A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Measurement of 3-Dimensional cAMP Distributions in Living Cells using 4-Dimensional (x, y, z, and λ) Hyperspectral FRET Imaging and Analysis
In This Article
Summary
Due to inherent low signal-to-noise ratio (SNR) of Fӧrster resonance energy transfer (FRET) based sensors, measurement of cAMP signals has been challenging, especially in three spatial dimensions. Here, we describe a hyperspectral FRET imaging and analysis methodology that allows measurement of cAMP distribution in three spatial dimensions.
Abstract
Cyclic AMP is a second messenger that is involved in a wide range of cellular and physiological activities. Several studies suggest that cAMP signals are compartmentalized, and that compartmentalization contributes to signaling specificity within the cAMP signaling pathway. The development of Fӧrster resonance energy transfer (FRET) based biosensors has furthered the ability to measure and visualize cAMP signals in cells. However, these measurements are often confined to two spatial dimensions, which may result in misinterpretation of data. To date, there have been only very limited measurements of cAMP signals in three spatial dimensions (x, y, and z), due to the technical limitations in using FRET sensors that inherently exhibit low signal to noise ratio (SNR). In addition, traditional filter-based imaging approaches are often ineffective for accurate measurement of cAMP signals in localized subcellular regions due to a range of factors, including spectral crosstalk, limited signal strength, and autofluorescence. To overcome these limitations and allow FRET-based biosensors to be used with multiple fluorophores, we have developed hyperspectral FRET imaging and analysis approaches that provide spectral specificity for calculating FRET efficiencies and the ability to spectrally separate FRET signals from confounding autofluorescence and/or signals from additional fluorescent labels. Here, we present the methodology for implementing hyperspectral FRET imaging as well as the need to construct an appropriate spectral library that is neither undersampled nor oversampled to perform spectral unmixing. While we present this methodology for measurement of three-dimensional cAMP distributions in pulmonary microvascular endothelial cells (PMVECs), this methodology could be used to study spatial distributions of cAMP in a range of cell types.
Introduction
Cyclic adenosine monophosphate (cAMP) is a second messenger involved in key cellular and physiological processes including cell division, calcium influx, gene transcription, and signal transduction. A growing body of evidence suggests the existence of cAMP compartments in the cell through which signaling specificity is achieved1,2,3,4,5,6,7. Until recently, cAMP compartmentalization was inferred based upon distinct physiological or cellular effects induced by different G-coupled receptor agonists8,9,10,11. More recently, FRET based fluorescence imaging probes have provided new approaches for the direct measurement and observation of cAMP signals in a cell12,13,14.
Förster resonance energy transfer (FRET) is a physical phenomenon in which energy transfer occurs between donor and acceptor molecules in a non-radiative fashion when the molecules are in close proximity15,16. With the development of FRET based fluorescent indicators, this physical phenomenon has been used in biological applications to study protein-protein interactions17, protein co-localization18, Ca+2 signaling19, gene expression20, cell division21 and cyclic nucleotide signaling. FRET based cAMP indicators typically consist of a cAMP binding domain, a donor fluorophore and an acceptor fluorophore22. For example, the H188 cAMP sensor12,22 used in this methodology consists of a cAMP binding domain obtained from Epac, sandwiched between Turquoise (donor) and Venus (acceptor) fluorophores. At basal conditions (unbound), Turquoise and Venus are in an orientation such that FRET occurs between the fluorophores. Upon binding of cAMP to the binding domain, a conformational change occurs such that Turquoise and Venus move apart resulting in a decrease in FRET.
FRET based imaging approaches offer a promising tool for investigating and visualizing cAMP signals within a cell. However, current FRET based microscopic imaging techniques are often only partially successful in achieving sufficient signal strength to measure FRET with subcellular spatial clarity. This is due to several factors, including the limited signal strength of many FRET reporters, the high level of precision required to accurately quantify changes in FRET efficiency, and the presence of confounding factors, such as cellular autofluorescence23,24. The result is often a FRET image that is plagued by weak SNR, making visualization of subcellular changes in FRET very difficult. In addition, investigation of spatially localized cAMP signals has been almost exclusively performed in only two spatial dimensions and the axial cAMP distribution has been rarely considered25. This is likely because low SNR impeded the ability to measure and visualize cAMP gradients in three spatial dimensions. To overcome limitations of using FRET sensors with low SNR, we have implemented hyperspectral imaging and analysis approaches to measure FRET in single cells25,26,27.
Hyperspectral imaging approaches were developed by NASA to differentiate terrestrial objects present in satellite images28,29. These techniques have since been translated to the fluorescence microscopy field30, with several commercial confocal microscope systems offering spectral detectors. In traditional (non-spectral) fluorescence imaging, the sample is excited using a band-pass filter or a laser line, and the emission is collected using a second band-pass filter, often selected to match the peak emission wavelength of the fluorophore(s). By contrast, hyperspectral imaging approaches seek to sample a complete spectral profile of either the fluorescence emission26,31,32 or excitation33,34 at specific wavelength intervals. In our previous studies, we showed that hyperspectral imaging and analysis approaches can offer improved quantification of FRET signals in cells when compared to traditional filter-based FRET imaging techniques26. Here, we present a methodology for performing 4-dimensional (x, y, z, and λ) hyperspectral FRET imaging and analysis to measure and visualize cAMP distributions in three spatial dimensions. These approaches have allowed visualization of agonist-induced cAMP spatial gradients in single cells25. Interestingly, depending on the agonist, cAMP gradients may be apparent in cells. The methodology presented here utilizes spectral unmixing of non-uniform background and cellular autofluorescence to improve the accuracy of the FRET measurements. While this methodology is demonstrated in pulmonary microvascular endothelial cells (PMVECs) using a cAMP FRET biosensor, the methodology could easily be modified for use with alternative FRET reporters or alternative cell lines.
Protocol
This protocol follows procedures approved by the University of South Alabama Institutional Animal Care and Use Committee.
1. Cell, sample, and reagent preparation for imaging
- Isolate rat pulmonary microvascular endothelial cells (PMVECs) as described previously35.
NOTE: Cells were isolated and cultured by the Cell Culture Core at the University of South Alabama, Mobile, AL on 100 mm cell culture dishes. - Seed isolated PMVECs on 25 mm round glass coverslips and let them grow in the incubator at 37 °C until cells attain at least 80% confluency (at least 24 hours).
NOTE: Cells and cell type may vary from study to study and hence cell-specific cell culture procedures should be followed to seed and grow cells. The cell seeding and culturing protocol used in these studies is available as supplemental information in the file named “Supplemental File_Cell Culture and Transfection”. - Transfect PMVECs with a FRET biosensor and incubate for 48 hours at 37 °C.
NOTE: The protocol to transfect PMVECs is also described in the supplemental information file named “Supplemental File_Cell Culture and Transfection”. - On the day of imaging, warm Tyrode’s buffer to 37 °C in a water bath.
NOTE: Tyrode’s buffer consists of 145 mM NaCl, 4 mM KCl, 10 mM HEPES, 10 mM Glucose, 1 mM MgCl2 and 1 mM CaCl2 - Mount a coverslip containing transfected cells into a cell chamber and secure the top with a mounting gasket to prevent leaking.
- Wipe the bottom of the coverslip using a delicate task wipe to clean any excess media or adherent cells.
- Add 800 µL of working buffer and 4 µL of 5 mM nuclear label to the cell chamber and gently rock for 5 – 10 seconds.
NOTE: When adding buffer or reagent solutions to coverslips mounted in the cell chamber, make sure to add the solution gently and at the side of the cell chamber so as not to dislodge adherent cells. Adding 4 µL of 5 mM nuclear label to 800 µL of buffer makes 25 µM final concentration of nuclear label. For loosely adherent cells such as HEK293 cells, mix nuclear label and buffer in a vial first and then add to coverslips mounted in the cell chamber. This will prevent lifting the cells off the coverslip. - Cover the cell chamber with aluminum foil to protect from light and incubate for 10 minutes at room temperature.
- Reagent Preparation: Add 1 µL of 50 mM forskolin to 199 µL of buffer. This will produce a final concentration of forskolin of 50 µM when added to cells that were prepared with 800 µL of buffer. 1 µL of DMSO in 199 µL of buffer should also be prepared to be used as a vehicle control.
NOTE: In these studies, forskolin is used as an adenylyl cyclase activator to stimulate cAMP production. If desired, this methodology can easily be modified to allow treatment with alternative reagents for stimulating or inhibiting adenylyl cyclase, phosphodiesterases, etc.
2. Image acquisition
- Use a confocal microscope equipped with a spectral detector.
NOTE: All image acquisition steps outlined here were developed using a commercially available Nikon A1R microscope system. These steps may need to be adjusted if using an alternative spectral microscope. Ensure that all equipment is turned on at least 30 minutes prior to the start of the experiment so as to reach stable operating conditions. - Select the 60x water immersion objective and add a drop of water to the objective.
NOTE: For high-resolution live-cell imaging, it is recommended to use a high numerical aperture objective. Please refer to the List of Materials for information about the objective used in these studies. - Place the loaded cell chamber (from step 1.7) onto the microscope stage.
- Select the DFT (DAPI/FITC/TRITC) filter set by tuning the filter knob on the right side of the microscope.
- Operate the microscope in fluorescence widefield mode using the eyepieces to select a field of view containing cells expressing the cAMP FRET sensor.
NOTE: Ensure that the average intensity of the FRET signal at the donor or acceptor emission peak wavelength in the selected cell is at least 100 intensity units (A.U.) or at least 4X the baseline signal of a region with no expressing cells. This can be confirmed using the spectrum profile viewer available in NIS Elements software. When looking for a cell with good signal, it is advisable to discard excessively bright cells (they may be compromised). - Open NIS software, switch to confocal mode, unlock the laser interlock button and click on Live.
- Use the focus knob to focus on the cells by looking at the preview on the screen.
- Configure device, acquisition, and z-stack settings in the software, as described below.
- Acquisition settings:
NOTE: Camera and device acquisition settings can be applied using a previously acquired image. Open the image, right click and select Reuse Camera Settings.- Open the A1 settings menu, check the boxes corresponding to 405 nm and 561 nm laser lines, select SD for spectral detector, select 10 for resolution and 31 for channels.
NOTE: A1 settings menu is shown as a small gear icon on the top left corner of the A1 Plus Settings window. 405 nm laser is used for donor excitation and 561 nm laser is used for nuclear label excitation. - Set the wavelength range (410 – 730 nm) by selecting start and end wavelength values.
- Click the binning/skip icon in the A1 settings menu and select the box that is numbered 15, then click OK on the A1 settings menu.
NOTE: This is to remove the wavelength channel that corresponds to the 561 nm excitation laser (this is typically the 15th wavelength channel). It is important not to use this wavelength band to avoid an artificially low signal, which can create a spectral artifact. The signal is lower in this band because of the mechanical finger that covers the detector element to protect it from laser damage. - Set the laser intensities to 8% and 2% for the 405 nm and 561 nm lasers, respectively, Si Hv (detector gain) at 149, and a pinhole radius of 2.4 airy disk units (AU).
NOTE: Laser intensities may have to be adjusted depending on the age of the instrument and condition of the lasers. If adjusting laser intensities between different samples or experimental groups, it is important to maintain the same ratio of laser intensities (e.g., 8:2). In addition, it is important to select a laser intensity that is not so bright as to create rapid photobleaching. The detector gain should be adjusted to maximize signal intensity while minimizing detector noise. For these studies, a gain of 149 was used. A pinhole size of 2.4 AU was selected as a balance between acquiring images with sufficient signal to noise ratio (SNR) and maintaining optical sectioning (confocality). An increase in pinhole size increases SNR but decreases confocality. - Set the scan speed to 0.25 spectral frames per second, select the icon corresponding to unidirectional for scan direction, enter 4 for the count, and set 1024 x 1024 for scan size.
NOTE: FRET signals are weak, and a slow scan speed is often required. Using scan speed of 0.25, acquisition of a spectral z-stack is completed in ~3 minutes. Scan speed can be increased or decreased depending on the fluorophores used. For example, for brighter fluorophores like eGFP, faster scan speed (2 frames/second) can be used. The number entered under count corresponds to a frame averaging value of 4, which helps in noise reduction during image acquisition. For very stable samples and where time is not a constraint, higher averaging values (up to 16) can be used to obtain images with improved SNR.
- Open the A1 settings menu, check the boxes corresponding to 405 nm and 561 nm laser lines, select SD for spectral detector, select 10 for resolution and 31 for channels.
- Define z-stack acquisition parameters:
NOTE: The values entered in steps 2.10 may need to be adjusted to accommodate changes in fluorescent label binding or concentration, type of label, number of labels used, cell line, and other changes in sample preparation that may affect cell labeling density and/or cellular autofluorescence. When adjusting acquisition parameters, care should be taken to achieve a sufficient SNR while minimizing photobleaching. In addition, when configuring a spectral FRET assay, care should be taken to ensure that parameters work well across all treatment groups. It is advisable to run a trial of each treatment group with the proposed parameter settings to ensure that SNR is sufficient and photobleaching is minimized.- Open the ND acquisition window by clicking view → acquisition control → ND acquisition.
- Enter the path/destination and a file name to save the ND file on the popup window.
- Check the box corresponding to z-series.
- Click on live in the A1 Plus Settings window. This will open a live viewing window.
- Adjust the focus knob on the microscope to select the top of the cell and click Top in the ND acquisition window to set the current position as the top.
NOTE: It is suggested to focus slightly above the top of the cell to ensure that all of the cell is sampled in the z series. - Adjust the focus knob on the microscope to select the bottom of the of the cell and click on Bottom in the ND acquisition window to set the current position as the bottom.
NOTE: Focus slightly below the bottom of the cell to ensure that all of the cell is sampled. - Enter 1 µm for step size, select top-bottom for the z-scan direction and click run on the ND Acquisition window to acquire a z-stack.
NOTE: Step size determines the number of z-slices that will be acquired depending on top and bottom locations (i.e., the distance traveled). A 1 µm step size was selected as a compromise between imaging speed, z-axis sampling, and photobleaching. Using the confocal pinhole diameter of 2.4 AU and the 60x water immersion objective resulted in optical section thickness of 1.73 µm. Hence, a 1 µm step size is slightly below the Nyquist sampling criteria, but this is a compromise that was made to reduce the time needed to acquire a z-stack. For very stable samples, for which speed is not critical, a smaller z-axis step and possibly a smaller confocal pinhole diameter may be used to increase z-axis resolution. Bottom-top should yield similar results and can be used to evaluate any effects of photobleaching that may occur during the z scan.
- Set up the Perfect Focus System (PFS) if available:
NOTE: PFS allows the system to compensate for fluctuations in the focal depth during image acquisition. The following steps may be used to set up PFS, and these steps may vary slightly depending on the version of the Nikon A1R and the version of NIS Elements used.- Highlight symmetric mode defined by the range icon in the ND acquisition window.
- Turn on the PFS button on the front face of the microscope (make sure the dichroic mirror knob located on the section below the sample stage is ‘in’).
- Redefine the top (rotate counterclockwise) and bottom (rotate clockwise) using the knob on the front face of the PFS offset controller.
- Define a relative z-position/z-depth by clicking ‘relative’ on the ND acquisition window.
- Click memory on the front face of the microscope so that the software memorizes the relative z-depth.
- After the z-stack acquisition is complete, gently add the desired reagent (forskolin or vehicle control) using a pipette and wait for 10 minutes.
NOTE: Add the reagent very gently so as not to disturb the cells or move the position of the cell chamber within the microscope XY stage; it is helpful to verify in a subsequent live view or image that the field of view has not shifted during reagent addition. The 10-minute wait time is for the forskolin treatment to take effect. If an alternative treatment is used, the wait time may need to be adjusted. - After 10 minutes, change the filename and click run in the ND acquisition window.
- Repeat steps 2.11 – 2.13 as outlined above for at least 5 coverslips so as to achieve sufficient results for statistical analysis (n=5 for each treatment group – forskolin and vehicle control).
- Prepare samples and sample blanks to construct the spectral library and acquire spectral images using similar acquisition settings as outlined in steps 2.9 and 2.10.
3. Image analysis
NOTE: These images will be used to construct a spectral library containing the pure spectra of all individual endmembers present in the study. The endmembers in the spectral library might vary from study to study if different fluorophores are used. A detailed procedure to construct the spectral library is provided in a supplemental information file named “Supplemental File_Spectral Library”. Here, we describe exporting data to .tiff files, linear spectral unmixing, FRET efficiency measurements, three-dimensional reconstruction, and cAMP levels estimation. Image analysis can be performed using different image analysis and programming platforms such as ImageJ, Python, MATLAB, or CellProfiler. In these studies, MATLAB scripts were used.
- Export image data:
- Create new folders with the same filename corresponding to the spectral z-stack images acquired in steps 2.13 and 2.14.
NOTE: The following steps outlined to export data are specific for NIS Elements AR version 4.30.01. These steps may vary slightly depending on the version of the software. - Click File, which will open a File Window. Browse and select the spectral image file acquired in step 2.12 and click Open.
- Once the file loads, click File→ Import/Export→ Export ND document.
- On the popup window: browse and select the folder created in step 3.1.1, select Tagged Image Format (TIF) for File type, then select Mono image for each channel and Keep bit depth.
NOTE: The File prefix will be pre-generated; change this value for convenience. The Index order will change depending on the Channels that are selected, and should display “z, c” for indexing according to z-slice location first and wavelength band number second. Make sure that the boxes corresponding to Apply LUTs or Insert Overlays or Use Point Names are unselected. - Click Export to export the tiff files to a destination folder as individual tiff files.
- Repeat steps 3.1.2 – 3.1.5 to export spectral image files acquired in step 2.13.
- Create new folders with the same filename corresponding to the spectral z-stack images acquired in steps 2.13 and 2.14.
- Linear spectral unmixing:
- Open the programming software.
NOTE: Custom developed programming script to unmix raw spectral images is provided on the University of South Alabama BioImaging and BioSystems website, under the Resources tab (https://www.southalabama.edu/centers/bioimaging/resources.html). - Open the file labeled “Linear Unmixing.m” and click the run button in the editor toolbar.
- Browse and select the folder containing the exported *.tif file sequence generated by the NIS Elements software.
- Click OK to continue, which will open a new window called Wavelength and Z-Slice.
- Copy the filename of the first file (without z-slice and channel number) in the folder selected in step 3.2.4 and paste it into the first step of the dialog box labeled “Enter the Image Name”.
- Enter the number of channels in the second step labeled “Enter the number of wavelength bands”, number of z-slices in the third step labeled “Enter the number of Z-slices” and click OK.
NOTE: The number of wavelengths bands may change if changes are made to the acquisition settings, such as adjusting the wavelength range or the wavelength step size. The number of Z-slices may also change depending on the height of the cell. - Browse and select the wavelength file called “Wavelength.mat” in the popup window labeled “Select the wavelength information file” and click open.
- Browse and select the “Library.mat” file in the new popup window labeled “Select the spectral library file”, click open and wait until the unmixing of the slices is finished.
NOTE: Library.mat file is a file containing pure spectra for each endmember fluorophore along with cell autofluorescence and background spectral signatures. In this case, endmember fluorophores include Turquoise, Venus, and DRAQ5. Background spectral signatures include cellular or matrix autofluorescence, coverslip fluorescence, and coverslip diffraction. Wavelength.mat file is a file containing wavelength channel information used to acquire the spectral image. An example library file and wavelength file are available on Bioimaging and Biosystems website (see the note under 3.2.1). For more information on how to generate spectral library and wavelength files, refer to supplemental information file named “Supplemental File_Spectral Library”. Unmixed images corresponding to each z-slice will be saved into the folder called “Unmixed” created during the unmixing process within the folder that was selected in step 3.2.3.
- Open the programming software.
- FRET Efficiency Calculation:
- Open the programing script called “multiFRRCF.m” and click run.
NOTE: This programming file is available from the University of South Alabama Bioimaging and Biosystems website (see note under step 3.2.1). - Enter the number of experimental trials to analyze in the popup dialogue box called “how many folders to reslice” and click OK.
NOTE: Image data from each experiment should be saved in a separate unmixed image folder. This step simply allows the analysis code to loop over many folders as a time saving step. - Browse and select the unmixed folder(s) and click OK.
NOTE: The number of times that the “Browse for folder” pop up window opens depends on the number entered in “How many folders to reslice” dialogue box in the previous step. Browse and select the folders one after the other. - On the new popup window, enter the following information into the respective boxes: scaling factor is 12.4, Threshold is 5.6, X, Y, and Z Frequency are 5, 5, and 1 respectively, and smoothing algorithm is Gaussian.
NOTE: The scaling factor is a value in pixels/µm and will be used to scale the Z-direction sampling to that of the XY direction. The scaling factor is obtained from the image pixel size, which is usually provided as metadata in the image for most confocal microscope systems. For example, if the image is acquired with 0.08 µm/pixel spacing, the scaling factor should be 12.5 pixels/µm. Threshold value will be used to threshold the images and generate a binary mask of the cell. We created a list of optimum values based on the image donor+acceptor intensity. Use 4.5 as a thresholding value if the image has bright donor+acceptor intensity and low background, a value between 5.6 to 6.5 for images having only moderate donor+acceptor intensity and/or higher background, and a value of 7.5 and above for images having a donor+acceptor intensity that is lower than the background. Frequency value corresponds to the interval, in number of pixels, at which the slicing is performed in the subsequent steps. For example, if the Z-depth of the cell is 17 µm with a 1 μm step size and a scaling factor of 12.5 pixels/μm is used in the XY direction, then the depth of the 3-dimensional image dataset will be resampled at 212 pixels (Z direction). Based on the Z frequency value entered (for example, 1 pixel), the 3-dimensional image data set will be re-sliced beginning at the top of the image data set and then moving in increments of 1 pixel downward. This results in 212 resliced images. If a larger frequency value interval were entered for Z Frequency, then fewer resliced images would be generated. Resliced images are saved in subsequent steps. - Click run and wait until all the FRET measurements and reslicing are performed.
NOTE: A separate folder is created within the parent directory to which resliced grayscale FRET efficiency images and colored (a colormap applied) FRET efficiency images are saved. For example, all grayscale and colormap FRET images resliced in the X direction (YZ plane) are saved into a folder called “Resliced_XFRET”. - Repeat the analysis with similar settings for all the experiments – before and after forskolin treatments and vehicle controls.
NOTE: Steps mentioned in section 3.3 describe the values to enter for the custom FRET analysis programming script to generate 3-dimensional FRET image data. However, this script executes several operations while running, including: loading image data, creating image stacks, smoothing, FRET efficiency calculations, creating and applying a cell border mask, 3-dimensional image reconstruction, reslicing 3-dimensional images at specified intervals (frequencies), applying a colormap for visualizing FRET changes, and saving the resliced image data to the same directory. Additional details have been included as comments in the program script.
- Open the programing script called “multiFRRCF.m” and click run.
4. Mapping FRET efficiency to cAMP levels
- Open the programming file named ‘Mapping_FRET Efficiency_to_cAMP_concentration.m’ and click run on the main window.
NOTE: The file is available on the BioImaging and BioSystems website (see note under 3.2.1). This file reads grayscale FRET efficiency images and converts them to cAMP levels based on a characteristic curve. This characteristic curve uses a cAMP-to-FRET relationship documented in literature15,36 that is described by the Hill equation (the third equation shown below). However, Kd of the probe in intact cells is difficult to estimate and we have assumed it to be 1 μM in our calculations. Hence, results are shown as a function of Kd. (i.e., [cAMP] = x* Kd). Equations used to measure FRET efficiency and mapping FRET to cAMP levels are shown below:
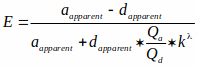
Where E is the FRET efficiency, and aapparent and dapparent are unmixed pixel intensities of acceptor and donor images, respectively.
Qa and Qd are quantum yields of acceptor and donor. Note that Qa and Qd cancel out when the equation for kλ is incorporated in the FRET efficiency equation, kλ is a correction factor:
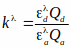
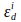 and
and 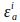 are extinction coefficients of donor and acceptor at the donor excitation wavelength, i (405nm).
are extinction coefficients of donor and acceptor at the donor excitation wavelength, i (405nm).
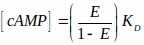
E is FRET efficiency and KD = Dissociation constant = 1 μM. - Navigate and select the first grey scale FRET image (saved in step 3.3.5) and click OK.
- Open the FRET/cAMP images to inspect the distribution of cAMP signals in three dimensions.
Results
This protocol describes the use of hyperspectral FRET imaging and analysis approaches to measure cAMP gradients in three spatial dimensions in living cells. There are several key steps involved in generating these results, for which careful attention is required while analyzing and quantifying the data. These key steps include construction of an appropriate spectral library, background spectral unmixing, thresholding to identify cell borders, and FRET efficiency calculations. Figure 1 illust...
Discussion
The development of FRET biosensors has allowed the measurement and visualization of cyclic nucleotide signals in single cells, and there is great promise for visualizing subcellular signaling events13,22,37,38. However, the use of FRET biosensors presents several limitations, including the low signal-to-noise characteristics of many fluorescent protein-based FRET reporters and the weak transfec...
Disclosures
Drs. Leavesley and Rich disclose financial interest in a start-up company, SpectraCyte, LLC, that was formed to commercialize spectral imaging technologies. However, all procedures described in this protocol were conducted using commercially available products not associated with SpectraCyte, LLC.
Acknowledgements
The authors would like to acknowledge Dr. Kees Jalink (The Netherlands Cancer Institute and van Leeuwenhoek Center for Advanced Microscopy, Amsterdam, the Netherlands) for providing us with the H188 cAMP FRET biosensor and Kenny Trinh (College of Engineering, University of South Alabama) for technical help in reducing the time taken to run our custom developed programming scripts.
The authors would like to acknowledge the funding sources: American Heart Association (16PRE27130004), National Science Foundation; (1725937) NIH, S100D020149, S10RR027535, R01HL058506, P01HL066299).
Materials
| Name | Company | Catalog Number | Comments |
| Attofluor Cell Chamber | Invitrogen | A7816 | Attofluor contains steel cell chambers and a rubber O-ring. Cell chamber holds the coverslip and O-ring provides a lock in mechanism to hold the buffer in cell chamber with out leakage |
| Dimethyl Sulfoxide (DMSO) | Fisher Scientific | BP231-100 | Solvent used to prepare stock solution forskolin. |
| DRAQ5 Fluoroscent Probe Solution | Thermo Scientific | 62252 | Nuclear label |
| Dulbecco Modified Eagle Medium (DMEM) | Gibco | 11965-092 | Contains nutrients and growth factors for the cells to grow and divide in the culture dishes. |
| Fetal Bovine Serum (FBS) | Sigma | F6178 | Growth factor suppliment that is added to culture medium, DMEM |
| Forskolin | Sigma | F3917 | Adenyly cyclase activator. |
| H188 Cyclic AMP FRET biosensor | Netherland Cancer Institute, Dr. K. Jalink | Gift | Plasmid encoding Turquoise (donor fluorophore), Venus (acceptor fluorophore), and binding domain obtained from Epac. |
| Image J | image.net | Free download | Another image processing platform used to extact spectral information and image processing. |
| Integrating Sphere | Ocean Optics | FOIS-1 | Used to measure illumination intensity of the laser line at different laser intensities (?). |
| Laminin Mouse Protein, Natural | Invitrogen | 23017-015 | Coverslips are coated with laminin and this helps in cell attachment, growth and motility of the cell. |
| Lipofectamine 3000 Transfection Kit | Invitrogen | L3000-015 | Transfection reagent used to transfect cells with H188 FRET biosensor |
| MATLAB | Mathworks | R2019a | Image processing operations (linear unmixing and FRET efficiency calculations) are performed by writing custom programs in MATLAB programming environment |
| Nikon A1R confocal microscope | Nikon Instruments | Nikon A1R | Spectral image acquisition is performed using confocal microscope. |
| Nikon Elements Software | Nikon Instruments | Software dongle | used to export and handle nd2 image files (multidimensional image files) that are aquired using Nikon A1R |
| NIST-Traceable Calibration Lamp | Ocean Optics | LS-1-CAL-INT | A lamp with a known spectrum for use as a standard |
| PBS pH 7.4 (1X) | Gibco | 10010-023 | coomonly used buffer suring cell culture |
| Pulmonary Microvascular Endothelial Cells (PMVECs) | In house - Cell culture core, Univeristy of South Alabama | Isolated from Rat pulmonary microvasculature | PMVECs form inner lining of a blood vessel. |
| Penicillin-Streptomycin (10,000 U/ml) | Gibco | 15140-122 | antibiotics are added to culture medum to prevent contamination of the cells. |
| Pre-Cleaned Gold Seal Micro Slides | Clay Adams | 3010 | Microscope slides used for cell fixation |
| ProLong Diamond Antifade Mounting Media | Invitrogen | P36961 | If samples are fixed using antifade mountant, then the later protects fluoroscent dyes and chromophores from fading. |
| Spectrometer | Ocean Optics | QE65000 | Used to measure spectral response of the light source (?) |
| Trypsin-EDTA (0.25%) | Gibco | 25200-056 | Digests the protein-protein bond between the cell and cell matrix and helps to disscociate and lift the cells during cell plating. |
| Tyrodes Buffer | Made in-house | Made in-house | Tyrodes buffer is used to make working solutions and to maintain cells in aqueous solution during image acquisition. |
| 6 Well Cell Culture Plate | Corning | 3506 | Laminin coated coverslips are placed in 6-well culture dish (one coverlisps/well). Cells along with medium is added into each well. |
| 25 mm Round Microscope Cover Slips | Fisher Scientific | 12545102 | Cells were grown on round glass coverslips |
| 60X Ojective | Nikon Instruments | Plan Apo VC 60X/1.2 WI ∞/0.15-0.18 WD 0.27 | water immersion and commonly used objective for cells |
References
- Corbin, J. D., Sugden, P. H., Lincoln, T. M., Keely, S. L. Compartmentalization of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in heart tissue. The Journal of Biological Chemistry. 252, 3854-3861 (1977).
- Terrin, A., et al. PGE1 stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. The Journal of Cell Biology. 175, 441-451 (2006).
- Bacskai, B. J., et al. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 260, 222-226 (1993).
- Iancu, R. V., Ramamurthy, G., Harvey, R. D. Spatial and temporal aspects of cAMP signalling in cardiac myocytes. Clinical and Experimental Pharmacology & Physiology. 35, 1343-1348 (2008).
- Brunton, L. L., Hayes, J. S., Mayer, S. E. Functional compartmentation of cyclic AMP and protein kinase in heart. Advances in Cyclic Nucleotide Research. 14, 391-397 (1981).
- Hohl, C. M., Li, Q. Compartmentation of cAMP in adult canine ventricular myocytes. Relation to single-cell free Ca2+ transients. Circulation Research. 69, 1369-1379 (1991).
- Rich, T. C., et al. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proceedings of the National Academy of Sciences of the United States of America. 98, 13049-13054 (2001).
- Sayner, S. L., Alexeyev, M., Dessauer, C. W., Stevens, T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circulation Research. 98, 675-681 (2006).
- Rich, T. C., Tse, T. E., Rohan, J. G., Schaack, J., Karpen, J. W. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. The Journal of General Physiology. 118, 63-78 (2001).
- Blackman, B. E., et al. PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. Journal of Biological Chemistry. 286, 12590-12601 (2011).
- Sayner, S. L., et al. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circulation Research. 95, 196-203 (2004).
- Klarenbeek, J., Jalink, K. Detecting cAMP with an EPAC-based FRET sensor in single living cells. Methods in Molecular Biology. 1071, 49-58 (2014).
- Surdo, N. C., et al. FRET biosensor uncovers cAMP nano-domains at β-adrenergic targets that dictate precise tuning of cardiac contractility. Nature Communications. 8, 15031 (2017).
- Ponsioen, B., et al. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Reports. 5, 1176-1180 (2004).
- Vogel, S. S., Thaler, C., Koushik, S. V. Fanciful FRET. Science's STKE. 2006, (2006).
- Clegg, R. M. The History of FRET: From conception through the labors of birth. Reviews in Fluorescence. 3, (2006).
- Giepmans, B. N. G., Adams, S. R., Ellisman, M. H., Tsien, R. Y. The fluorescent toolbox for assessing protein location and function. Science. 312, 217-224 (2006).
- Manzella-Lapeira, J., Brzostowski, J. A. Imaging protein-protein interactions by Förster resonance energy transfer (FRET) microscopy in live cells. Current Protocols in Protein Science. 93, 58 (2018).
- Cooper, D. M. F., Mons, N., Karpen, J. W. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 374, 421-424 (1995).
- Sassone-Corsi, P. Coupling gene expression to cAMP signalling: role of CREB and CREM. The International Journal of Biochemistry & Cell Biology. 30, 27-38 (1998).
- Rebhun, L. I. Cyclic nucleotides, calcium, and cell division. International Review of Cytology. 49, 1-54 (1977).
- Klarenbeek, J., Goedhart, J., Van Batenburg, A., Groenewald, D., Jalink, K. Fourth-generation Epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PLOS ONE. 10, 0122513 (2015).
- Leavesley, S. J., Rich, T. C. FRET: signals hidden within the noise. Cytometry Part A. 85, 918-920 (2014).
- Rich, T. C., Webb, K. J., Leavesley, S. J. Can we decipher the information content contained within cyclic nucleotide signals. The Journal of General Physiology. 143, 17-27 (2014).
- Annamdevula, N. S., et al. Spectral imaging of FRET-based sensors reveals sustained cAMP gradients in three spatial dimensions. Cytometry Part A. 93 (10), 1029-1038 (2018).
- Leavesley, S. J., Britain, A. L., Cichon, L. K., Nikolaev, V. O., Rich, T. C. Assessing FRET using spectral techniques. Cytometry Part A. 83, 898-912 (2013).
- Leavesley, S. J., Rich, T. C. Overcoming limitations of FRET measurements. Cytometry Part A. 89, 325-327 (2016).
- Fink, D. J. Monitoring Earcths Resources from Space. Technology Review. 75, 32-41 (1973).
- Goetz, A. F. H., Vane, G., Solomon, J. E., Rock, B. N. Imaging Spectrometry for Earth Remote Sensing. Science. 228, 1147-1153 (1985).
- Bücherl, C. A., Bader, A., Westphal, A. H., Laptenok, S. P., Borst, J. W. FRET-FLIM applications in plant systems. Protoplasma. 251, 383-394 (2014).
- Chen, Y., Mauldin, J. P., Day, R. N., Periasamy, A. Characterization of spectral FRET imaging microscopy for monitoring nuclear protein interactions. Journal of Microscopy. 228, 139-152 (2007).
- Zimmermann, T., Rietdorf, J., Girod, A., Georget, V., Pepperkok, R. Spectral imaging and linear un-mixing enables improved FRET efficiency with a novel GFP2-YFP FRET pair. FEBS Letters. 531, 245-249 (2002).
- Griswold, J. R., Annamdevula, N., Deal, J., Rich, T., Leavesley, S. Estimating FRET Efficiency using Excitation-Scanning Hyperspectral Imaging. Biophysical Journal. 112, 586 (2017).
- Favreau, P. F., et al. Excitation-scanning hyperspectral imaging microscope. Journal of Biomedical Optics. 19, 046010 (2014).
- King, J., et al. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvascular Research. 67, 139-151 (2004).
- Thaler, C., Koushik, S. V., Blank, P. S., Vogel, S. S. Quantitative multiphoton spectral imaging and its use for measuring resonance energy transfer. Biophysical Journal. 89, 2736-2749 (2005).
- Agarwal, S. R., et al. Compartmentalized cAMP signaling associated with lipid raft and non-raft membrane domains in adult ventricular myocytes. Frontiers in Pharmacology. 9, 332 (2018).
- Johnstone, T. B., Agarwal, S. R., Harvey, R. D., Ostrom, R. S. cAMP signaling compartmentation: Adenylyl cyclases as anchors of dynamic signaling complexes. Mol Pharmacol. , (2017).
- Zhang, J., Li, H., Chai, L., Zhang, L., Qu, J., Chen, T. Quantitative FRET measurement using emission-spectral unmixing with independent excitation crosstalk correction. Journal of Microscopy. 257, 104-116 (2015).
- Zhang, J., Lin, F., Chai, L., Wei, L., Chen, T. IIem-spFRET: improved Iem-spFRET method for robust FRET measurement. Journal of Biomedical Optics. 21, 105003 (2016).
- Levy, S., et al. SpRET: highly sensitive and reliable spectral measurement of absolute FRET efficiency. Microscopy and Microanalysis. 17, 176-190 (2011).
- West, S. J., et al. Hyperspectral Measurements Allow Separation of FRET Signals from Non-Uniform Background Fluorescence. Biophysical Journal. 112, 453 (2017).
- Annamdevula, N. S., et al. An approach for characterizing and comparing hyperspectral microscopy systems. Sensors. 13, 9267-9293 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved