Method Article
Luminescence Lifetime Imaging of O2 with a Frequency-Domain-Based Camera System
* These authors contributed equally
In This Article
Summary
We describe the use of a novel, frequency-domain luminescence lifetime camera for mapping 2D O2 distributions with optical sensor foils. The camera system and image analysis procedures are described along with the preparation, calibration and application of sensor foils for visualizing the O2 microenvironment in the rhizosphere of aquatic plants.
Abstract
We describe a method to image dissolved oxygen (O2), in 2D at high spatial (< 50-100 µm) and temporal (< 10 s) resolution. The method employs O2 sensitive luminescent sensor foils (planar optodes) in combination with a specialized camera system for imaging luminescence lifetime in the frequency-domain. Planar optodes are prepared by dissolving the O2-sensitive indicator dye in a polymer and spreading the mixture on a solid support in a defined thickness via knife coating. After evaporation of the solvent, the planar optode is placed in close contact with the sample of interest - here demonstrated with the roots of the aquatic plant Littorella uniflora. The O2 concentration-dependent change in the luminescence lifetime of the indicator dye within the planar optode is imaged via the backside of the transparent carrier foil and aquarium wall using a special camera. This camera measures the luminescence lifetime (µs) via a shift in phase angle between a modulated excitation signal and emission signal. This method is superior to luminescence intensity imaging methods, as the signal is independent of the dye concentration or intensity of the excitation source, and solely relies on the luminescence decay time, which is an intrinsically referenced parameter. Consequently, an additional reference dye or other means of referencing are not needed. We demonstrate the use of the system for macroscopic O2 imaging of plant rhizospheres, but the camera system can also easily be coupled to a microscope.
Introduction
The distribution and dynamics of dissolved gases and ions in sediments and soils provide key information on biogeochemical processes such as microbial respiration1,2, or radial oxygen loss from plant roots3,4,5, and the chemical microenvironment of microbes6,7, plant rhizospheres5,8,9 and animal burrows10,11,12. Biological and chemical activity in such diffusion-limited environments can create steep gradients of chemical substrates or products of biogeochemical processes. In particular, O2 availability has a huge impact on biogeochemical processes and thus the biology and ecology of a system13. Therefore, analyzing O2 concentrations at high spatial and temporal resolution is of prime importance in aquatic and terrestrial sciences. First, electrochemical and optical microsensors14,15 were developed to measure this important analyte. Later, 2 dimensional (2D) imaging of O2 with planar optodes was introduced12,16,17,18,19, which enabled the visualization and quantification of the heterogeneous O2 distribution in soils and sediments.
Planar O2 optodes consist of an O2-sensitive indicator dye20, which is dissolved in a suitable polymer21. The indicator dye is excited at specific optical wavelengths and emits red-shifted light upon relaxation in the form of luminescence. In the presence of O2, the excited indicator dye can transfer its energy to the O2 molecule upon collision, which is referred to as collision-based luminescence quenching22. Therefore, the luminescence intensity as well as the luminescence lifetime are reduced with increasing O2 concentration23. In an ideal case the change in intensity and lifetime follows the Stern-Volmer equation (equation 1) using either the luminescence intensity or lifetime in the absence (I0; τ0) or presence (I, τ) of O2 at a given concentration [Q]. The Stern-Volmer constant (Ksv) is a measure for the sensitivity of the optode towards O2; KSV is dependent on environmental variables such as temperature and pressure.
(1) 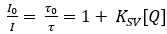
Recording such changes in luminescence over a planar sensor foil with a camera system can be used to visualize the corresponding changes in O2 distribution. Initially, simple luminescence intensity-based O2 imaging was used18. However, such methodology is very sensitive to external interferences, which compromise the reliability of the results due to heterogeneous illumination, fluctuations in the excitation source or camera, as well as uneven distribution of the indicator dye within the planar optode.
Some of these limitations can be alleviated by using planar optodes for ratiometric imaging17,24, where the O2-sensitive indicator dye is co-immobilized in the polymer layer of the planar optode with an insensitive reference dye emitting in a different spectral range than the O2-indicator. Based on emission images acquired in two spectral windows, the O2-sensitive emission signal is divided by the reference signal, generating a ratio image that is less prone to the above mentioned interferences5,17. The method requires the use of a second dye, which ideally can be excited by the same excitation source, but emits at a different wavelength (without significant spectral overlap), in another spectral window of the camera (e.g., in another color channel of an RGB camera).
Alternatively, O2 imaging can be based on quantifying the O2-dependent change in luminescence lifetime of the indicator dye, which is not affected by uneven illumination or heterogeneities in the indicator concentration25. First luminescence lifetime-based O2 imaging systems were based on time-domain measurements with a gate-able charged coupled device (CCD) camera system26, where a pulsed excitation source is used and luminescence images are taken over defined time intervals within the excitation or emission of the indicator8,23,27. From such images, the luminescence lifetime can be determined and correlated to the corresponding O2 concentration in a calibration. Subsequently, luminescence lifetime images for a given sample pressed against the planar optode can be converted to images of the corresponding 2D distribution of O2 concentration. This system has been used in many applications both in the laboratory and in situ16,28, but the essential gate-able CCD camera is no longer commercially available.
Recently, a different luminescence lifetime camera system was released, which acquires images in the frequency-domain8. The system relies on a continuously modulated light source for excitation. This can either be a sinusoidal or square wave instead of a pulsed excitation, which is used for image acquisition in the time-domain. This modulation results in a modulated luminescence emission of the O2 indicator dye, which is phase-shifted by an angle, φ, which is depended on the luminescence lifetime of the indicator dye (τ) (see equation 2).
(2) 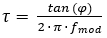
The change between excitation and emission amplitude (i.e., the so-called modulation index or depth (amplitude divided by the constant luminescence part)) is also dependent on the luminescence lifetime. So, by setting a known modulation frequency the special CMOS image sensor within the camera is able to measure the luminescence lifetime in the ns to µs range as described in detail elsewhere 8,29,30. A general guide on the operation principle can be found (using the following link https://www.youtube.com/watch?v=xPAB_eVWOr8).
In the following protocol, we demonstrate the use of the novel camera system for imaging the distribution of O2 concentration around the roots of the aquatic freshwater plant Littorella uniflora in 2D9,31. We would like to emphasize that this method is by no means limited to this application. Oxygen-sensitive optodes or sensor particles27 in combination with various imaging methods have been used in medical research32, in bioprinting33, for pressure sensitive paints34,35, or to study photosynthetic systems2,36,37, just to name a few other fields of application.
Protocol
1. Fabrication of planar O2 optode
- Dissolve 1.5 mg of the luminescent O2 indicator dye platinum(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin (PtTFPP) and 100 mg of polystyrene (PS) in 1 g of chloroform to get the so-called 'sensor cocktail'.
NOTE: The cocktail can be kept in a closed, gas-tight glass vial for a few hours in the fridge and darkness until further use. - Fix a clean, dust free polyethylene terephthalate (PET) foil (size dependent on the application) on a cleaned glass plate with the help of a water or ethanol (70%) film (Figure 1A).
- Place the cleaned knife coating device (120 µm) on the foil and apply a line of the sensor cocktail in front of the device using a glass pipette (Figure 1B). Then, drag the knife-coating device slowly and uniformly over the PET foil to spread the cocktail evenly.
NOTE: All materials and tools have to be cleaned thoroughly and the fabrication should be done in a dust-free environment, such as a fume hood, flow bench or underneath a point suction device. To avoid heterogeneities in the final sensor foil, the steps following the application of the sensor cocktail onto the foil should be done swiftly, as the chloroform evaporates fast. - Dry the finished planar O2-sensitive optode in ambient air for 1 h and then over-night in a heating cabinet at 50-60 °C, resulting at a final layer thickness after solvent evaporation of ~12 µm. Store the produced optodes in darkness (e.g., in a paper envelope) until further use (Figure 1C).
NOTE: Planar O2 optodes can be stored dry and in darkness for several months to years prior to use. A final layer thickness ranging from 1-20 µm has proven to deliver good results, with sufficient luminescence signal and adequate response times.
2. Rhizo-sandwich chamber
- Clean two glass plates (24.5 x 14 cm2, thickness: 4 mm) with 96% ethanol.
- Use light-curable, acrylic-based instant adhesive (see Table of Materials) to glue microscope slides (76 x 26 mm2, thickness: 1 mm) along the edges of the first glass plate (i.e., the rear chamber side), while leaving one long edge open. Use a glasscutter to shorten microscope slides as needed.
CAUTION: Cutting glass can cause sharp edges and should be handled with care.
NOTE: The microscope slides function as spacers between the front and back, and depending on the thickness of the roots and plant size, multiple layers of microscope slides can be glued on top of each other. - Cut the planar optode into the required shape and size to fit into the space between the glued microscope slides. Place it on the inside of the front glass plate with the coated side upwards, to allow contact with the sample of interest when pressed against it.
- Tape one edge of the optode foil to the glass plate and add a few drops of tap water in between the glass plate and the optode foil (Figure 2A). Slowly lower the foil on these water droplets allowing it to straighten itself out on the glass surface.
- Carefully remove air bubbles trapped in-between the planar optode and the glass plate using a soft tissue, while avoiding scratching of the sensor coating. Wipe the glass plate dry and tape the remaining edges of the optode foil to the glass plate (Figure 2B).
NOTE: A tape with suitable adhesion under water should be chosen. - Sieve the sediment using a mesh size of 0.5 mm. Place a spoon of wet sediment on the first glass plate (Figure 2C).
NOTE: The mesh size should not be larger than half the spacer thickness. - Distribute the sediment evenly and adjust it to the same thickness as the microscope slide spacers using a flat glass plate. Carefully clean the upper surface of the microscope slides to ensure that the second glass plate seals the chamber properly.
- Apply silicon grease to the microscope slide surface. Cover the sediment with a thin water film, whilst carefully avoiding the formation of air bubbles.
- Carefully wash a single shoot of Littorella uniflora and place it on the sediment, with the plant leaves sticking out from the upper open side (Figure 2D).
- Place the second glass plate, with the optode attached to it, on the sediment and apply gentle pressure to bring the optode in close contact with the plant roots and the surrounding sediment.
NOTE: Air bubbles trapped in the sediment can be removed by tilting the glass plates while bringing them together. - Fasten the glass plates together using clamps (Figure 2E). Dry the outer edges with tissue paper. Keep the leaves moisturized throughout the entire assembly of the rhizo-sandwich (e.g., by frequent addition of a few drops of water).
- Tighten the rhizo-sandwich chamber using vinyl electrical tape. Seal the edges with modeling clay and additionally tape them with vinyl electrical tape (Figure 2F).
NOTE: If there are many air bubbles in the sediment, or sediment grains in between the spacer microscope slides and the second glass plate, the chamber should be reassembled as pore water can leak out (repeat steps 2.4 - 2.8). - Use an opaque plastic to cover the rhizo-sandwich, but leave a slit in the foil for the plant leaves to stick out. Cut a window in the plastic foil, so it can be opened for the experiments by unfolding. Close the window during acclimatization times using rubber bands (Figure 2G) to protect the optode from photo bleaching while the plant is incubated.
NOTE: As algal growth could potentially interfere with O2 concentrations measured, we recommend trying to minimize it, by using filtered water, pre-cleaned experimental equipment and removing algae upon formation.
3. Rhizo-sandwich chamber incubation
- Place the rhizo-sandwich chamber in a water tank (32 x 7 x 28 cm3) in a slightly tilted position to encourage root growth against the planar optode.
- Fill the water tank with enough water to fully submerge the plant leaves.
- Establish a 14 h light, 10 h dark cycle for acclimatization of the plant using a time-controlled lamp. Place an air-stone or a water pump in the tank to ensure aeration and mixing of the water (Figure 2H).
4. Imaging
- Imaging setup
- Remove the plastic foil covering the planar optode in the rhizo-sandwich chamber. Position the chamber with the glass wall with the optode upright against the aquarium wall. Use a spacer to press the rhizo-sandwich chamber against the aquarium wall.
NOTE: The overall thickness of the aquarium wall plus the rhizo-sandwich chamber wall should not get too thick, however, glass thicknesses for aquaria walls for luminescence imaging are recommended with > 1 cm, in order to reduce spatial cross-talk by increasing the attenuation of the scattered light. It is important, to use the same material for both glass walls (same refractory index), in order to minimize light scattering at the material interface; as this would lead to a blurred image as well12. - Place the frequency-domain-based luminescence lifetime camera equipped with an objective (see Table of Materials) in front of the aquarium and the area of interest (roots of the aquatic plant Littorella uniflora, which are in direct contact with the planar optode) (Figure 3).
NOTE: The camera might be placed on a lab stand to allow easy height adjustment of the camera. The position of the lab stand should be marked and kept fixed. Additionally, the camera can be taped to the lab stand to avoid accidental movement of the camera during the experiment. - Screw a suitable emission filter for imaging PtTFPP as indicator dye (see Table of Materials) on the camera objective, to remove inferences from the excitation source.
NOTE: Screw-on filters are ideal, but square filters may also be used with either an appropriate adaptor, or by careful taping them to the objective. - Connect a LED excitation source (see Table of Materials) to the modulation and dark gate output of the camera.
NOTE: The former delivers the modulation signal for the light source, while the latter switches off the light during image readout of the image sensor. Connect the LED excitation source and camera to a computer. Background light should be minimized during image readout, by either darkening the entire room or putting a dense, black cloth over the entire set-up. In the latter case, it is important to ensure sufficient ventilation to avoid heating of the camera. - Fix the light guide in the LED excitation source and position it to evenly illuminate the planar optode foil covering the area of interest.
NOTE: In the used LED excitation source it is possible to switch between 3 different LEDs (460 nm, 528 nm, 625 nm), the intensity of which can be adjusted via the control software.
- Remove the plastic foil covering the planar optode in the rhizo-sandwich chamber. Position the chamber with the glass wall with the optode upright against the aquarium wall. Use a spacer to press the rhizo-sandwich chamber against the aquarium wall.
- Settings and camera operation
NOTE: For the described experiments, we used a frequency-domain-based lifetime camera in combination with a dedicated module for lifetime imaging in a commercially available software package (see Table of Materials).- Select the camera in the chosen software prior to use.
NOTE: The software and camera drivers need to be installed prior to imaging following the manufacturers guidelines. - Open the LED control software (again installed prior to starting the experiment) and choose the suitable LED (here: 528 nm) by ticking off standby. Set the LED intensity as needed (here to 30%). Ensure that the LED is triggered by the external TTL; this is done by ticking analog and sync for the LED.
NOTE: The LED intensity needs to be adjusted individually, as too high laser power can lead to accelerated photo bleaching of the indicator or reference dye. - Focus the camera and adjust the aperture of the objective manually (in the present study use f = 2.8).
NOTE: It is important to focus the camera on the planar optode and not on the aquarium glass; this can be ensured by taking an image with a ruler for scale, and focusing on the shadow of the ruler on the optode, rather than on the actual ruler. - Set the following parameters within the software's camera control panel: internal modulation source; sine wave for the output waveform; additional phase sampling (Yes); 8 phase samples, phase order opposite, Tap A + B readout; 5 kHz modulation frequency.
NOTE: These parameters affect image quality and can be changed if needed. The manufacturer of the camera provides guidelines about the individual parameters (The camera manufacturer is releasing guidelines and updates whenever the software gets updated). - Take a reference image prior to experiments.
NOTE: This can be done either by imaging a calibration standard (a luminescent dye with a known lifetime (ns or µs)), or by using the reflected light of the LED. In the latter case, the emission long pass filter needs to be removed from the objective and the known lifetime can be set to 1 ns. - Adjust the exposure time in the calibration section of the dedicated imaging software until the ROI Statistics readout (in the bottom of this panel) for the normalized luminescence intensity image is in the range of 0.68 - 0.72.
NOTE: Now the reference lifetime (e.g., 1 ns) is given as input to the software. - Press Capture reference to start the acquisition of a reference measuring series.
NOTE: When finished, the reference data are stored and either single or time lapse measurements can be done on samples.
- Select the camera in the chosen software prior to use.
- Calibration of the O2 optode
- Position a piece of a planar O2-sensitive optode in a (small) glass aquarium. Fix the planar optode on the glass wall of the calibration chamber as described previously (see section 2.3). Place the calibration aquarium in front of the camera. Ensure even illumination by the LED, as well as that the optode fills the entire field of view.
NOTE: The planar optode should be from the same piece of foil or made from the same sensor cocktail as the foil used in the actual experiment. - Fill the aquarium with the same liquid medium as used in the experiments.
NOTE: Using different media for calibrations and experiments can influence the measurement, (e.g., by changing the sensor response and/or the O2 solubility). Thus, calibration should be done in the same medium, and at the same temperature as the actual experiment. Fluctuations in temperature will affect the luminescence signal and should be avoided. However, if the temperature cannot be kept stable, temperature compensation needs to be done by calibrating the O2-sensitive optode (multiple points) at different (relevant) temperatures and subsequent recalculation of the values. - Adjust the O2 concentration within the calibration aquarium by flushing the water with an air/N2 gas-mixture of known O2 concentration, using a gas-mixing device. Ensure that the water is well equilibrated with the used gas-mixture by aerating for a sufficient time (depends on the flow rate and size of the aquarium).
NOTE: We recommend monitoring the O2 level in the calibration aquarium with an external, calibrated O2 sensor with temperature compensation (e.g., using a fiber optic or electrochemical O2 sensor). - Take a series of images at different O2 concentrations in the calibration chamber.
NOTE: At least five different O2 concentrations should be measured in order to enable a proper curve fit to the acquired calibration data. It is important to measure at 0 hPa (anoxic conditions), and then distribute the other values over the dynamic range of your specific indicator dye. Here we used PtTFPP as the O2-sensitive indicator dye immobilized in a polystyrene matrix. Images were taken at 0, 48, 102, 156, and 207 hPa; 207 hPa corresponds to 100% air saturation at the given salinity and pressure.
- Position a piece of a planar O2-sensitive optode in a (small) glass aquarium. Fix the planar optode on the glass wall of the calibration chamber as described previously (see section 2.3). Place the calibration aquarium in front of the camera. Ensure even illumination by the LED, as well as that the optode fills the entire field of view.
- Imaging the sample
- Place the sample in front of the camera and ensure even illumination.
- Switch off the light supplying irradiation to the plant (and all other light sources) just prior to acquiring the luminescence lifetime image of the plant. Adjust the acquisition time based on the intensity image, ensuring that the signal is neither oversaturated nor too weak for a good signal to noise (S/N) ratio in the lifetime determination.
- Expose the plant to varying light conditions (e.g., light/ dark) and acquire a set of images.
- Switch on the light in the room to acquire a Structural image.
NOTE: When the background light is switched on, the camera will not measure a realistic lifetime image. However, the intensity image now shows the entire field of view as seen through the semitransparent optode. - Take an image with a ruler or alike in the field of view to enable later scaling of the acquired images.
5. Data analysis
- Export the phase lifetime and intensity images directly from the dedicated imaging software, using the macro provided by the camera manufacturer.
- Perform further image analysis using a freely available image analysis software (see Table of Materials).
- Open the phase lifetime images of the calibration in the image analysis software and determine the mean of the entire image using the measure function. Plot the measured lifetimes against the known O2 concentrations to determine the calibration function (Figure 4A).
- Calculate τ0/τ from all the data (τ0 is the measured phase lifetime in the absence of O2). Plot these values versus the known O2 concentrations (Figure 4B).
- Determine the parameters Ksv and f from the calibration plot, using the simplified two-site model for dynamic collisional quenching (equation 3)38,39 where [Q] is the O2 concentration. Define the fit-function in the data analysis software, which then determines Ksv and f.
(3) 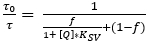
- Open the acquired sample images in the image analysis software to convert the imaged lifetimes into O2 concentrations, using the determined parameters Ksv, f and τ0.
NOTE: As an alternative approach also the acquired calibration phase lifetime values (Figure 4A) can be used directly. In this case, an exponential fit using the curve fit function is used for calibration. - Open the image with the ruler next in the image analysis software and measure a known distance using the measuring tool. Set this measurement as global scale under Set scale.
Results
As an application example for the new imaging system, we show 2D O2 imaging of a complex biological sample (i.e., the rhizosphere of the aquatic plant Littorella uniflora).
First, the method describes the fabrication of a planar sensor film, a so-called planar optode. As seen in Figure 1, such an optode is made of a thin layer of an optical indicator in a polymer matrix that is spread on a transparent support. By following the described protocol, a homogeneous sensor film with a uniform thickness, as defined by the gap of the knife coating device, is obtained. If the produced optode has a patchy sensor material distribution (e.g., holes in the coating, shows stripes, or dye aggregates (this can be evaluated visually, and visually with the help of an UV lamp)), the protocol needs to be repeated and all materials need to be thoroughly cleaned using acetone.
Once the planar optode is prepared, the sample can be brought in close contact with the sensing layer of the planar optode, as shown here with the planar optode integrated in a rhizo-sandwich chamber, where the roots of a plant within a surrounding sediment matrix can be positioned in close contact to the planar optode (Figure 2). If prepared correctly, the rhizo-sandwich chamber should be easily moveable from one aquarium (incubation) to the other (measurement). If not constructed correctly, the rhizo-sandwich chamber may be instable, lose sediment or contain air bubbles. Visual examination of the rhizo-sandwich chamber directly after assembly is thus recommended.
The given protocol enables frequency-domain-based luminescence lifetime imaging of the sample in contact with the planar optode using the frequency-domain-based luminescence lifetime camera. More details on this camera system such as the mode of image acquisition and scientific complementary metal-oxide-semiconductor (SCMOS) camera characteristics are given in recent publications8,29.
The setup itself is rather simple and only includes the camera that controls a light source (in this case, a LED excitation source) and the sample with the optode (Figure 3). Ensure that all parts are correctly connected and that the sample is illuminated homogeneously. Background light needs to be avoided while preforming measurements.
Prior to imaging the sample, the optode needs to be calibrated. As seen in Figure 4A, the measured luminescence lifetime decreases with increasing O2 concentration following a quasi-exponential decay. This relationship can also be described using the simplified two-site model (Figure 4B and equation 3). In the given example, the parameters needed to subsequently calculate the O2 concentration were as followed; τ0 = 56.26 µs, Ksv = 0.032 hPa-1 and f = 0.86.
Performing a calibration is also an ideal way to test that the system is working correctly. If all components are installed as described here (or within the manufacturers guidelines), the measured lifetime should show the same O2 dependence as seen in Figure 4. In addition, for the same combination of O2 sensing materials (polymer and dye), the measured τ0 should be in the same range (± a few µs) as measured here (mainly influenced by the experimental temperature). If unable to obtain a similar calibration curve, ensure that all steps were followed correctly. Sometimes the optode is accidentally fixed with the sensitive side facing the glass wall rather than the sample, or the acquired images are over- or underexposed.
With the calibration parameters, it is possible to determine the O2 concentration by imaging the luminescence lifetime (τ). This is demonstrated in Figure 5A,B, where the distribution of O2 concentration in the rhizosphere of Littorella uniflora was imaged in darkness and after light exposure to 500 µmol photons m-2 s-1 for 12 h, respectively. Due to the photosynthetic activity of the plant, the O2 concentration in the rhizosphere increased after light exposure. Besides lifetime images, also "structural" images can be acquired under external illumination, while keeping the imaging geometry fixed. In this way, O2 images can be precisely correlated to the structural image (Figure 5C), cross sections or regions of interest. As an example, O2 concentration profiles across a single root were extracted from the image acquired in darkness and light, respectively (Figure 5D).
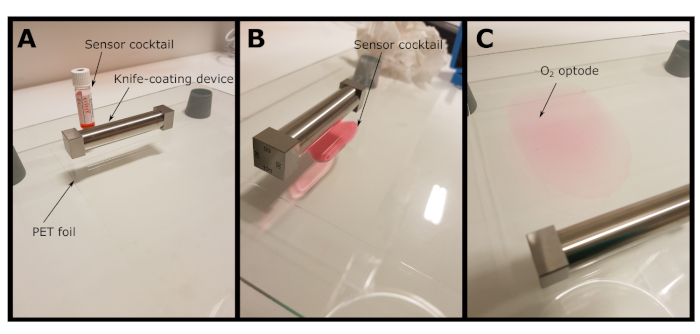
Figure 1: Fabrication of a planar O2 optode. (A) A PET foil is fixed on a glass plate and the knife-coating device is placed on the foil. (B) The prepared sensor cocktail is spread on the PET foil as a thin line in front of the knife-coating device. (C) The knife-coating device is moved downwards to spread the sensor cocktail as a thin film on the PET foil, which after solvent evaporation results in a ready to use planar optode. Please click here to view a larger version of this figure.
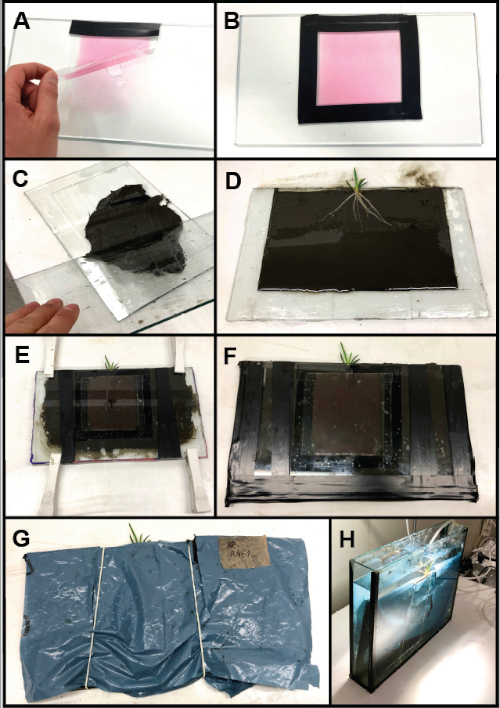
Figure 2: Rhizo-sandwich chamber assembly with integration of a planar O2 optode. (A) The optode is fixed on one of the glass plates using a water film. (B) The optode is glued to the plate with electric tape. (C) Sediment is filled into the opposing plate with the attached spacers (i.e., microscope slides). (D) The plant roots are placed on the evenly spread out sediment. (E) The rhizo-sandwich chamber is closed and temporarily fixed with clamps. (F) Fully closed and assembled rhizo-sandwich chamber. (G) To protect the optode from light exposure by the incubation lamp and to avoid algal growth a plastic cover is placed over the assembled rhizo-sandwich chamber. (H) The rhizo-sandwich chamber incubated in an aquarium. Please click here to view a larger version of this figure.
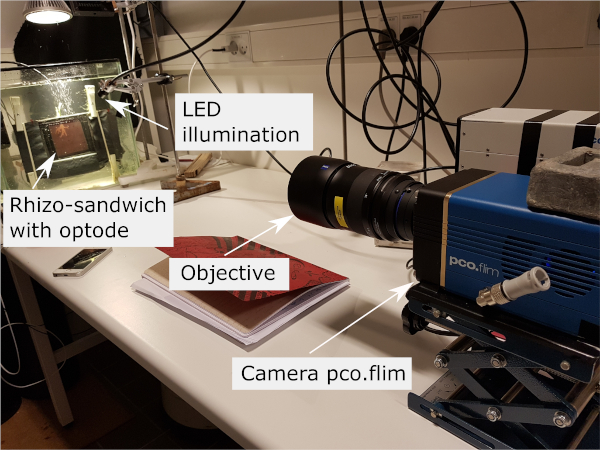
Figure 3: Imaging setup containing the frequency-domain-based luminescence lifetime camera, with the objective focused at the sample with the optode from behind via the transparent aquarium and rhizo-sandwich chamber walls. The light guide of the LED excitation source is positioned to illuminate the sample evenly. Please click here to view a larger version of this figure.
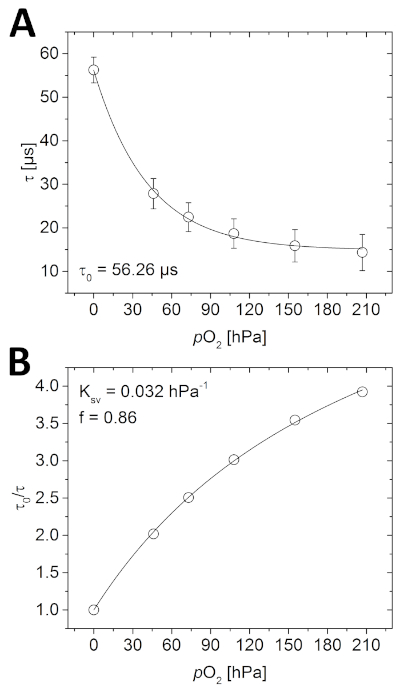
Figure 4: Calibration curves for planar O2 optode. (A) Different phosphorescence lifetimes measured at the respective O2 concentrations in the water-filled calibration chamber. (B) Stern-Volmer plot of the calibration data fitted using the simplified two-site model for dynamic collisional quenching (equation 3). Please click here to view a larger version of this figure.
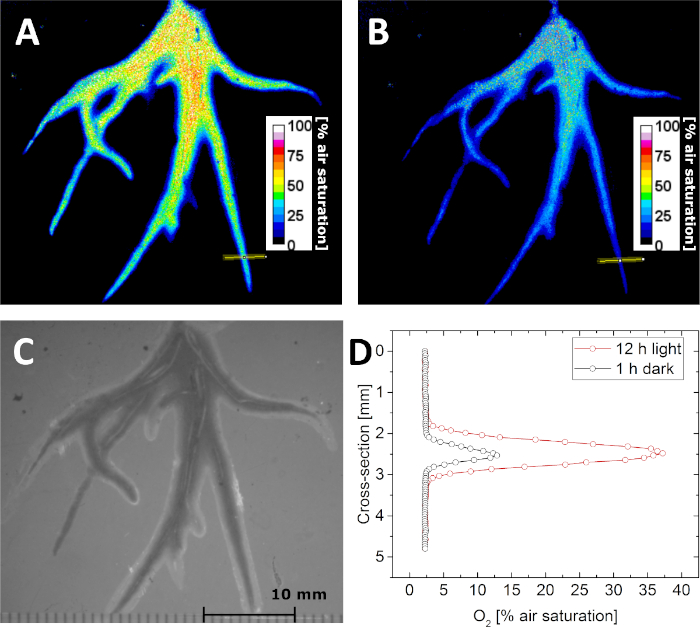
Figure 5: Lifetime imaging of the O2 distribution in the rhizosphere of the aquatic plant Littorella uniflora. (A) O2 distribution after keeping the plant under light for 12 h at approximately 500 µmol photons m-2 s-1. (B) O2 distribution after keeping the plant in darkness for 1 h. (C) Structural image of the plant roots as seen through the planar optode. (D) Cross-sectional O2 concentration profile (the location is indicated by the yellow line in panel A and B) after 12 h in light (red) and 1 h in darkness (black). Adapted with permission from (Koren, K., Moßhammer, M., Scholz, V. V., Borisov, S.M., Holst, G., Kühl, M. Luminescence Lifetime Imaging of Chemical Sensors - A Comparison between Time-Domain and Frequency-Domain Based Camera Systems. Analytical Chemistry. 91 (5), 3233-3238, doi: 10.1021/acs.analchem.8b05869 (2019)). Copyright (2019) American Chemical Society. Please click here to view a larger version of this figure.
Discussion
In this protocol, the entire work-flow from optode preparation to O2 image analysis is covered. By following this protocol, chemical images can be obtained using the novel frequency-domain-based luminescence lifetime camera. Depending on the application, the planar optodes can be fabricated in various sizes and layer thickness of the sensor layer ranging from robust 50-100 µm thick planar optodes of several tenths of square centimeter to microscope cover slips with <1 µm thick sensor layers6,40. The potential of this method was demonstrated with a particular application, but is not only limited to O2 imaging in plant rhizospheres12,28.
This method has several benefits when compared to pure luminescence intensity-based chemical imaging methods. Luminescence lifetime imaging is not, or at least far less, affected by uneven illumination, uneven optode thickness, and photo bleaching25. Also, this method avoids the use of an additional reference dye common in ratiometric imaging17,37. In comparison to other lifetime based camera systems, such as commonly used gated time-domain cameras8,26, the novel camera system and protocol presented here can deliver comparable results. In a recent publication, the analytical characteristics of those two systems were compared and it was found that the frequency-domain-based luminescence lifetime camera system is at least comparable to the discontinued time-domain-based predecessor8.
We have presented the simplest O2 optode consisting only of an indicator in a polymer matrix. Besides multiple other possible O2 indicators20 that might be used additives can be included, i.e., scattering agents such as TiO2 or diamond powder2 to increase sensor signal while reducing transparency of the optode. Also additional dyes might be used to enhance signal intensity via energy transfer41.
For planar optode fabrication, we recommend using a gap in the knife-coating device of 75 - 120 µm to yield a final sensor layer thickness of around 7.5 to 12 µm after solvent evaporation (around 10% of the used gap), when using the described sensor cocktail composition. This is a good compromise between signal intensity, which can be modified by higher dye loading, or by choosing indicator and reference dyes of higher brightness, and response time. An increase in layer thickness results in an increase in response time, as the time span required for the analyte to reach a thermodynamic equilibrium in the sensing layer with the surrounding media increases12.
Optodes, as described here, react to changes in O2 concentration within a few seconds17 while still having a sufficiently strong luminescence signal. Ultrathin sensor coatings with sub-second response times can be realized with spin-coating6. If the support or the knife-coating device are not well-cleaned, it might result in inhomogeneous sensor layers. Also, when the cocktail is not completely homogenous or applied too rapidly after spreading in front of the coating device such an unwanted result can be observed. Therefore, it might need some practice to prepare optimal optodes.
The method can be used to image samples which can be put in close contact to the optode, such as certain marine animals42, biofilms6 and soils31 just to name a few. We present a standalone setup using an objective, however, the camera can easily be coupled to a microscope for higher resolution chemical imaging43.
While time-domain based luminescence lifetime imaging enabled suppression of background fluorescence26, this is an issue when using the new frequency-domain-based camera system8. Due to the continuous image acquisition, this camera will record any background fluorescence of the sample that can be excited by the selected LED and emits in the selected spectral window as defined by the emission filter on the camera objective. This will result in an apparently lower lifetime and consequently in false readings. In case you work with samples with a significant intrinsic fluorescence overlapping with the O2 sensor excitation and emission, it is essential to apply an extra optical isolation on top of the optode, by coating an additional layer containing carbon black2,17. Thus, only luminescence emitted from the planar optode will reach the camera. In order to check for background luminescence an image without the optode can be taken, which then would exclusively show intrinsic luminescence of the sample. It is also possible to add scattering agents such as TiO2 or diamond powder2,44, to the sensor cocktail, to increase luminescence intensity of the indicator dye. However, this can also lead to faster photo bleaching and TiO2 is a known photo-catalyst, which can impair photostability of a dye41. A further aspect to consider is background light. When imaging luminescence lifetimes, background light needs to be avoided as efficiently as possible. Therefore, this imaging method requires the setup to be placed in a dark environment and any external light source needs to be temporarily switched off during image acquisition.
In summary, luminescence lifetime imaging is a robust chemical imaging method that can be adapted to many different applications. This protocol (see section 1 - 5) covers all the essential steps to generate an O2 image and uses the currently most flexible frequency-domain luminescence lifetime imaging system, which can replace the discontinued gated time-domain camera for 2D O2 imaging with planar optodes.
Disclosures
The author Gerhard Holst is an employee of PCO AG which manufactures the camera system used in this article. PCO AG financially contributed to the publication and open access costs of this article.
Acknowledgements
We thank Sofie Lindegaard Jakobsen (University of Copenhagen) and Lars Borregaard Pedersen (Aarhus University) for technical assistance. Funding for this study was obtained from a Sapere-Aude Advanced grant from the Independent Research Fund Denmark (DFF-1323-00065B; MK), project grants from the Independent Research Fund Denmark | Natural Sciences (DFF-8021-00308B; MK) & Technical and Production Sciences (DFF-8022-00301B and DFF-4184-00515B; MK), the Danish National Research Foundation (DNRF136), and the Poul Due Jensen Foundation (KK).
Materials
| Name | Company | Catalog Number | Comments |
| Air pump with air stone and water pump | Local aquarium store | ||
| Chloroform | Sigma Aldrich | 67-66-3 | |
| DC4 silicone compound | Dow Corning GmbH | 2793695 | |
| Gas mixer | Vögtlin Instruments GmbH | red-y compact meter GCM | This is just one possible instrument. Several companies offer gas mixing devices |
| Glass plates and aquaria | Local aquarium or hardware store | ||
| ImageJ Software | ImageJ | Freely available imaging software (imagej.nih.gov/ij/index.html) | |
| Knife-coating device | BYK-GARDNER GMBH byk.com | 2021 | This is a four sided film applicator enabling easy variation of the film thickness. Other versions are also available. We recommend a thickness of the applied film between 75-120 µm, which yields a final sensor layer thickness of ~10% of the applied thickness before solvent evaporation. |
| LED lamp, Reflector PAR38 | Megaman | MM17572 | |
| LED LEDHUB | Omicon Laserage, Germany | Can be configured with a variety of LEDs. For the presented example, the green LED (528 nm) is essential | |
| LOCTITE AA 3494 | Henkel AG & Co. KGaA | NA | Acrylic-based instant adhesive |
| NIS Elements AR Software | Nikon Inc | Software package used for image acquisition | |
| pco.flim | PCO AG, Germany | Frequency domain based luminescence lifetime camera | |
| platinum(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin (PtTFPP) | Frontier Scientific | PtT975 | O2 indicator |
| polyethylene terephthalate (PET) foil | Goodfellow | 320-992-72 | Such foils might also be found from other providers and serve as solid support |
| Polystyrene (PS) | Sigma Aldrich | 9003-53-6 | Polymer matrix |
| Schott RG610 filter | www.uviroptics.com | Here 52mm screw on Filters can obtained. Other sources offer square glass filters from Schott glass that can be fixed in front of the objective | |
| Vinyl electrical tape | Scotch, Super 33+ | NA | |
| Zeiss Makro Planar 2/100 with Hama C for Nikon adaptor | delivered with the camera | Here any other objective might also be used in combination with an adaptor if the objective does not have a C-mount |
References
- Glud, R. N., Kühl, M., Kohls, O., Ramsing, N. B. Heterogeneity of oxygen production and consumption in a photosynthetic microbial mat as studied by planar optodes. Journal of Phycology. 35 (2), 270-279 (1999).
- Moßhammer, M., Strobl, M., Kühl, M., Klimant, I., Borisov, S. M., Koren, K. Design and Application of an Optical Sensor for Simultaneous Imaging of pH and Dissolved O2 with Low Cross-Talk. ACS Sensors. 1 (6), 681-687 (2016).
- Jensen, S. I., Kühl, M., Glud, R. N., Jørgensen, L. B., Priemé, A. Oxic microzones and radial oxygen loss from roots of Zostera marina. Marine Ecology Progress Series. , 49-58 (2005).
- Larsen, M., Santner, J., Oburger, E., Wenzel, W. W., Glud, R. N. O2 dynamics in the rhizosphere of young rice plants (Oryza sativa L.) as studied by planar optodes. Plant and Soil. 390 (1-2), 279-292 (2015).
- Brodersen, K. E., Koren, K., Moßhammer, M., Ralph, P. J., Kühl, M., Santner, J. Seagrass-Mediated Phosphorus and Iron Solubilization in Tropical Sediments. Environmental Science and Technology. 51, 14155-14163 (2017).
- Kühl, M., Rickelt, L. F., Thar, R. Combined imaging of bacteria and oxygen in biofilms. Applied and Environmental Microbiology. 73 (19), 6289-6295 (2007).
- Sønderholm, M., et al. Tools for studying growth patterns and chemical dynamics of aggregated Pseudomonas aeruginosa exposed to different electron acceptors in an alginate bead model. npj Biofilms and Microbiomes. 3, 1-11 (2018).
- Koren, K., Moßhammer, M., Scholz, V. V., Borisov, S. M., Holst, G., Kühl, M. Luminescence Lifetime Imaging of Chemical Sensors - A Comparison between Time-Domain and Frequency-Domain Based Camera Systems. Analytical Chemistry. 91 (5), 3233-3238 (2019).
- Brodersen, K. E., Koren, K., Lichtenberg, M., Kühl, M. Nanoparticle-based measurements of pH and O2 dynamics in the rhizosphere of Zostera marina L.: effects of temperature elevation and light-dark transitions. Plant, Cell & Environment. 39 (7), 1619-1630 (2016).
- Zhu, Q., Aller, R. C., Fan, Y. High-Performance Planar pH Fluorosensor for Two-Dimensional pH Measurements. in Marine Sediment and Water. Environmental Science & Technology. 39, 8906-8911 (2005).
- Murniati, E., Gross, D., Herlina, H., Hancke, K., Glud, R. N., Lorke, A. Oxygen imaging at the sediment-water interface using lifetime-based laser induced fluorescence (τLIF) of nano-sized particles. Limnology and Oceanography: Methods. 14 (8), 506-517 (2016).
- Santner, J., Larsen, M., Kreuzeder, A., Glud, R. N. Two decades of chemical imaging of solutes in sediments and soils - a review. Analytica Chimica Acta. , 9-42 (2015).
- Glud, R. N. Oxygen dynamics of marine sediments. Marine Biology Research. 4 (4), 243-289 (2008).
- Revsbech, N. P., Jorgensen, B. B., Blackburn, T. H. Oxygen in the Sea Bottom Measured with a Microelectrode. Science. 207 (4437), 1355-1356 (1980).
- Klimant, I., Meyer, V., Kuhl, M. Fiberoptic oxygen microsensors, a new tool in aquatic biology. Limnology and Oceanography. 40 (6), 1159-1165 (1995).
- Glud, R. N., Tengberg, A., Kühl, M., Hall, P. O. J., Klimant, I., Holst, G. An in situ instrument for planar O2 optode measurements at benthic interfaces. Limnology and Oceanography. 46 (8), 2073-2080 (2001).
- Larsen, M., Borisov, S. M., Grunwald, B., Klimant, I., Glud, R. N. A simple and inexpensive high resolution color ratiometric planar optode imaging approach: application to oxygen and pH sensing. Limnology and Oceanography: Methods. 9, 348-360 (2011).
- Glud, R., Ramsing, N., Gundersen, J., Klimant, I. Planar optrodes:a new tool for fine scale measurements of two-dimensional O2 distribution in benthic communities. Marine Ecology Progress Series. 140, 217-226 (1996).
- Frederiksen, M. S., Glud, R. N. Oxygen dynamics in the rhizosphere of Zostera marina: A two-dimensional planar optode study. Limnology and Oceanography. 51 (2), 1072-1083 (2006).
- Quaranta, M., Borisov, S. M., Klimant, I. Indicators for optical oxygen sensors. Bioanalytical Reviews. 4, 115-157 (2012).
- Koren, K., Hutter, L., Enko, B., Pein, A., Borisov, S. M., Klimant, I. Tuning the dynamic range and sensitivity of optical oxygen-sensors by employing differently substituted polystyrene-derivatives. Sensors and Actuators B: Chemical. 176 (100), 344-350 (2013).
- Borisov, S. M. Fundamentals of Quenched Phosphorescence O2 Sensing and Rational Design of Sensor Materials. Quenched-phosphorescence Detection of Molecular Oxygen: Applications in Life Sciences. , 1-18 (2018).
- Wang, X., Wolfbeis, O. S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chemical Society Reviews. 43, 3666-3761 (2014).
- Ehgartner, J., Wiltsche, H., Borisov, S. M., Mayr, T. Low cost referenced luminescent imaging of oxygen and pH with a 2-CCD colour near infrared camera. The Analyst. 139 (19), 4924 (2014).
- Meier, R. J., Fischer, L. H., Wolfbeis, O. S., Schäferling, M. Referenced luminescent sensing and imaging with digital color cameras: A comparative study. Sensors and Actuators B: Chemical. 177, 500-506 (2013).
- Holst, G., Kohls, O., Klimant, I., König, B., Kühl, M., Richter, T. A modular luminescence lifetime imaging system for mapping oxygen distribution in biological samples. Sensors and Actuators B. 51, 163-170 (1998).
- Moßhammer, M., Brodersen, K. E., Kühl, M., Koren, K. Nanoparticle- and microparticle-based luminescence imaging of chemical species and temperature in aquatic systems: a review. Microchimical Acta. , 1-28 (2019).
- Koren, K., Kühl, M. CHAPTER 7. Optical O2 Sensing in Aquatic Systems and Organisms. Quenched-phosphorescence Detection of Molecular Oxygen: Applications in Life Sciences. 1, 145-174 (2018).
- Chen, H., Holst, G., Gratton, E. Modulated CMOS camera for fluorescence lifetime microscopy. Microscopy Research and Technique. 78, 1075-1081 (2015).
- Franke, R., Holst, G. A. Frequency-domain fluorescence lifetime imaging system (pco.flim) based on a in-pixel dual tap control CMOS image sensor. Proceedings of SPIE 93281, Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XIII. , 1-19 (2015).
- Williams, P. N., et al. Localized flux maxima of arsenic, lead, and iron around root apices in flooded lowland rice. Environmental Science and Technology. 48 (15), 8498-8506 (2014).
- Schreml, S., et al. 2D luminescence imaging of physiological wound oxygenation. Experimental dermatology. 20 (7), 550-554 (2011).
- Trampe, E., et al. Functionalized Bioink with Optical Sensor Nanoparticles for O2 Imaging in 3D-Bioprinted Constructs. Advanced Functional Materials. 1804411, 1804411 (2018).
- Gouterman, M. Oxygen Quenching of Luminescence of Pressure Sensitive Paint for Wind Tunnel Research. Journal of Chemical Education. 74 (6), 697 (1997).
- Fischer, L. H., et al. Referenced dual pressure- and temperature-sensitive paint for digital color camera read out. Chemistry. 18 (49), 15706-15713 (2012).
- Fabricius-Dyg, J., Mistlberger, G., Staal, M., Borisov, S. M., Klimant, I., Kühl, M. Imaging of surface O2 dynamics in corals with magnetic micro optode particles. Marine Biology. 159 (7), 1621-1631 (2012).
- Koren, K., Jakobsen, S. L., Kühl, M. In-vivo imaging of O2 dynamics on coral surfaces spray-painted with sensor nanoparticles. Sensors and Actuators B: Chemical. 237, 1095-1101 (2016).
- Carraway, E. R., Demas, J. N., DeGraff, B. A., Bacon, J. R. Photophysics and Photochemistry of Oxygen Sensors Based on Luminescent Transition-Metal Complexes. Analytical Chemistry. 63 (4), 337-342 (1991).
- Klimant, I., Ruckruh, F., Liebsch, G., Stangelmayer, A., Wolfbeis, O. S. Fast response oxygen micro-optodes based on novel soluble ormosil glasses. Mikrochimica Acta. 131, 35-46 (1999).
- Askaer, L., Elberling, B., Glud, R. N., Kühl, M., Lauritsen, F. R., Joensen, H. P. Soil heterogeneity effects on O2 distribution and CH4 emissions from wetlands: In situ and mesocosm studies with planar O2 optodes and membrane inlet mass spectrometry. Soil Biology and Biochemistry. 42 (12), 2254-2265 (2010).
- Mayr, T., Borisov, S. M., Abel, T., Enko, B., Waich, K. Light Harvesting as a Simple and Versatile Way to Enhance Brightness of Luminescent Sensors. Analytical Chemistry. 81, 6541-6545 (2009).
- Kühl, M., et al. Microenvironmental Ecology of the Chlorophyll b-Containing Symbiotic Cyanobacterium Prochloron in the Didemnid Ascidian Lissoclinum patella. Frontiers in microbiology. 3, 1-18 (2012).
- Dalfen, I., Dmitriev, R. I., Holst, G., Klimant, I., Borisov, S. M. Background-Free Fluorescence-Decay-Time Sensing and Imaging of pH with Highly Photostable Diazaoxotriangulenium Dyes. Analytical Chemistry. 91 (1), 808-816 (2019).
- Chatni, M. R., Maier, D. E., Porterfield, D. M. Evaluation of microparticle materials for enhancing the performance of fluorescence lifetime based optrodes. Sensors and Actuators B: Chemical. 141, 471-477 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved