at room temperature.
- Fix the cells attached to the construct with 2 mL of 10% formalin for 15 min at room temperature.
- Wash the cells 3x with 3 mL of PBS for 5 min at room temperature. For long-term storage, leave the samples in PBS with 0.1% sodium azide at 4 °C.
- Permeabilize the cells with 3 mL of PBS + 0.5% detergent (3x, each time 5–10 min, at room temperature).
- Incubate the cells with 100 µL of PBS + 10% horse serum + 0.2% detergent + 1% bovine serum albumin at room temperature for 1 h, to block nonspecific antibody binding.
- Incubate the cells with 100 µL of PBS + 10% horse serum + 0.2% detergent + 1% bovine serum albumin + primary antibody at room temperature for 1 h. For example, primary antibodies against Cx43 (1:100), sarcomeric α-actinin (1:100), GATA-4 (1:50), MEF2 (1:25), and SERCA2 (1:50).
- Wash 3x with 3 mL of PBS at room temperature for 5 min.
- Incubate the cells with 100 µL of PBS + secondary antibody at room temperature in the dark for 1 h.
NOTE: Secondary antibodies conjugated with different fluorophores and a counterstaining agent were used. - Wash them 3x with 3 mL of PBS at room temperature in the dark for 5 min.
- Incubate the cells with 100 µL of nuclear staining (0.1 µg/mL) in PBS at room temperature in the dark for 15 min.
- Wash them 3x with 3 mL of PBS at room temperature in the dark for 5 min.
- Store the samples in 3 mL of PBS with 0.1% sodium azide at 4 °C until the acquisition.
NOTE: Microscope acquisition is possible on inverted fluorescent and confocal microscopes with long-working distance objectives because the construct thickness is about 0.5 mm.
Results
Figure 2 represents the general schema followed for the cell stimulation. Briefly, cells were seeded on the PDMS construct and subjected to electromechanical stimulation, with a media change performed twice a week. Nonstimulated cells were used as a control for the electromechanical conditioning. Additionally, we added an extra control to the experiment, and subcutaneous ATDPCs were used as a control for cardiac ATDPCs. Subcutaneous ATDPCs are obtained from s...
Discussion
Electromechanical stimulation appears to be a safe alternative for preparing cells for a hostile cardiac environment and enhancing their cardiac commitment. Here, a protocol described for cardiac progenitor cells increased the expression of main cardiac markers and was reported to be beneficial for their next implantation on infarcted murine myocardium30. In general, electromechanically stimulated cardiac ATDPCs increased the expression of genes related to early, structural, and calcium regulation...
Disclosures
The authors have nothing to disclose, except that the stimulation device and protocol were previously patented (WO-2013185818-A1, WO-2017125159-A1).
Acknowledgements
The authors want to thank the members of the ICREC Research Program (IGTP, Badalona) and the Electronic and Biomedical Instrumentation Group (UPC, Barcelona), especially Prof. J. Rosell-Ferrer. In addition, the authors acknowledge STEM CELLS Translational Medicine journal and AlphaMed Press for permitting the adaptation of previously published figures (Llucià-Valldeperas, et al.30). The development of this prototype and the design of the protocol were supported by Ministerio de Educación y Ciencia (SAF 2008-05144), Ministerio de Economía y Competitividad (SAF 2014-59892), the European Commission 7th Framework Programme (RECATABI, NMP3-SL-2009-229239), Fundació La Marató de TV3 (080330, 201516, 201502), and Fundación para la Innovación y la Prospectiva en Salud en España (FIPSE; 06-00001396-15).
Materials
| Name | Company | Catalog Number | Comments |
| Stimulator | |||
| nickel plated neodymium magnets | Supermagnete | Q-10-10-05-N | |
| nickel-plated neodymium magnets | Supermagnete | Q-06-04-02-HN | |
| polydimethylsiloxane (PDMS) SYLGAR 184 Silicone Elastomer Kit | Dow Corning Corp | 184 | |
| ruled diffraction grating (1250 grooves/mm) | Newport | 05RG150-1250-2 | |
| Motor controller | Faulhaber | MCLM-3006-S | |
| Labview | National Instruments | ||
| Cell culture | |||
| phosphate-buffered saline (PBS) | Gibco | 70013-065 | |
| 0.05% trypsin-EDTA | Gibco | 25300-120 | |
| 35 mm cell culture dish | BD Falcon | 45353001 | |
| fetal bovine serum (FBS) | Gibco | 10270-106 | |
| L-Glutamine 200 mM, 100x | Gibco | 25030-024 | |
| Penicilina/Streptomicine, 10.000 U/mL | Gibco | 15140-122 | |
| Minimum essential medium eagle (alfa-MEM) | Sigma | M4526-24x500ML | |
| Protein & RNA analyses | |||
| protease inhibitor cocktail | Sigma | P8340 | |
| QIAzol Lysis Reagent | Qiagen | 79306 | |
| AllPrep RNA/Protein Kit | Qiagen | 50980404 | |
| Rneasy mini kit | Qiagen | 74104 | |
| iTaq Universal Probes One-Step Kit | Bio-Rad Laboratories | 172-5140 | |
| Random hexamers | Qiagen | 79236 | |
| TaqMan PreAmp MasterMix 2x | Applied Biosystems | 4391128 | |
| TaqMan Universal PCR MasterMix | Applied Biosystems | 4324018 | |
| Immunostaining | |||
| 10% formalin | Sigma | HT-501128-4L | |
| horse serum | Sigma | H1138 | |
| Triton X-100 | Sigma | X100-500ML | |
| Bovine Serum Albumina (BSA) | Sigma | A7906-100G | |
| PARAFILM | Sigma | P6543 | |
| 4’,6-diamidino-2-phenylindole (DAPI) | Sigma | D9542 | |
| Phalloidin Alexa 568 | Invitrogen | A12380 | |
| sodium azide | Sigma | S8032-100g | |
| Hoechst 33342 | Sigma | 14533 | |
| Connexin-43 rabbit primary antibody | Sigma | C6219 lot#061M4823 | |
| sarcomeric α-actinin mouse primary antibody | Sigma | A7811 lot#080M4864 | |
| GATA-4 goat primary antibody | R&D | AF2606 VAZ0515101 | |
| MEF2 rabbit primary antibody | Santa Cruz | sc-313 lot#E0611 | |
| SERCA2 goat primary antibody | Santa Cruz | sc-8095 lot#D2709 | |
| Cy3 secondary antibody | Jackson ImmunoResearch | 711-165-152 | |
| Cy3 secondary antibody | Jackson ImmunoResearch | 715-165-151 | |
| Cy3 secondary antibody | Jackson ImmunoResearch | 712-165-150 | |
| Cy2 secondary antibody | Jackson ImmunoResearch | 715-225-150 | |
| Cy2 secondary antibody | Jackson ImmunoResearch | 711-225-152 | |
| Cy2 secondary antibody | Jackson ImmunoResearch | 705-225-147 |
References
- McDonough, P. M., Glembotski, C. C. Induction of atrial natriuretic factor and myosin light chain-2 gene expression in cultured ventricular myocytes by electrical stimulation of contraction. Journal of Biological Chemistry. 267, 11665-11668 (1992).
- Tandon, N., et al. Electrical stimulation systems for cardiac tissue engineering. Nature Protocols. 4, 155-173 (2009).
- Serena, E., et al. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Experimental Cell Research. 315, 3611-3619 (2009).
- Tandon, N., et al. Optimization of electrical stimulation parameters for cardiac tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 5, 115-125 (2011).
- Zhang, X., Wang, Q., Gablaski, B., Lucchesi, P., Zhao, Y. A microdevice for studying intercellular electromechanical transduction in adult cardiac myocytes. Lab on a Chip. 13, 3090-3097 (2013).
- Chan, Y. C., et al. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. Journal of Cardiovascular Translational Research. 6, 989-999 (2013).
- Pietronave, S., et al. Monophasic and biphasic electrical stimulation induces a precardiac differentiation in progenitor cells isolated from human heart. Stem Cells and Development. 23, 888-898 (2014).
- Pavesi, A., et al. Electrical conditioning of adipose-derived stem cells in a multi-chamber culture platform. Biotechnology and Bioengineering. 111, 1452-1463 (2014).
- Baumgartner, S., et al. Electrophysiological and morphological maturation of murine fetal cardiomyocytes during electrical stimulation in vitro. Journal of Cardiovascular Pharmacology and Therapeutics. 20, 104-112 (2015).
- Llucià-Valldeperas, A., et al. Electrical stimulation of cardiac adipose tissue-derived progenitor cells modulates cell phenotype and genetic machinery. Journal of Tissue Engineering and Regenerative Medicine. 9 (11), 76-83 (2015).
- Llucià-Valldeperas, A., et al. Physiological conditioning by electric field stimulation promotes cardiomyogenic gene expression in human cardiomyocyte progenitor cells. Stem Cell Research and Therapy. 5, 93 (2014).
- Radisic, M., et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 101 (52), 18129-18134 (2004).
- Fink, C., et al. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB Journal. 14, 669-679 (2000).
- Zimmermann, W. H., et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Medicine. 12 (4), 452-458 (2006).
- Birla, R. K., Huang, Y. C., Dennis, R. G. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Engineering. 13, 2239-2248 (2007).
- Salameh, A., et al. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circulation Research. 106, 1592-1602 (2010).
- Galie, P. A., Stegemann, J. P. Simultaneous application of interstitial flow and cyclic mechanical strain to a three-dimensional cell-seeded hydrogel. Tissue Engineering Part C: Methods. 17 (5), 527-536 (2011).
- Leychenko, A., Konorev, E., Jijiwa, M., Matter, M. L. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One. 6, 29055 (2011).
- Tulloch, N. L., et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation Research. 109, 47-59 (2011).
- Mihic, A., et al. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 35, 2798-2808 (2014).
- Llucià-Valldeperas, A., et al. Unravelling the effects of mechanical physiological conditioning on cardiac adipose tissue-derived progenitor cells in vitro and in silico. Scientific Reports. 8, 499 (2018).
- Stoppel, W. L., Kaplan, D. L., Black, L. D. Electrical and mechanical stimulation of cardiac cells and tissue constructs. Advanced Drug Delivery Reviews. 96, 135-155 (2016).
- Nunes, S. S., et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nature Methods. 10, 781-787 (2013).
- Barash, Y., et al. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Engineering Part C: Methods. 16, 1417-1426 (2010).
- Maidhof, R., et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. Journal of Tissue Engineering and Regenerative Medicine. 6, 12-23 (2012).
- Feng, Z., et al. An electro-tensile bioreactor for 3-D culturing of cardiomyocytes. A bioreactor system that simulates the myocardium's electrical and mechanical response in vivo. IEEE Engineering in Medicine and Biology Magazine. 24 (4), 73-79 (2005).
- Wang, B., et al. Myocardial scaffold-based cardiac tissue engineering: application of coordinated mechanical and electrical stimulations. Langmuir. 29 (35), 11109-11117 (2013).
- Morgan, K. Y., Black, L. D. Mimicking isovolumic contraction with combined electromechanical stimulation improves the development of engineered cardiac constructs. Tissue Engineering Part A. 20 (11-12), 1654-1667 (2014).
- Godier-Furnémont, A. F., et al. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 60, 82-91 (2015).
- Llucià-Valldeperas, A., et al. Electromechanical Conditioning of Adult Progenitor Cells Improves Recovery of Cardiac Function After Myocardial Infarction. Stem Cell Translational Medicine. 6 (3), 970-981 (2017).
- Ronaldson-Bouchard, K., et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 556 (7700), 239-243 (2018).
- Gelmi, A., et al. Direct Mechanical Stimulation of Stem Cells: A Beating Electromechanically Active Scaffold for Cardiac Tissue Engineering. Advanced Healthcare Materials. 5 (12), 1471-1480 (2016).
- Poulin, A., et al. An ultra-fast mechanically active cell culture substrate. Scientific Reports. 8 (1), 9895 (2018).
- Bayes-Genis, A., et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. Journal of Molecular and Cellular Cardiology. 49 (5), 771-780 (2010).
- Bagó, J. R., et al. Bioluminescence imaging of cardiomyogenic and vascular differentiation of cardiac and subcutaneous adipose tissue-derived progenitor cells in fibrin patches in a myocardium infarct model. International Journal of Cardiology. 169, 288-295 (2013).
- Zimmermann, W. H., et al. Tissue engineering of a differentiated cardiac muscle construct. Circulation Research. 90 (2), 223-230 (2002).
- Rosell Ferrer, F. X., Sánchez Terrones, B., Bragós Bardia, R., Bayés Genís, A., Llucià Valldeperas, A. Methods and devices for mechanical and electrical stimulation of stem cell monolayer and 3d cultures for tissue engineering applications. Spanish patent. , (2013).
- Bayés Genís, A., Llucià Valldeperas, A., Soler Botija, C., Bragós Bardia, R., Rosell Ferrer, F. X. Method for Conditioning Stem Cells. Spanish patent. , (2017).
- Roura, S., Gálvez-Montón, C., Bayes-Genis, A. Myocardial healing using cardiac fat. Expert Review of Cardiovascular Therapy. 16 (4), 305-311 (2018).
- Zhang, Y. M., Hartzell, C., Narlow, M., Dudley, S. C. Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation. 106 (10), 1294-1299 (2002).
- Liu, J., Fu, J. D., Siu, C. W., Li, R. A. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 25 (12), 3038-3044 (2007).
- Wipff, P. J., et al. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials. 30 (9), 1781-1789 (2009).
- Kim, C. iPSC technology--Powerful hand for disease modeling and therapeutic screen. Biochemistry and Molecular Biology Reports. 48 (5), 256-265 (2015).
- Ronaldson-Bouchard, K., Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell. 22 (3), 310-324 (2018).
- Bruyneel, A. A., McKeithan, W. L., Feyen, D. A., Mercola, M. Will iPSC-cardiomyocytes revolutionize the discovery of drugs for heart disease. Current Opinion inPharmacology. 42, 55-61 (2018).
- Farley, A., Johnstone, C., Hendry, C., McLafferty, E. Nervous system: part 1. Nursing Standard. 28 (31), 46-51 (2014).
- Brotto, M., Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone. 80, 109-114 (2015).
- Park, S. J., et al. Neurogenesis Is Induced by Electrical Stimulation of Human Mesenchymal Stem Cells Co-Cultured With Mature Neuronal Cells. Macromolecular Bioscience. 15 (11), 1586-1594 (2015).
- Vianney, J. M., Miller, D. A., Spitsbergen, J. M. Effects of acetylcholine and electrical stimulation on glial cell line-derived neurotrophic factor production in skeletal muscle cells. Brain Research. 1588, 47-54 (2014).
- Shima, A., Morimoto, Y., Sweeney, H. L., Takeuchi, S. Three-dimensional contractile muscle tissue consisting of human skeletal myocyte cell line. Experimental Cell Research. 370 (1), 168-173 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved