A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Nuclear Migration in the Drosophila Oocyte
In This Article
Summary
In Drosophila, the oocyte nucleus undergoes microtubule-dependent migration during oogenesis. Here, we describe a protocol that was developed to follow the migration by performing live imaging on egg chambers ex-vivo. Our procedure maintains egg chambers alive for 12 h to acquire multi-position 3D time-lapse movies using spinning-disk confocal microscopy.
Abstract
Live cell imaging is particularly necessary to understand the cellular and molecular mechanisms that regulate organelle movements, cytoskeleton rearrangements, or polarity patterning within the cells. When studying oocyte nucleus positioning, live-imaging techniques are essential to capture the dynamic events of this process. The Drosophila egg chamber is a multicellular structure and an excellent model system to study this phenomenon because of its large size and availability of numerous genetic tools. During Drosophila mid-oogenesis, the nucleus migrates from a central position within the oocyte to adopt an asymmetric position mediated by microtubule-generated forces. This migration and positioning of the nucleus are necessary to determine the polarity axes of the embryo and the subsequent adult fly. One characteristic of this migration is that it occurs in three dimensions (3D), creating a necessity for live imaging. Thus, to study the mechanisms that regulate nuclear migration, we have developed a protocol to culture the dissected egg chambers and perform live imaging for 12 h by time-lapse acquisitions using spinning-disk confocal microscopy. Overall, our conditions allow us to preserve Drosophila egg chambers alive for a long period of time, thereby enabling the completion of nuclear migration to be visualized in a large number of samples in 3D.
Introduction
For several years, the Drosophila oocyte has emerged as a model system to study nuclear migration. The Drosophila oocyte develops in a multicellular structure called the egg chamber. Egg chambers encompass 16 germ cells (15 nurse cells and the oocyte) surrounded by an epithelial layer of follicular somatic cells. Egg chamber development has been subdivided into 14 stages (Figure 1A), during which the oocyte will grow and accumulate reserves necessary for the early development of the embryo. During the development, upon microtubule reorganization and asymmetric transport of maternal determinants, the oocyte polarizes along the antero-dorsal and dorso-ventral axes. These axes determine the subsequent polarity axes of the embryo and the adult arising from the fertilization of this oocyte1. During oogenesis, the nucleus adopts an asymmetric position in the oocyte. In stage 6, the nucleus is centered in the cell. Upon a yet to be identified signal emitted by the posterior follicular cells which is received by the oocyte, the nucleus migrates toward the intersection between the anterior and lateral plasma membranes in stage 7 (Figure 1B)2,3. This asymmetric position is required to induce the determination of the dorso-ventral axis.
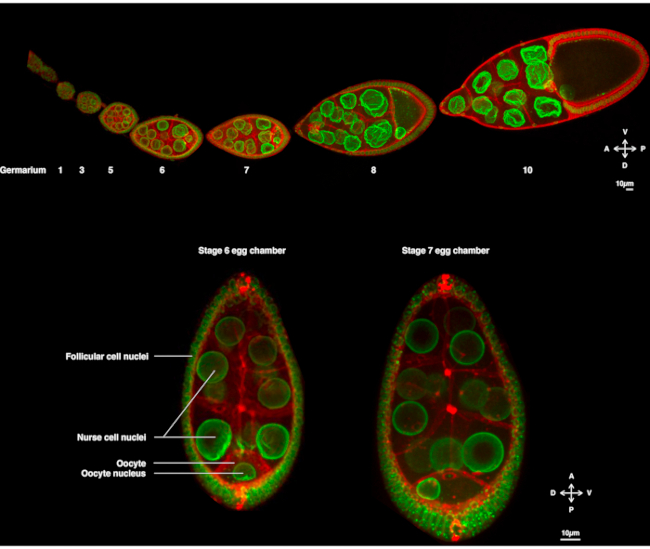
Figure 1: Drosophila melanogaster egg chambers. (A) Fixed ovariole from transgenic flies expressing Fs(2)Ket-GFP that labels the nuclear envelopes and ubi-PH-RFP that labels the plasma membranes. The ovariole is composed of developing egg chambers at different stages. Maturation increases along the antero-posterior axis with the germarium at the anterior tip (left) where the germ stem cell resides and the older stage at the posterior tip (right). (B) Z-projection of living egg chamber by spinning disk confocal microscopy at stage 6 of oogenesis (left), in which the nucleus is centered in the oocyte. The nucleus will migrate to adopt an asymmetrical position at stage 7 (right) in contact with the anterior plasma membrane (between the oocyte and the nurse cell) and the lateral plasma membrane (between the oocyte and the follicular cells). This position will induce the determination of the dorsal side and, thus, the dorso-ventral axis of the egg chamber. Please click here to view a larger version of this figure.
For many decades, this nuclear migration has been studied on fixed tissues by immunostaining. This approach has notably made it possible to demonstrate that this process depends on a dense network of microtubules4,5. More recently, we developed a protocol offering conditions compatible with live imaging of the oocyte during several hours making it possible to study this process dynamically6.
Hence, for the first time, we have been able to describe that the nucleus has preferential and characteristic trajectories during its migration, one along the anterior plasma membrane (APM) and another along the lateral plasma membrane (LPM) of the oocyte (Figure 2). These latest results underline the importance of live-imaging protocols when studying dynamic processes such as nuclear migration.
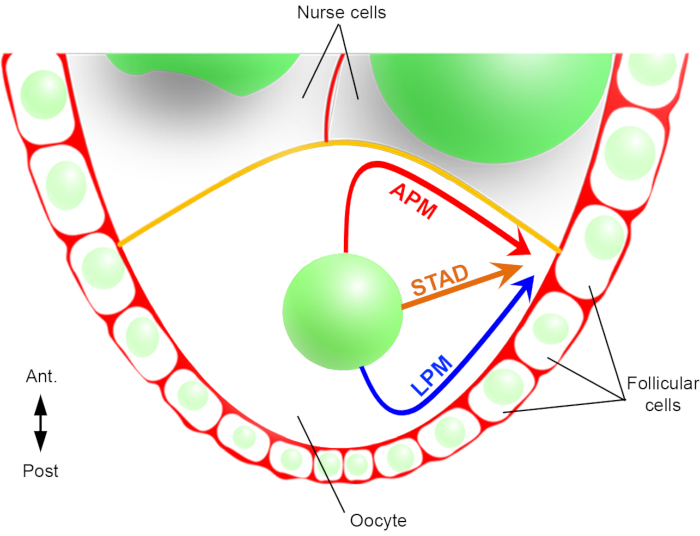
Figure 2: Schematic representation of the different migration paths of the nucleus. At stage 6 of the oogenesis, the oocyte is a large cell with a central nucleus. At this stage, the antero-posterior polarity axis is set with a posterior/lateral plasma membrane of the oocyte in contact with the follicular cells and the anterior plasma membrane (in yellow) is in contact with the nurse cells2. We have previously reported that the nucleus could migrate either along the anterior plasma membrane (APM), along the lateral plasma membrane (LPM), or through the cytoplasm (STAD, straight to the antero-dorsal cortex)6. Please click here to view a larger version of this figure.
The oocyte nucleus migration is a phenomenon of about 3 h6, and so far, the event triggering the start of the actual migration is unknown. The start of the migration can also be delayed by protein mutants used to study this mechanism. These unknown variables motivated us to acquire images over long time periods (10-12 h). It is, therefore, important to ensure that the oocytes remain alive. As the egg chamber develops, it elongates along the antero-posterior axis from a spherical to an elliptical shape. This elongation is driven by the rotation of follicular cells, which occurs from stage 1 to stage 8, perpendicular to the antero-posterior axis7. In addition, a tubular sheath of muscle with pulsatile property surrounds the egg chambers. Its physiological function is to push the developing follicles toward the oviduct continuously8. In order to limit the movements that induce oscillations of the egg chambers after their dissection, we designed an observation micro-chamber measuring 150 µm in height (Figure 3A). This height is marginally higher than the size of a follicle at stages 10 and 11. It considerably limits the vertical movements of the sample while preserving the rotation of the egg chamber, thereby resulting in limited defects in follicle development. We then perform live imaging for 12 h on dissected egg chambers by multi-position time-lapse acquisitions using a spinning-disk confocal microscope. Here we describe our protocol for studying the oocyte nuclear migration between stages 6 and 7.
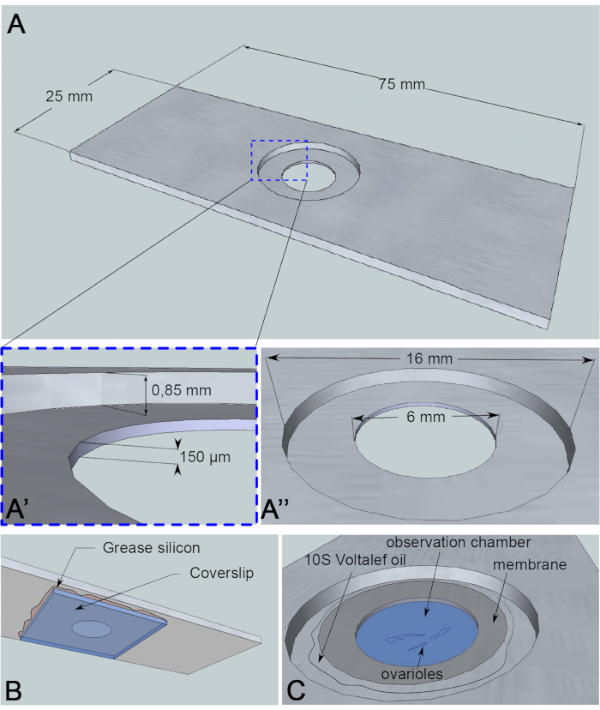
Figure 3: Schematic representation of the observation chamber. (A) (Top view) Precise dimensions of the aluminum slide with the heights (A') and circumferences (A'') of the well drilled in the middle of the slide. (B) (Bottom view) A coverslip blocking the well is sealed to the slide with silicon grease. (C) (Top view) Dissected ovarioles develop in an imaging medium that is covered by a gas permeable membrane. Halocarbon oil is used to stabilize the membrane. Please click here to view a larger version of this figure.
In order to follow the nuclear migration and precisely assess trajectories in the oocyte, markers for both the nuclear envelope and plasma membrane are needed. With this aim, two transgenes that have a high signal/noise ratio and do not fade over the course of live imaging have been selected. To label the plasma membrane, the use of a P[ubi-PH-RFP] that encodes the Pleckstrin Homology (PH) domain of the Human Phospholipase C ∂1 (PLC∂1) fused to RFP is recommended. This PH domain binds to the phosphoinositide PI(4,5)P2 distributed along the plasma membrane of the oocyte9. For the nuclear envelope, the P[PPT-un1]Fs(2)Ket-GFP protein-trap strain where GFP is inserted within the gene encoding the Drosophila ß-importin displays a homogeneous and an intense signal10. Young flies (1-2 days old) are placed in fresh vials containing dry yeast 24-48 h prior to ovary dissection.
For this live-imaging assay, a 1 mm thick piece of aluminum, which is nonreactive for the sample, has been cut into the dimensions of a microscopy slide. It has a 16 mm diameter hole in the center of the slide that has been counterbored to 0.85 mm. This counterbore has an additional 6 mm diameter hole with a depth of 150 µm (Figure 3A). A coverslip is glued with silicone grease (inert for the sample) at the bottom of the aluminum chamber (Figure 3B). After placing the samples in the medium-filled well, a membrane permeable to O2/CO2 exchange is placed over the medium and surrounded by halocarbon oil (Figure 3C).
For the dissection, it is recommended to use stainless steel forceps with a tip dimension of 0.05 x 0.02 mm, and 0.20 mm diameter needles for the separation of the ovarioles (Figure 4B,C). The migrating nuclei are imaged on a spinning-disk confocal inverted microscope CSU-X1 equipped with a camera. Multi-position images were acquired by time-lapse every 15 min at 24 °C. A 15 min interval allows performing multi-position acquisitions with limited photobleaching of the fluorescent proteins and phototoxicity for the samples. Furthermore, a shorter interval would not provide much more informative data to follow the nuclear trajectories. The movies are processed and analyzed via Fiji software11.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Imaging medium preparation
- Prepare fresh media on the day of use. Pipette 200 µL of Schneider medium (containing L-Glutamine and 0.40 g/L of NaHCO3 complemented with 10% heat-inactivated fetal calf serum, 100 U/mL of penicillin, and 100 mg/mL of streptomycin).
- Supplement with 30 µL of insulin 10 mg/mL.
- Add 4 µL of heat-inactivated fetal calf serum.
2. Observation-chamber preparation
- With a pipette tip, apply a small amount of silicone grease all around the hole on the underside of the punctured slide (Figure 4D).
- Position a 24 x 50 mm coverslip of 0.13-0.16 mm thickness.
- With the wide end of a pipette tip, apply pressure on the coverslip to flatten the silicone in order to seal the coverslip and create a silicone ring interior to the slide (Figure 4F,G).
3. Ovary dissection
- Anesthetize a female fly of the desired phenotype on a CO2 pad.
- Transfer the female in 150 µL of the imaging medium in a dissecting well (Figure 4H).
- Open one female by grabbing its thorax with forceps and pinching the dorsal abdomen cuticle with a second pair of forceps.
- Isolate and detach the pair of ovaries, which should be readily visible upon cuticle opening.
- Carefully remove the uterus, oviduct, and muscle sheath (Figure 4I).
- Place a drop of 10-15 µL of the imaging medium and transfer one ovary in the imaging chamber (Figure 4J,K).
4. Egg chamber isolation
- To separate the ovarioles, hold the posterior end of the ovary (toward the older stages) with the needle. Tease apart the ovarioles by carefully pulling on the germarium with another needle.
- Remove the remaining muscle sheath on the egg chambers; one needle holding the sheath and the other pulling on the ovariole through the larger chambers (stage 9 or older).
- Allow the unsheathed ovariole to sink and contact the coverslip.
- Remove late stages and the rest of the ovaries from the micro-chamber with the help of forceps. Carefully distance the ovarioles from the others with needles to facilitate the acquisition (Figure 4L).
5. Observation chamber closing
- Cut a small square (10 x 10 mm) of permeable membrane (Figure 4M,N).
- Carefully apply the membrane on top of the imaging medium to expel any air bubbles (Figure 4O).
- Hermetically seal the chamber with a thin layer of halocarbon oil around the well on the contour of the membrane (Figure 4P,Q).
6. Imaging
- Place the imaging set-up on the slide holder of the inverted microscope using a 40x objective (HCX PL Apo, 1.25NA, oil immersion).
- Locate and save positions of different stage 6 oocytes in which the nucleus is ready to migrate.
- Set-up the 488 and 561 nm lasers. With a measured output laser power of 150 mW, use 30% of the laser power and 300 ms and 500 ms exposure, respectively.
- Set-up the experiment. Take a time-lapse of 12 h with the interval of 15 min-41 sections with an interval of 1 µm centered on the nucleus.
NOTE: According to the exposure time described above, this setting allows the acquisition of one position in around 45 s. Since there is a delay due to position changing, it is recommended to set a maximum of 12 positions in these conditions.
7. Image analysis
- Process the movies on the software Fiji, using the plug-in Orthogonal view and manually track the nuclei.

Figure 4: Step by step micro-chamber mounting pictures. (A,B,C) Preparation of the needed tools: dissecting well plate, forceps, needles, imaging media, silicon grease, permeable membrane, and the aluminum slide. (D) Application of the silicon grease at the back of the aluminum slide with a pipette tip. (E) A glass coverslip is glued on the silicon grease to create the bottom of the chamber. (F,G) Pressure application on the coverslip with the wider extremity of a pipette tip to create a joint inside the chamber. (H,I) Dissection of the fly ovaries in the imaging media. (J) Pipetting of a drop of imaging media in the micro-chamber. (K,L) Separation of the ovarioles in the micro-chamber using needles. (M,N,O) Permeable membrane cut into a 10 x 10 mm square and placing over the drop of the medium in the micro-chamber. (P,Q) Sealing of the micro-chamber with halocarbon oil. The samples are ready to be imaged. Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Results
Before migration, the nucleus is dynamic and oscillates around a central position during a period defined as pre-migration. These small movements reflect a balance of pushing and pulling forces that maintain equilibrium in the middle of the oocyte. By quantifying the trajectories of the nuclei, we have shown that the APM and LPM trajectories had similar proportions. We define the nature of the trajectory by the first contact between the nucleus and the plasma membrane6. Thus, the nucleus reaches e...
Access restricted. Please log in or start a trial to view this content.
Discussion
Other protocols describe how to prepare and culture Drosophila egg chambers ex vivo for live-imaging assay12,13. The novelty of this protocol is the use of an imaging chamber constructed using a hollowed aluminum slide, a coverslip, and an O2/CO2 permeable membrane. The main advantage of this set-up is to limit the movement in Z without exerting pressure on the sample. Thus, the oocyte can still move freely, and this is why first, t...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors declare no competing interests.
Acknowledgements
We are extremely grateful to Jean-Antoine Lepesant and Nicolas Tissot who originally developed the protocol and shared some graphical elements of Figure 3 with us. We thank Fanny Roland-Gosselin who took the photos of Figure 4. We also thank other lab members for helpful discussions that contributed to the amelioration of this technique and Nathaniel Henneman for his comments that helped to improve this manuscript. We acknowledge the ImagoSeine core facility of the Institute Jacques Monod, member of France-BioImaging (ANR-10-INBS-04). Maëlys Loh is supported by a PhD fellowship from the French Ministry of Research (MESRI). Antoine Guichet and Fred Bernard were supported by the ARC (Grant PJA20181208148), the Association des Entreprises contre le Cancer (Grant Gefluc 2020 #221366) and by an Emergence grant from IdEx Université de Paris (ANR-18-IDEX-0001).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Anesthetize CO2 pad | Dutscher | 789060 | Anesthetize flies |
| Coverslip (24x50 mm) | Knittel Glass | VD12450Y100A | Observation-chamber preparation |
| Forceps Dumont #5 | Carl Roth | K342.1 | Dissection |
| Stainless steel needles | Entosphinx | 20 | Dissection |
| Heat-inactivated fetal calf serum | SIGMA-ALDRICH | F7524 | Imaging medium |
| Insulin solution bovine pancreas | SIGMA-ALDRICH | 10516 - 5ml | Imaging medium |
| Penicilin/Streptomycin solution | SIGMA-ALDRICH | P0781 | Imaging medium |
| Permeable membrane | Leica | 11521746 | Observation-chamber preparation |
| Schneider Medium | Pan Biotech | P04-91500 | Imaging medium |
| Silicon grease | BECKMAN COULTER | 335148 | Observation-chamber preparation |
| Spinning disk confocal | Zeiss | CSU-X1 | Nuclear migration observation |
| Voltalef oil 10S | VWR | 24627 - 188 | Observation-chamber preparation |
References
- Merkle, J. A., Wittes, J., Schüpbach, T. Signaling between somatic follicle cells and the germline patterns the egg and embryo of Drosophila. Current Topics in Developmental Biology. 140, 55-86 (2020).
- Roth, S., Lynch, J. A. Symmetry breaking during drosophila oogenesis. Cold Spring Harbor Perspectives in Biology. 1 (2), 001891(2009).
- Bernard, F., Lepesant, J. -A., Guichet, A. Nucleus positioning within Drosophila egg chamber. Seminars in Cell and Developmental Biology. 82, 25-33 (2017).
- Koch, E. A., Spitzer, R. H. Multiple effects of colchicine on oogenesis in Drosophila: Induced sterility and switch of potential oocyte to nurse-cell developmental pathway. Cell and Tissue Research. 228 (1), 21-32 (1983).
- Januschke, J., et al. The centrosome-nucleus complex and microtubule organization in the Drosophila oocyte. Development. 133, Cambridge, England. 129-139 (2006).
- Tissot, N., et al. Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte. Nature Communications. 8, 15168(2017).
- Cetera, M., Horne-Badovinac, S. Round and round gets you somewhere: collective cell migration and planar polarity in elongating Drosophila egg chambers. Current Opinion in Genetics & Development. 32, 10-15 (2015).
- Hudson, A. M., Petrella, L. N., Tanaka, A. J., Cooley, L. Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Developmental Biology. 314, 329-340 (2008).
- Gervais, L., Claret, S., Januschke, J., Roth, S., Guichet, A. PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte. Development. 135 (23), 3829-3838 (2008).
- Villányi, Z., Debec, A., Timinszky, G., Tirián, L., Szabad, J. Long persistence of importin- b explains extended survival of cells and zygotes that lack the encoding gene ' n Villa. Mechanisms of Development. 3-4 (125), 196-206 (2008).
- Schindelin, J., et al. Fiji: An open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Prasad, M., Jang, A. C. C., Starz-Gaiano, M., Melani, M., Montell, D. J. A protocol for culturing drosophila melanogaster stage 9 egg chambers for live imaging. Nature Protocols. 2 (10), 2467-2473 (2007).
- Weil, T. T., Parton, R. M., Davis, I. Preparing individual Drosophila egg chambers for live imaging. Journal of Visualized Experiments: JoVE. (60), (2012).
- Chanet, S., Huynh, J. R. Collective cell sorting requires contractile cortical waves in germline cells. Current Biology. 30 (21), 4213-4226 (2020).
- Zhao, T., Graham, O. S., Raposo, A., St Johnston, D. Growing microtubules push the oocyte nucleus to polarize the drosophila dorsal-ventral axis. Science. 336 (6084), New York, N.Y. 999-1003 (2012).
- Legent, K., Tissot, N., Guichet, A. Chapter 7 Oogenesis using fixed and live imaging. Drosophila Oogenesis: Methods and Protocols. 1328, 99-112 (2015).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved