Method Article
In Vivo Surface Electrocardiography for Adult Zebrafish
In This Article
Summary
Here, we present a reliable, minimally invasive, and cost-effective method to record and interpret electrocardiograms in live anesthetized adult zebrafish.
Abstract
The electrocardiogram waveforms of adult zebrafish and those of humans are remarkably similar. These electrocardiogram similarities enhance the value of zebrafish not only as a research model for human cardiac electrophysiology and myopathies but also as a surrogate model in high throughput pharmaceutical screening for potential cardiotoxicities to humans, such as QT prolongation. As such, in vivo electrocardiography for adult zebrafish is an electrical phenotyping tool that is necessary, if not indispensable, for cross-sectional or longitudinal in vivo electrophysiological characterizations. However, too often, the lack of a reliable, practical, and cost-effective recording method remains a major challenge preventing this in vivo diagnostic tool from becoming more readily accessible. Here, we describe a practical, straightforward approach to in vivo electrocardiography for adult zebrafish using a low-maintenance, cost-effective, and comprehensive system that yields consistent, reliable recordings. We illustrate our protocol using healthy adult male zebrafish of 12-18 months of age. We also introduce a rapid real-time interpretation strategy for quality validation to ensure data accuracy and robustness early in the electrocardiogram recording process.
Introduction
The zebrafish (Danio rerio) heart is located anteroventrally to the thoracic cavity between the operculum and the pectoral girdles. The heart is enclosed rather loosely within a silver-colored pericardial sac. Anatomically, the zebrafish heart is different from the four-chambered human and other mammalian hearts because of its diminutive scale (100-fold smaller than the human heart) and its two-chambered structure consisting of only one atrium and one ventricle. Nonetheless, the electrocardiogram (ECG) waveforms and the duration of the QT interval of both species are remarkably similar (Figure 1). Accordingly, zebrafish has emerged as a popular model for studying human inherited arrhythmias1,2,3 and for high-throughput drug screening of potential human cardiotoxicities4,5, such as QT prolongation.
In the routine evaluation of human cardiac diseases, the body-surface ECG has become the most extensively used first-line non-invasive diagnostic tool since its invention by Einthoven in 1903. In contrast, since the first adaptation of the body-surface ECG recording method for adult zebrafish in 20066 and several modifications thereafter7, this technique has remained largely inaccessible to many researchers in the field despite the popularity of this animal model. For other researchers who performed in vivo ECG interrogation for adult zebrafish, wide variations among operators led to inconsistency in ECG findings from different studies. Common reasons include cumbersome and expensive specialized devices and software, low signal-to-noise ratio, and confusion regarding the electrode placement, all further aggravated by an incomplete understanding of the adult zebrafish ECG features and underlying tissue mechanisms. Given that in vivo ECG is the only diagnostic tool to electrically phenotype live zebrafish, there is a clear need for a standardized method to improve sensitivity and specificity, reproducibility and accessibility.
Here, we present a practical, reliable, and validated approach to record and interpret zebrafish in vivo electrocardiograms (Figure 2). Using a single bipolar lead in the frontal plane, we investigated the changes in ECG waveforms and interval durations of live anesthetized healthy wild-type AB adult zebrafish.
Protocol
All experiments in this study were conducted in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal protocols in this study were approved by the UCLA Institutional Animal Care and Use Committee.
1. Preparation of the experimental set up
- Maintain zebrafish in flow-through aquarium systems on a 14 h light, 10 h dark photoperiod at 28 °C ± 0.5 °C. Feed with flake food daily and live brine shrimp (Artemia nauplii) twice daily. Zebrafish in this study were maintained and fed by the UCLA Zebrafish Core.
- On the day of the experiment, transport zebrafish from the aquarium to the laboratory.
- Set up the in vivo ECG recording system by connecting the essential pieces of equipment and inserting the three color-coded stainless-steel electrodes into the three color-matched access portals of the amplifier (Figure 3). Start the system at the start of an ECG recording and/or analysis session.
- Procure necessary tools, such as a timer/stopwatch, a wet sponge with a slit to hold the fish, forceps, scissors, Pasteur pipettes, and culture dishes (100 mm x 20 mm).
2. Anesthesia induction
- Prepare immersion anesthesia for pain control and fish immobilization to avoid motion artifacts during ECG data acquisition. Most laboratories use immersion tricaine (ethyl 3-aminobenzoate methanesulfonate, MS-222).
- To make the tricaine 0.4% stock solution, combine the following items in a screw-capped dark glass bottle: 400 mg of tricaine powder, 98 mL of double distilled water, and 2 mL of 1 M Tris (pH 9). Adjust to pH 7.0 using 1 N NaOH or 1 N HCl as needed8.
- To make the tricaine final immersion solution, determine the minimum concentration that is appropriate for the zebrafish age9, size, metabolic state, strain, disease model, scientific objectives, and procedural duration.
- Perform a tricaine concentration-response study, titrating up or down from the recommended concentration of 168 mg/L (or 0.0168%)9 if necessary, to attain level 4 of anesthesia within 3 min with the fewest possible cardiorespiratory toxicities. For example, in this study, the immersion of wild-type AB zebrafish of 12-18 months of age into a 0.02-0.04% tricaine solution will induce level 4 of anesthesia within 3 min.
NOTE: At level 4 of anesthesia, equilibrium and muscle tone are completely lost and opercular movement rate is reduced8. - If necessary, consult the veterinarian in the Institutional Animal Care and Use Committee (IACUC) for additional guidance on the appropriateness of the selection of anesthetic(s) and route of administration.
- Immerse an adult zebrafish into a dish containing tricaine solution of the lowest predetermined and IACUC-approved concentration (e.g., 0.02-0.04% in this study) to induce level 4 of anesthesia within 3 min (Figure 2).
- For survival ECG protocol, keep the ECG recording session as brief as possible (under 10 min). For brief ECG recording sessions lasting less than 15 min, anesthesia maintenance is not necessary.
- For long ECG recording sessions lasting hours, use a long-acting intramuscular paralytic and an oral perfusion system to provide ample hydration and oxygenation6.
3. ECG lead placement
- Once the zebrafish maintains level 4 of anesthesia for 3 s, use a pair of blunt forceps to transfer the fish immediately onto the damp sponge slit with its ventral surface uppermost for placement of ECG lead electrodes (Figure 4).
- Gently insert the three ECG lead electrodes into the fish musculature to approximately 1 mm in depth to establish a bipolar lead in the frontal plane that parallels the left caudal-right cranial orientation of the cardiac main axis.
- Position the positive (red) electrode in the ventral midline at the level of the bulbus arteriosus, i.e., at 1-2 mm above an imaginary line connecting the two lower edges of the operculums (Figure 4A).
- Position the negative (black) electrode caudally and 0.5-1.0 mm left laterally to the positive electrode, at a distance greater than the maximal apicobasal length of the adult zebrafish ventricle (Figure 4A).
- Position the reference (green) electrode caudally, near the anal region.
NOTE: Since the cardiac main axis varies somewhat from fish to fish, to maximize the R and T wave amplitudes, adjust the lead positions by making only small, systematic changes through trial and error. For example, change one electrode (positive or negative), instead of both electrodes, at a time and make gradual changes in one specified direction before changing to another direction instead of making erratic changes in random directions.
4. ECG recording
- Open the ECG data acquisition program. Select a desired setting from the drop-down menus for range, low pass, and high pass. For example, the following setting in the in vivo ECG recording system used in this experiment yields consistent, satisfactory signal-to-noise ratio for a normal adult zebrafish: range “2 mV”, low pass “120 Hz”, and high pass “0.03 s”.
- Press Start to start continuous gap-free ECG recording at a sampling rate of 1 kHz.
- To optimize lead positioning for maximal signal-to-noise ratio, press Stop to stop ECG recording and review the ECG trace soon after the very first recording attempt for each heart. To diagnose that an adult zebrafish ECG is normal, confirm that all of the following four validating criteria are satisfied (Figure 1):
- Criterion 1: Ensure that all ECG waveforms (P, QRS, and T) are distinct and readily visible.
- Criterion 2: Ensure that the P wave is positive.
- Criterion 3: Ensure that the net QRS complex is positive (i.e. the R wave amplitude is larger than the sum of Q and S wave amplitudes).
- Criterion 4: Ensure that the T wave is positive.
- If a normal ECG is expected, reposition the electrodes (try the negative electrode first) if necessary, until all four validating criteria are satisfied.
- If a normal T wave is expected, but the T wave is too small, reposition the electrodes to maximize the T wave amplitude.
- Resume ECG recording after optimizing lead positioning. Save the ECG sweeps for subsequent analysis.
5. Recovery from anesthesia
- At the end of the ECG recording session, carefully remove the electrodes without injuring the fish. Transfer the fish to fresh, oxygenated fish water free of tricaine.
- To facilitate recovery from anesthesia, squirt water over the gills vigorously with a Pasteur pipette until the fish resumes regular gill movement or swimming.
- Monitor the fish for full recovery from anesthesia (typically 1-2 min), as indicated by the fish ability to swim upright for at least 5 s.
6. ECG interpretation
- Define the analysis settings.
- Know the software interface (Table of Materials) by reading the operating manual of the ECG data analysis software.
NOTE: Although the directions below are specific to the commercial software used in our laboratory, the basic tasks to accomplish are essentially the same in any software package for ECG analysis. - Open the ECG data analysis program. From the File menu, select Open to open the ECG file of interest and display the full ECG trace. Use the mouse to drag out a section of interest in the ECG trace to analyze.
- From the ECG Analysis menu, select ECG Settings to open a dialogue box to pre-define various parameter settings for software automatic analysis (Figure 5A).
- Know the software interface (Table of Materials) by reading the operating manual of the ECG data analysis software.
- Analyze the heart rhythm and rate.
NOTE: Heart rate depends on several factors, including zebrafish age and strain, anesthesia agents (e.g., tricaine, isoflurane, etc.) and concentration, anesthesia usage (single agent5,7 vs. combined agents5) and exposure time5. For example, in this study the heart rate of 12-18 month-old wild-type AB zebrafish after 3-5 min of immersion in 0.02-0.04% tricaine solution was 116 ± 17 beats per minute (n = 9), consistent with literature reports of heart rate for this age group and anesthetic5,7.- Determine whether the heart rhythm is sinus or not, regular or irregular.
NOTE: The presence (or absence) of sinus rhythm is based on the presence (or absence) of an upright P wave preceding each QRS by a normal PR interval (e.g., 60-65 ms for Liu et al.’s 10-12 month-old7 and 12-18 month-old wild-type AB zebrafish in this study). The atrial and ventricular rhythm regularity (or irregularity) is based on the regularity (or irregularity) of successive PP or RR intervals, respectively. - To determine the heart rate, ensure that the software correctly identifies all the P and R waves. Based on these automatic identifications (or manual corrections) of the P and R waves, the software automatically measures all the PP and RR intervals in the ECG selection, calculates the interval averages to generate the atrial and ventricular rate.
NOTE: The atrial rate is the average PP interval whereas the ventricular rate is the average RR interval. To determine the heart rate, correct identification of the P and R waves is critical. - Correct any auto-identification mistakes by moving the misplaced cursors to the appropriate P and R waves (Figure 5B).
NOTE: If the heart is in sinus rhythm, the atrial rate and ventricular rate are the same because of the one-to-one correspondence between the sinus P waves and the QRS complexes. However, in the case of atrioventricular dissociation (e.g., in ventricular tachycardia or third-degree atrioventricular block), this one-to-one correspondence between the P waves and QRS complexes is lost; therefore, there are two heart rates because the atrial rate is different from the ventricular rate. - Determine the heart rate based on at least five consecutive complete cardiac cycles if the heart rhythm is regular, or a strip of at least six seconds if the heart rhythm is irregular.
- Determine whether the heart rhythm is sinus or not, regular or irregular.
- Calculate intervals and wave durations.
- Go to ECG Analysis > Averaging View to concatenate n (e.g., 5) consecutive cardiac cycles into a single average signal (Figure 5C).
NOTE: If the ECG waveforms of an individual cardiac cycle diverge substantially from the average signal, study that cardiac cycle separately without concatenation. - Ensure that the software correctly identifies the start and end of the P wave, QRS complex, and T wave displayed in the Averaging View window (Figure 5C). Based on these automatic identifications (or manual corrections) of these waves and intervals, the software automatically measures the durations as defined conventionally.
NOTE: The PR interval extends from the start of the P wave to the start of the QRS complex (or the RS complex if the Q wave is not visible). The QRS duration extends from the start of the Q wave (or the R wave if the Q wave is not visible) to the end of the S wave (i.e., the J point; Figure 1). The QT interval extends from the start of the Q wave (or the R wave if the Q wave is not visible) to the end of the T wave. Therefore, to calculate intervals and durations, correct identification of the start and end of the P wave, QRS complex, and R wave is critical. - Correct any auto-identification mistakes by moving the misplaced cursors to the appropriate positions.
- Select the negative peak of the S wave as the end of the QRS complex7 because the zebrafish J point that signals the end of the S wave can be particularly difficult to identify accurately. This will cause a slight underestimate of the true QRS duration.
NOTE: The ECG analysis software automatically corrects the QT interval to the ventricular rate (or RR interval) to generate the corrected QT interval QTc using the method pre-selected by the user in step 6.1.3, for example, Bazett (Figure 5A). The Bazett’s formula (1920) QTc = QT / √RR is the most popular and the first of several methods proposed to correct the human QT interval for heart rate. Because the accuracy of the Bazett’s formula has been questioned, refer to other methods proposed for humans10,11 and zebrafish6 (Figure 5D).
- Go to ECG Analysis > Averaging View to concatenate n (e.g., 5) consecutive cardiac cycles into a single average signal (Figure 5C).
- Interpret ECG abnormalities by recognizing exceptions for the four validating criteria in step 4.3.
- Recognize exceptions for criterion 1. In the absence of any P waves (which indicates the absence of sinus rhythm), rely on the RR intervals and QRS duration to diagnose the heart rhythm. For example, if the RR intervals are irregularly irregular, diagnose atrial fibrillation; if the RR intervals are regular and the QRS is normally narrow, diagnose junctional escape rhythm; on the other hand, if the RR intervals are regular and the QRS is abnormally prolonged, diagnose ventricular escape rhythm.
- Recognize exceptions for criterion 2. When the P wave is negative (or inverted), diagnose retrograde atrial activation from an ectopic pacemaker (such as an atrial site downstream of the sinus node, the atrioventricular node, or the ventricle).
- Recognize exceptions for criterion 3. When tall and narrow Q waves present with negative P and negative T waves, diagnose lead reversal due to an erroneous switch of the positive and negative electrode positions because those tall and narrow Q waves were true R waves mistakenly inverted (Figure 6D). In contrast, when broad Q waves present with positive P waves following significant cardiac injury, diagnose myocardial infarction because those broad Q waves are true pathologic Q waves.
- Recognize exceptions for criterion 4. When the T wave is inverted, inspect ventricular activation to identify whether the ventricular repolarization abnormality is primary or secondary. Rely on the clinical scenario to narrow down the correct diagnosis from a differential list of primary ventricular repolarization abnormality (from drug effects or myocardial ischemia; Figure 6C) vs. secondary ventricular repolarization abnormality (due to aberrant ventricular activation from pre-excitation, ventricular ectopy, or ventricular pacing).
- Export ECG findings.
- Select Table View to review all ECG measurements. Select the measurements of interest to copy and paste into the desired document (e.g., Excel spreadsheet).
- To export an ECG trace, highlight a section of interest in the ECG sweep using the magnifier icon. Copy and paste into the desired document (e.g., Word or PowerPoint).
Results
Figure 1 illustrates the clinical relevance of the method presented here. In vivo surface electrocardiography for adult zebrafish is an essential electrical phenotyping tool because of the remarkable similarities between the zebrafish and human ECG despite their vast anatomical differences. The zebrafish heart has only one atrium and one ventricle in contrast to the human heart with two atria and two ventricles (top row; right and left, respectively). However, despite its apparent anatomical simplicity, the zebrafish heart shares several ECG features with the human heart (bottom row; right and left, respectively) Therefore, the zebrafish heart has emerged as a surrogate model for human cardiac electrophysiology5,12,13. Figure 1 illustrates a small but distinct Q wave from a live, healthy 14-month-old zebrafish. However, in zebrafish ECG, lead positioning is not commonly optimized to demonstrate the Q wave. Therefore, the Q wave is commonly invisible, and an RS complex is more commonly seen than the full QRS complex in zebrafish ECG.
Figure 2 summarizes the four essential action steps to conduct minimally invasive in vivo electrocardiography for adult zebrafish. Following anesthesia induction (step 1) and electrode placement (step 2), we recorded baseline ECG signals (step 3) from healthy wild-type AB zebrafish of 12 to 18 months of age (n = 9). Our electrode insertion technique was only minimally invasive because we did not need to peel fish scales or perform pericardiotomy. Following data acquisition, we manually reviewed and verified each ECG recording (step 4) to avoid potential misinterpretation by software automatic analysis.
Figure 3 shows the three indispensable components of a typical ECG data acquisition and processing system: a high-performance data acquisition hardware, a high-gain differential amplifier, and a computer uploaded with software for ECG data acquisition and analysis. In our laboratory, we adapted an existing commercial in vivo ECG recording system originally designed for small mammalian models (such as mice, rats, and rabbits) to accommodate the adult zebrafish model.
Figure 4 demonstrates that proper lead placement requires aligning the lead with the presumed cardiac main axis. In zebrafish in vivo ECG recording, because only one single lead is used, proper lead positioning to maximize concurrently both R and T wave amplitudes is critical. To maximize R and T wave amplitudes, we aligned the positive and negative lead electrodes with the cardiac main axis, presumably in the left caudal to right cranial orientation. Following thoracotomy and pericardiotomy to open the pericardial sac and expose the heart, the cardiac main axis becomes apparent (Figure 4B white dashed line). In fact, pericardiotomy to expose the heart is a commonly used strategy to increase the signal-to-noise ratio7 at the cost of converting the ECG recording from a minimally invasive into a highly invasive procedure.
Figure 5 illustrates critical steps in ECG analysis. First, we pre-defined the various parameter settings for software automatic analysis using the ECG Settings dialogue box (Figure 5A). Because we repurpose an existing ECG recording equipment designed for mammalian models to accommodate adult zebrafish, the Detection and Analysis setting for zebrafish is not available. We selected the Human Preset instead, given the remarkable similarity of zebrafish ECG to human ECG (Figure 5A). Second, we manually verified the software automatic ECG identification (in black) of the R wave peaks and correct (in red) any R wave auto-identification mistakes prior to commanding the software to recalculate the average ventricular rate. For example, in Figure 5B, a large P wave in relation to the R wave fooled the software into misidentifying the R waves, leading to the subsequent automatic miscalculation of the RR interval or ventricular rate. Therefore, human verification and appropriate corrections as needed are critical in ECG analysis. Third, we quickly assessed rhythm regularity and calculated the average duration of waves and intervals using the Averaging View (Figure 5C) to concatenate several consecutive cardiac cycles (green) into one single average signal (black). Here in Figure 5C, the negligible deviation between each of the nine cardiac cycles and the average signal argues for the excellent rhythm regularity of this zebrafish heart. Lastly, we enabled the software to automatically correct the QT interval for heart rate using Bazett, one of the seven different methods available (Figure 5D).
Figure 6A-C demonstrates how the depth of electrode placement affects the amplitudes of the ECG signals. When we incorrectly inserted the electrodes too superficially in the dermis (Figure 6A), the lead was “indirect”-like (more than two cardiac diameters from the heart, similar to the indirect standard human ECG limb leads I, II, and III) and the voltage signals were small. When we appropriately inserted the electrodes 1 mm deeper into the pectoralis musculature (Figure 6B), the lead became “semidirect” (in close proximity but not in direct contact with the heart) and the voltage signals increased. The ECG waveforms became readily visible. However, when we incorrectly inserted the electrodes even deeper into the ventricle (Figure 6C), the lead became “direct” (in direct contact with the heart) and the voltage signals increased further. The R wave amplitude in Figure 6C increased by eight-fold compared to Figure 6A and by four-fold compared to Figure 6B. However, the ECG trace in Figure 6C revealed new signs of injury to the ventricular myocardium, such as new ST depression and new T wave inversion.
Figure 6D demonstrates how the unusual inversions of all ECG waveforms (P, Q, R, S, and T) should signal a lead reversal mistake, in which the positive and negative electrodes switched place. Note that by definition Q and S are always negative whereas R is always positive.
Figure 6E-F shows how inappropriate anesthesia depth can impair the quality of in vivo ECG recording. In Figure 6E, inadequate anesthesia (0.017% tricaine) led to failure to immobilize the zebrafish completely. The resultant motion artifacts lowered the signal-to-noise ratio by both contaminating the signal (asterisk) and increasing the noise (arrows). In contrast, in Figure 6F, overdosed anesthesia (0.08% tricaine) induced severe sinus bradyarrhythmia as well as changes of the ST segment and T wave.
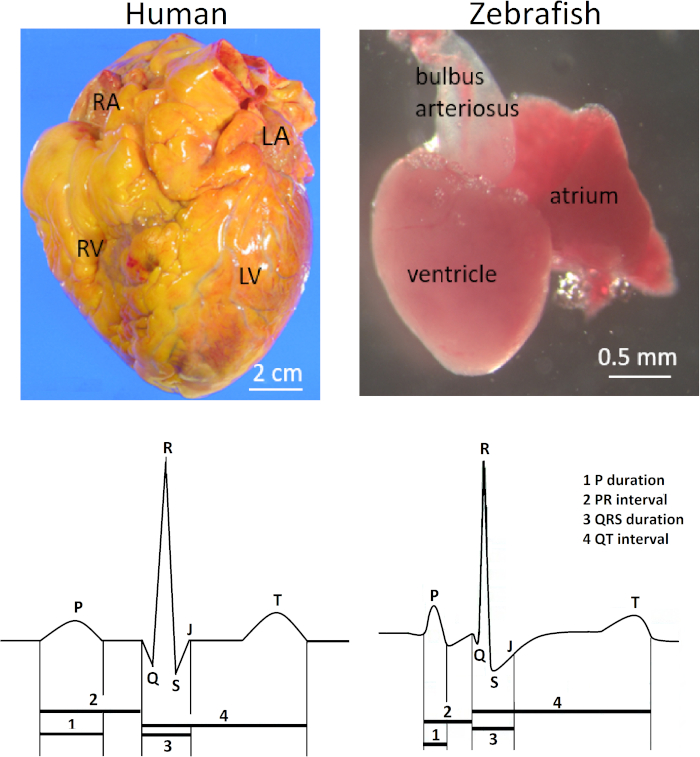
Figure 1: Contrasting anatomy and ECG of human and zebrafish hearts. In contrast to the human heart with two atria and two ventricles, the zebrafish heart has only one atrium and one ventricle (top row). Abbreviations: RA, right atrium; LA, left atrium; RV, right ventricle; LV: left ventricle. The zebrafish heart shares several common ECG features with the human heart (bottom row). Please click here to view a larger version of this figure.
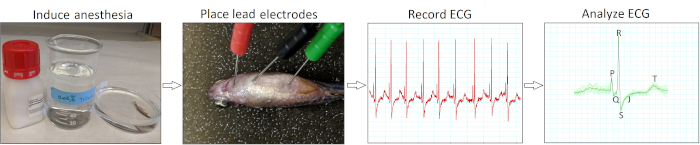
Figure 2: Minimally invasive in vivo ECG recording protocol. A schematic flow chart illustrates four critical action steps in conducting an in vivo ECG interrogation: induce anesthesia, place ECG lead electrodes, record ECG, and analyze the ECG recordings. Please click here to view a larger version of this figure.
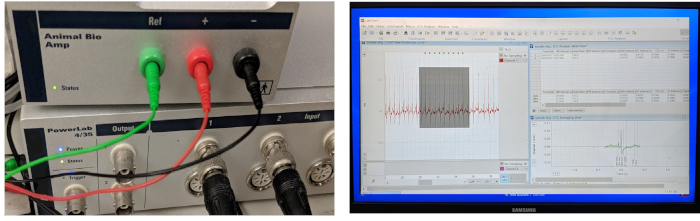
Figure 3: ECG data acquisition and processing system. The three key components of an integrated in vivo ECG recording system include a hardware to acquire data, an amplifier, and computer software for data acquisition and analysis. The amplifier comes with three ready-to-use 29-gauge stainless steel microelectrodes. Please click here to view a larger version of this figure.
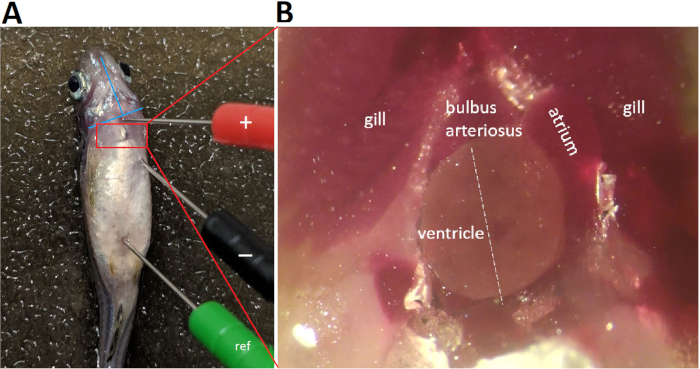
Figure 4: ECG lead placement. Three 29-gauge color-coded stainless steel electrodes are inserted securely into the fish musculature to approximately 1 mm in depth. Placement of the negative (black) electrode and the positive (red) electrode establishes a bipolar lead in the frontal plane, along a left caudal to right cranial orientation. Abbreviation: ref, reference electrode Please click here to view a larger version of this figure.
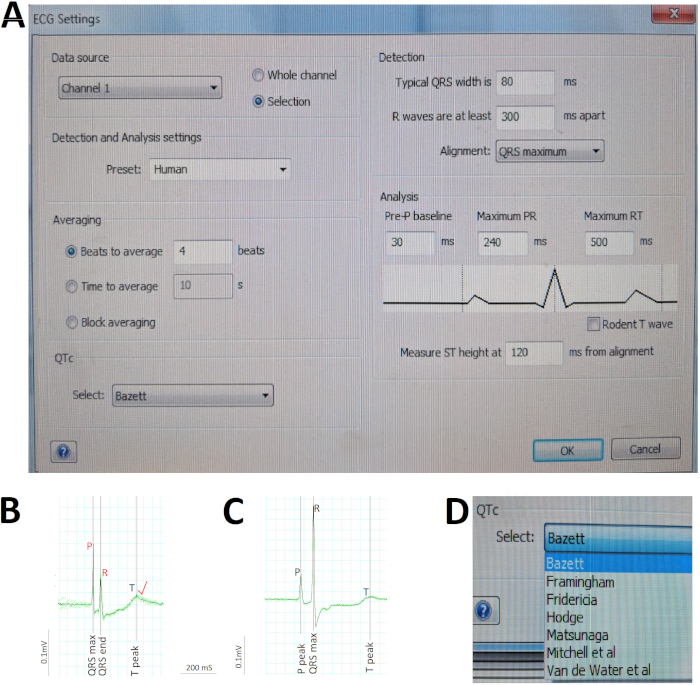
Figure 5: Critical steps in ECG analysis. (A) Pre-define the various parameter settings for software automatic analysis. (B) Manually correct (red) two automatic misidentifications by the software (black) of the P and R waves to rectify software miscalculation of the atrial and ventricular rate. (C) Concatenate nine consecutive cardiac cycles (green) into a single average signal (black) to quickly assess rhythm regularities/irregularities and calculate average durations of waves and intervals. (D) Correct the QT interval for heart rate using one of the various methods, such as Bazett. Please click here to view a larger version of this figure.
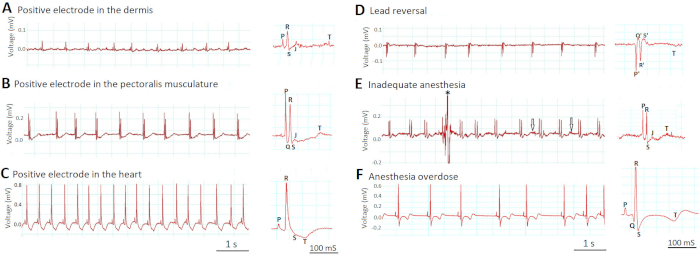
Figure 6: Effects of lead placement and anesthesia depth on ECG signals. Two most critical steps that determine the success of in vivo ECG recording are lead placement (A-D) and anesthesia depth (E-F). Please click here to view a larger version of this figure.
Discussion
When recording in vivo ECG for adult zebrafish by means of a single lead as we demonstrated in this study, there are a number of caveats concerning the quality and validity of the ECG recording results. First, in choosing the appropriate anesthetics and determining the minimal needed anesthesia concentration, depth, and duration, balance the anesthetic cardiotoxicities against the critical need to suppress motion artifacts and the a priori determination for a survival vs. terminal experimental design. Capitalizing on the synergistic potency of a combination of multiple anesthetics from different drug classes5,14 and paralytics1,6 to lower the dose of individual agents5 or administering a low maintenance dose following a higher induction dose are typical strategies. However, despite its well-known potential cardiorespiratory toxicities, including death8, tricaine is still the most widely used, the best available, and the only anesthetic approved by the US Food and Drug Administration (FDA) for zebrafish anesthesia. Tricaine has been popularly used in ECG recording of adult zebrafish either as a single agent or in combination with other anesthetics or paralytics.
Second, lead placement accuracy can be ensured at least for healthy normal zebrafish using our four validating criteria for a normal adult zebrafish ECG. Of the four validating criteria that we propose here, the last two criteria together confirm the fundamental concordance between the polarity of the R wave and that of the T wave in a normal ECG5,7,15. This R and T wave concordance is a fortuitous, yet critical, similarity between zebrafish and human16,17 normal ECG that contributes to the clinical relevance of the zebrafish heart model as a surrogate for human cardiac electrophysiology. However, several benign or malignant conditions may invalidate any of the four validating criteria. For example, the R and T wave concordance is lost in myocardial ischemia7,15. This loss of R and T wave concordance in myocardial ischemia is another striking resemblance between zebrafish and human ECG that contributes to the clinical relevance of the zebrafish myocardial infarction model.
Lastly, we recommend a standard practice in ECG analysis. With the advent of technology, ECG analysis software can generate automatic ECG interpretation. However, we strongly recommend that trained humans should always re-interpret and verify all ECGs based on the respective clinical scenario leading to ECG recording. Routine over-reliance solely on automatic interpretation by an ECG analysis software is inadvisable, particularly in the presence of common normal ECG variants, cardiac pathologies, or suboptimal lead placement.
This study focuses on the minimally invasive method for brief ECG recording sessions. However, should the need arise for terminal prolonged ECG recording sessions lasting hours, modifications are necessary to provide adequate oxygenation, hydration, and anesthesia by continuous perfusion6.
Additionally, enhance the signal-to-noise ratio by one of at least three ways. Choosing a more powerful amplifier is often a costly, if not impractical, option. Opening the pericardial sac to reduce the volume conductor is a reasonable, although invasive, approach that has been adopted7. Strategic lead placement to align the lead axis in a direction parallel to the main cardiac axis (Figure 4B) will maximize the ECG voltage signals but may require trial and error, especially in the absence of pericardiotomy.
The in vivo ECG interrogation method for adult zebrafish that we presented here offers four main advantages. First, our minimally invasive approach requires only electrode insertion, but no fish scale removal or thoracotomy-pericardiotomy. Therefore, by minimizing pain for the fish, our approach enables repeated ECG interrogations in longitudinal survival studies. Second, when anesthetics adequately suppress fish motion, the in vivo ECG recording system in our study consistently yields a satisfactory signal-to-noise ratio with noise-free raw signals. Third, the four-criterion quality validation that we propose here ensures data accuracy and robustness early in the ECG data acquisition and minimizes operator-dependent variations. Lastly, in particular, our last validating criterion (the normal T wave is upright) encapsulates the concordance of the R wave and T wave, an important human-like feature of zebrafish normal ECG (Figure 1).
However, there still exist four major limitations to current in vivo ECG methodology for adult zebrafish by our group and others.
First, the lack of subject cooperation necessitates the need for anesthesia with its limiting cardiorespiratory toxicity consequences. For in vivo ECG interrogation, whereas human patients never need sedation, zebrafish always require anesthetics or paralytics, all of which cause variable cardiorespiratory toxicities.
Second, the need to secure the attached ECG leads slightly elevates the invasiveness of an otherwise non-invasive procedure. Whereas lead placement in body-surface ECG recording of humans is entirely non-invasive because electrodes adhere to the human epidermis, lead placement for in vivo ECG recording of zebrafish is more invasive because, at the minimum, steel electrodes must puncture the fish skin for secure insertion into the fish musculature.
The last two limitations stem from the anatomical constraints of the zebrafish chest and heart. Third, the minuscule size of the adult zebrafish heart necessitates a drastic reduction in the number of ECG leads. While humans readily accommodate twelve leads in a standard ECG recording, adult zebrafish can typically accommodate only a single unipolar or bipolar lead. The ramification of a single ECG lead is the challenge to optimize concurrently the amplitudes of all three P, R, and T waves. Hence, the importance of optimal and accurate lead placement in zebrafish ECG interrogation cannot be overstated. In zebrafish, the T wave presents a unique detection challenge because it is often the smallest of these three waves. Therefore, the zebrafish T wave amplitude should receive optimization priority over the typically larger P and R waves.
Fourth, determining the zebrafish main cardiac axis to maximize the R wave amplitude can be challenging. The reason is that the zebrafish heart has more freedom of motion within its loose pericardial sac compared to the human heart within its form-fitting glove-like pericardium.
Overall, these limitations will stimulate future method innovation. With the advent of 3D printing and deformable electronics18, there is hope for direct lead implantation one day in awake, alert, swimming zebrafish using a ‘cardiac sock’ of wireless electrode sensors.
Disclosures
Open Access for this video-article is sponsored by AD Instruments.
Acknowledgements
This work was supported by the National Institutes of Health R01 HL141452 to TPN. ADInstruments kindly provided generous funding to defray the cost of open access publishing but had no role in either experimental design, data acquisition, data analysis of this study or any access to the manuscript prior to publication.
Materials
| Name | Company | Catalog Number | Comments |
| Culture dishes | Fisher Scientific | FB087571 | 100 mm x 20 mm |
| Dumont Forceps | Fine Sciense Tools | 11253-20 | 0.1 x 0.06 mm |
| FE136 Animal Bio Amp | AD Instruments | FE231 | |
| Iris Forceps | Fine Sciense Tools | 11064-07 | 0.6 x 0.5 mm |
| LabChart 8 Pro | AD Instruments | Software with ECG Module | |
| Needle electrodes for Animal Bio Amp | AD Instruments | MLA1213 | 29 gauge |
| Plastic Disposable Transfer Pipets | Fisher Scientific | 13-669-12 | 6 in., 1.2 mL |
| PowerLab 4/35 | AD Instruments | 4//35 | |
| Scissors | Fine Sciense Tools | 15000-08 | 2.5 mm, 0.075 mm |
| Tricaine (Ethyl 3-aminobenzoate methanesulfonate) | Sigma | E10521-10G | MS-222 |
References
- Arnaout, R., et al. Zebrafish model for human long QT syndrome. Proceedings of the National Academy of Sciences of the United States of America. 104 (27), 11316-11321 (2007).
- Hassel, D., et al. Deficient zebrafish ether-a-go-go-related gene channel gating causes short-QT syndrome in zebrafish reggae mutants. Circulation. 117 (7), 866-875 (2008).
- Meder, B., et al. Reconstitution of defective protein trafficking rescues Long-QT syndrome in zebrafish. Biochemical and Biophysical Research Communication. 408 (2), 218-224 (2011).
- Sieber, S., et al. Zebrafish as a preclinical in vivo screening model for nanomedicines. Advanced Drug Delivery Reviews. , (2019).
- Lin, M. H., et al. Development of a rapid and economic in vivo electrocardiogram platform for cardiovascular drug assay and electrophysiology research in adult zebrafish. Science Reports. 8 (1), 15986 (2018).
- Milan, D. J., Jones, I. L., Ellinor, P. T., MacRae, C. A. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. American Journal of Physiology-Heart and Circulation Physiology. 291 (1), H269-H273 (2006).
- Liu, C. C., Li, L., Lam, Y. W., Siu, C. W., Cheng, S. H. Improvement of surface ECG recording in adult zebrafish reveals that the value of this model exceeds our expectation. Science Reports. 6, 25073 (2016).
- Matthews, M., Varga, Z. M. Anesthesia and euthanasia in zebrafish. Ilar Journal. 53 (2), 192-204 (2012).
- Westerfield, M. . The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio). , (2007).
- Sagie, A., Larson, M. G., Goldberg, R. J., Bengtson, J. R., Levy, D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). American Journal of Cardiology. 70 (7), 797-801 (1992).
- Luo, S., Michler, K., Johnston, P., Macfarlane, P. W. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. Journal of Electrocardiology. 37 Suppl, 81-90 (2004).
- Vornanen, M., Hassinen, M. Zebrafish heart as a model for human cardiac electrophysiology. Channels (Austin). 10 (2), 101-110 (2016).
- Tsai, C. T., et al. In-vitro recording of adult zebrafish heart electrocardiogram - a platform for pharmacological testing). Clinica Chimica Acta. 412 (21-22), 1963-1967 (2011).
- Collymore, C., Tolwani, A., Lieggi, C., Rasmussen, S. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). Journal of American Association of Lab Animal Sciences. 53 (2), 198-203 (2014).
- Sun, Y., et al. Activation of the Nkx2.5-Calr-p53 signaling pathway by hyperglycemia induces cardiac remodeling and dysfunction in adult zebrafish. Disease Model and Mechanism. 10 (10), 1217-1227 (2017).
- Franz, M. R., Bargheer, K., Rafflenbeul, W., Haverich, A., Lichtlen, P. R. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation. 75 (2), 379-386 (1987).
- Chiale, P. A., et al. The multiple electrocardiographic manifestations of ventricular repolarization memory. Current Cardiology Reviews. 10 (3), 190-201 (2014).
- Xu, L., et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nature Communications. 5, 3329 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved