Method Article
Absolute Quantitation of Inositol Pyrophosphates by Capillary Electrophoresis Electrospray Ionization Mass Spectrometry
In This Article
Summary
A procedure for capillary electrophoresis electrospray ionization mass spectrometry for the absolute quantitation of inositol pyrophosphates from mammalian cell extracts is described.
Abstract
Inositol pyrophosphates (PP-InsPs) are an important group of intracellular signaling molecules. Derived from inositol phosphates (InsPs), these molecules feature the presence of at least one energetic pyrophosphate moiety on the myo-inositol ring. They exist ubiquitously in eukaryotes and operate as metabolic messengers surveying phosphate homeostasis, insulin sensitivity, and cellular energy charge. Owing to the absence of a chromophore in these metabolites, a very high charge density, and low abundance, their analysis requires radioactive tracer, and thus it is convoluted and expensive. Here, the study presents a detailed protocol to perform absolute and high throughput quantitation of inositol pyrophosphates from mammalian cells by capillary electrophoresis electrospray ionization mass spectrometry (CE-ESI-MS). This method enables the sensitive profiling of all biologically relevant PP-InsPs species in mammalian cells, enabling baseline separation of regioisomers. Absolute cellular concentrations of PP-InsPs, including minor isomers, and monitoring of their temporal changes in HCT116 cells under several experimental conditions are presented.
Introduction
Since the initial discovery of myo-inositol pyrophosphates (PP-InsPs) in 19931,2, significant progress has been made to elucidate their biosynthesis, turnover, and functions3. Inositol pyrophosphates ubiquitously occur in eukaryotic cells4 and serve as metabolic signaling molecules critically involved in, e.g., phosphate homeostasis5,6, insulin sensitivity7, calcium oscillations8,9, vesicular trafficking10, apoptosis11, DNA repair12, immune signalling13, and others. The plethora of important processes under the control of inositol pyrophosphates calls for a deeper understanding of their cellular abundance, fluctuation, and localization.
Although InsPs and PP-InsPs attracted attention across disciplines, the analysis of their abundance is routinely performed using a method developed during the '80s, consisting of labeling cells with tritiated inositol, resolving the extracted PP-InsPs by strong anion exchange chromatography Sax-HPLC with subsequent scintillation counting. Newer methods based on mass spectrometry still face significant challenges: inositol pyrophosphates with up to eight phosphate units harbor phosphate esters and anhydrides, leading to a significant negative charge and potential phosphate loss during ionization. There are four major types of PP-InsPs found in mammals (Figure 1): 1,5-(PP)2-InsP4 (or 1,5-InsP8), 5-PP-InsP5 (or 5-InsP7), 1-PP-InsP5 (or 1-InsP7), and 5-PP-Ins(1,3,4,6)P4 (or 5-PP-InsP4)3,14. The physiological levels of PP-InsPs are typically in the nano- to low-micromolar range, with 5-PP-InsP5 as the most abundant with cellular concentrations of 0.5 - 5 µM. 1,5-(PP)2-InsP4 and 1-PP-InsP5 are believed to be up to around 10% of the 5-PP-InsP5 pool and remain difficult to trace in many cells15. 5-PP-InsP4 with a free OH group is even lower in abundance and usually only becomes detectable when phosphate hydrolases are inhibited with sodium fluoride (NaF)16.
The high charge density of PP-InsPs makes their separation difficult, and the occurrence of PP-InsP regioisomers further complicates these efforts. As a result, most experiments relied on quantitation by metabolic radioactive labeling of cells using [3H]-inositol, as background from the matrix is excluded and high sensitivity is achieved17,18. However, this method is costly, time-consuming, and does not allow to properly distinguish related PP-InsP regioisomers. Moreover, [3H]-inositol labeling does not account for endogenous inositol synthesis from glucose. A polyacrylamide gel electrophoresis (PAGE)-based method is a widely applied inexpensive alternative but limited in its sensitivity19,20,21,22. Other approaches avoiding radiolabeling have been published, including ion chromatography followed by post-column derivatization UV-detection23, hydrophilic interaction chromatography (HILIC)24, or weak anion exchange (WAX) coupled with mass spectrometry (MS)25. However, they are not (yet) on par with the classic [3H]-inositol SAX-HPLC protocol.
Recently, capillary electrophoresis electrospray ionization mass spectrometry (CE-ESI-MS) was introduced as a transformative strategy for the analysis of InsPs and PP-InsPs metabolism, meeting all requirements discussed above16. Combined with current state-of-the-art InsP extraction by perchloric acid followed by enrichment with titanium dioxide beads26, CE-ESI-MS succeeded in every organism tested so far, from yeast to plants and mammals. Simultaneous profiling of InsPs and PP-InsPs, including all possible regioisomers, was easily achieved. Stable isotope-labeled (SIL) internal standards enabled a rapid and precise absolute quantitation, irrespective of matrix effects. Because MS can capture isotopic mass differences, CE-ESI-MS can also be applied to study compartmentalized cellular synthesis pathways of InsPs and PP-InsPs, e.g., by feeding cells with [13C6]-myo-inositol or [13C6]-D-glucose.
Described here is a detailed step-by-step protocol for the absolute quantitation of PP-InsPs and InsPs from mammalian cells by CE-ESI-MS. Apart from the major 5-PP-InsP5 isomer, 1,5-(PP)2-InsP4 and 1-PP-InsP5 are also quantified in this study, despite their lower abundance. Two HCT116 cell lines from different laboratories (NIH, UCL) are studied, and it is validated that HCT116UCL cells contain 7-fold higher levels of 1,5-(PP)2-InsP4 than found in HCT116NIH, while 5-PP-InsP5 concentrations are comparable. In addition, 1-PP-InsP5 synthesis in HCT116UCL is not significantly increased. Also, the increase of PP-InsP levels by blocking their dephosphorylation using sodium fluoride is studied quantitatively.
Protocol
1. Setting up the CE-ESI-MS system
- Set up a CE-ESI-MS system consisting of a commercial CE system - and a triple quadrupole tandem mass spectrometer, equipped with an Agilent Jet Stream (AJS) electrospray ionization (ESI) source. A CE-ESI-MS sprayer kit and an isocratic LC (Liquid Chromatography) pump are requisite.
- Connect the sheath flow 1:100 splitter (included in the CE-MS sprayer kit) and the isocratic LC pump outlet.
- Ensure that the CE system inlet vial is at the same height as the sprayer tip of the mass analyzer.
- Utilize the MassHunter Workstation (Version 10.1) or comparable MS software to control the entire system, and for data acquisition and analysis.
2. Preparing buffer, capillary, and CE-MS system
- Prepare CE running buffer: Adjust the pH of 40 mM ammonium acetate to 9.0 with ammonium hydroxide. A 250 mL volumetric flask is recommended. Filter the 250 mL of buffer with 0.2 µm pore-sized membrane filters. Ensure to use only ultra-pure deionized water and MS-grade reagents.
NOTE: This buffer can be kept at room temperature for 2-3 weeks or for several months in a fridge. - Prepare sheath liquid: Mix 100 mL of ultra-pure water and 100 mL of LC-MS grade isopropanol in a 250 mL bottle. Change the sheath liquid at least once a week. Add mass reference into the sheath liquid when employing a high-resolution mass spectrometer.
- Install sheath liquid: Purge at 5 mL/min for 5 min and set the flow rate at 1 mL/min (10 µL/min into the CE-MS sprayer). The pump pressure will be at ca. 180 bar. Ensure that the recycle tubing connects back into the sheath liquid bottle to reuse the solvent.
- Prepare capillary: Purchase a CE-MS capillary (50 µm i.d. and 365 µM o.d. with a length of 125 cm) with a UV detection window. The user can also obtain much cheaper bar-fused silica capillaries from specialized distributors. Cut a capillary with a length of 100 cm. Properly cut both capillary ends with a capillary column cutter with a rotating diamond blade and remove 2-3 cm of polyimide coating on both ends with a lighter. Clean the capillary surface with isopropanol.
- Install capillary: Match the capillary into the CE-MS cassette. Click on the Change Cassette button and install the cassette into the CE device. The inlet end of the capillary is around 2 mm lower than the electrode. Ensure that the inlet end is lower than the sample's surface during the injection process.
- Activate capillary: Prior to first use, flush the capillary with 1 M NaOH, followed by water for 10 min, and CE running buffer for 15 min.
- Insert the capillary end into the CE-MS sprayer: Gently put the capillary into the CE-MS sprayer and ensure that the capillary end protrudes approximately 0.1 mm out of the sprayer tip. Ensure to make precise adjustment of the capillary outlet end with a magnifying glass and the adjustment screw in the sprayer. Insert the sprayer back into the ion source and avoid touching the adjustment screw. The MS is on Standby mode when performing this operation.
- Check ESI spray: Check the stability of the ESI sprayer under full scan mode. The fluctuation of total ion electropherograms must be within 5%.
- Perform a test run with InsP standards: Employ a mixture of 2 µM InsP3-InsP8 standards (adjusted by quantitative 31P NMR15,27) for test runs with an injection at 50 mbar for 10 s (10 nL). Set the detailed ESI and MS parameters as shown in Table 1. CE current is ca. 26 µA. The peak width is around 0.4-0.5 min. Ensure that the signal-to-noise ratio reaches at least 400.
3. Extraction of soluble inositol phosphates from mammalian cells
NOTE: HCT116NIH cells were a kind gift from Stephen Shears28. HCT116UCL cells were from Saiardi's Lab26.
- Seeding cells
- Culture HCT116NIH or HCT116UCL cells in T75 flasks at 37 °C in a 5% high humidity CO2 atmosphere (further referred to as standard conditions) in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
- Wash the HCT116NIH and HCT116UCL stock cultures with phosphate-buffered saline (PBS) (5 mL) and incubate the cells with trypsin-ethylenediamine tetraacetic acid (EDTA) (3 mL, 0.25%) under standard conditions until they are completely detached. Quench trypsin activity by adding medium (7 mL), collect the cells into a centrifuge tube, and centrifuge (200 x g, 3 min).
- Remove the supernatant and resuspend the cells in medium (10 mL). Count the cells and determine the viability via trypan blue exclusion.
- Seed the cells (6 million HCT116 cells per assay) into a 150 mm dish and adjust 20 mL of cell culture medium in total. Premix the medium and the cells in a centrifuge tube prior to seeding to achieve an equal distribution of the cells in the dish. Prepare a parallel dish when normalization by cell number is required.
- Culture the cells under standard conditions for 72 h. The cells will reach about 80%-90% confluence.
- Modulation of inositol phosphate levels with NaF and cell harvesting
- NaF treatment: Add NaF (10 mM) 1 h before harvesting into the medium. Mix the medium by swirling the plate/pipetting and incubate the cells for 60 min under standard conditions.
- After the NaF treatment, remove the medium from the cells and place the cells on ice.
- Wash the cells twice with PBS (5 mL, 4 °C) and remove the PBS completely from the dish.
- Add perchloric acid (PA) (1 mL, 1 M, 4 °C). Ensure to cover the whole surface with PA (the cells will turn white as proteins precipitate). Incubate the cells for 10 min on a tilt table at 4 °C.
- Collect PA into a centrifuge tube and remove the contaminating debris by centrifugation (17,000 x g, 5 min, 4 °C). Add the supernatant to the prepared TiO2 beads for the pulldown of InsPs.
- Wash the post-extraction dish twice with PBS (5 mL, r.t.) for deacidification; remove PBS completely from the dish.
- Solubilize the proteins on the plate via the addition of cell lysis buffer (1.5 mL, r.t.; 0.1% sodium dodecyl sulfate [SDS] in 0.1 M NaOH). Incubate the dish for 15 min on a tilt table at r.t. Transfer the cell lysate into a centrifuge tube and centrifuge (17,000 x g, 5 min, 4 °C). Store the supernatant at -80 °C until the protein concentration is determined via the DC protein assay using bovine serum albumin as calibration standard (usually, one 150 mm dish contains around 10 mg of proteins).
- Determination of cell numbers from parallel dishes: Harvest the cells of the parallel dish via trypsin as described in step 3.1.2 (use 5 mL trypsin-EDTA for the 150 mm dish) and remove the medium. Resuspend the cell pellet in PBS (5 mL), mix properly, and count the cells. Perform this step right before harvest via direct quenching to obtain representative cell counts. Additionally, measure the volume of the cells with an appropriate method (e.g., with a multisizer machine).
- TiO2 enrichment of inositol phosphates
NOTE: To avoid acidic decomposition of phosphorylated compounds, perform all the enrichment steps until elution on ice, and cool all reagents to 4 °C. Keep the time for the extraction to a minimum (1.5-2 h). Perform all the extraction steps with 1 M PA.- Preparation of beads: Wash TiO2 beads (5 mg per sample) with ddH2O (1 mL) and centrifuge (3,500 x g, 1 min, 4 °C). Remove ddH2O and wash the beads with PA (1 mL). Remove PA by centrifugation (3,500 x g, 1 min, 4 °C). Resuspend the beads in PA (50 µL per sample).
- Add the supernatant containing phosphorylated compounds (compare section 3.2, step 3.2.5) to the bead suspension, vortex, and then rotate the sample for 20 min at 4 °C.
- Centrifuge the sample (3,500 x g, 1 min, 4 °C) and discard the supernatant. Wash the beads with PA (500 µL) and centrifuge (3,500 x g, 1 min, 4 °C). Discard the supernatant and repeat the washing step.
- Add NH4OH (200 µL, 3%) to the beads and resuspend. Rotate the sample for 5 min at r.t.
- Centrifuge the sample (3,500 x g, 1 min) and transfer the supernatant into a new centrifuge tube.
- Repeat elution steps 3.3.4 and 3.3.5 and combine the eluents. Discard the beads.
- Centrifuge the combined eluents (17,000 x g, 1 min, 4 °C) to remove any insoluble residues.
- Completely dry the supernatant under vacuum evaporation (70 min, 60 °C, V-AQ). Add ddH2O (50 µL) to the dried extracts containing InsPs. Vortex to mix the sample until completely dissolved. Store the sample at -20 °C until CE-ESI-MS analysis.
4. Performing the CE-ESI-MS runs
- Prepare a mixture of internal standards containing 40 µM [13C6]1,5-(PP)2-InsP4, 80 µM [13C6]5-PP-InsP5, 80 µM [13C6]1-PP-InsP5, 400 µM [13C6]InsP6 and 400 µM [13C6]Ins(1,3,4,5,6)P5. Determine the concentrations of SIL IS solutions by quantitative 31P and 1H NMR, with the aid of a certified reference standard, i.e., phosphoacetic acid.
NOTE: All above SIL internal standards (IS) with purities higher than 96% were synthesized and provided by Fiedler group15,27. Same as with nucleotides, these IS could carry many crystal water molecules and diverse counter ions. Instead of weighing the substance and calculating the concentration, concentration determination of 13C6 standard solutions by quantitative 31P NMR is recommended. - Mix 10 µL of the sample with 0.5 µL of internal standards mixture in a CE sample vial. 2 µM [13C6]1,5-(PP)2-InsP4, 4 µM [13C6]5-PP-InsP5, 4 µM [13C6]1-PP-InsP5, 20 µM [13C6]InsP6, and 20 µM [13C6]Ins(1,3,4,5,6)P5 are the final concentrations inside the samples.
- When using the replenishment system, click on the Change Bottle button, put the prepared 250 mL of CE running buffer into the electrolyte bottle, and click on Clean Tubes. Keep the replenishment needle in a water vial.
- Set ESI and MS parameters as shown in Table 1. Optimize the source parameters using a Source Optimizer with a mixture of inositol polyphosphate standards. Obtain the multiple reaction monitoring (MRM) settings using Masshunter Optimizer with all standards. Adjust the ESI and MS/MS settings for different instrumentation.
- Perform a run for the InsP extracts and check the result (Figure 2). Set a sequence when there are more samples.
- Let the MS be on Standby mode after measurements. Do not turn off the LC pump. The flow of sheath liquid protects the sprayer needle. Replace the CE-ESI-MS Sprayer with an LC-ESI-MS Sprayer when there is no sheath liquid supply.
5. Data analysis
- Open Quantitative Analysis (for QQQ) software, create a batch for all samples.
- Create a new method from the acquired MRM data. Set the [13C6]InsPs as internal standards - (ISTD). Check MRM compound setup, retention time setup, ISTD setup, concentration setup, and qualifier setup. Pass the validation and exit to apply the method to the current batch. Save the method.
- Check whether each peak in the batch is properly integrated; otherwise, manually integrate the peak.
- Export the results into a spreadsheet. Perform the quantitation of inositol (pyro)phosphates by comparing the analyte peak response with the respective peak response of SIL IS with known concentrations. Theoretical and experimental calibration curves are shown in Table 2 for 5-PP-InsP5, InsP6, and Ins(1,3,4,5,6)P5 with the linear range.
NOTE: The precondition applying theoretical calibration curve is the usage of a high-quality isotopic pattern of targeted analytes and with credible concentration. Evaluate the linear range. A calibration curve is essential for absolute quantitation when the full acquisition of the above-mentioned isotopic standards is impractical. - With the measured concentration in the InsP extract solution and its volume, calculate the absolute amounts. Further, normalize the amount by cell counts or protein content. Calculate the cellular concentration based on cell counts and average cell volume of HCT116 (1.68 fL).
Results
The results shown here aim to illustrate the potential of CE-ESI-MS analysis. The reported figures are descriptive of a technically flawless CE-ESI-MS run. Firstly, a mixture of inositol pyrophosphate standards (Figure 1) and a mammalian cell extract (Figure 2) are presented. Secondly, a comparison of two HCT116 cell lines (Figure 3) and NaF-treated HCT116 (Figure 4) cells are provided.
Extracted ion electropherograms (EIEs) of inositol (pyro)phosphate standards at a concentration of 2 µM are shown in Figure 1. Metabolism of inositol pyrophosphates in mammals with their simplified structures is inserted. The four inositol pyrophosphates in mammals, 1,5-(PP)2-InsP4, 5-PP-InsP5, 1-PP-InsP5, and 5-PP-Ins(1,3,4,6)P4 are well distinguished using the described method. A CE-ESI-MS run of HCT116UCL is depicted in Figure 2. With the aid of stable isotope-labeled (SIL) internal standards, an absolute quantitation can be readily achieved by comparing the signal response with the spiked-in SIL of known concentration. The integrated EIEs of the inositol phosphate from InsP5 to (PP)2-InsP4 and un-integrated EIEs of their isotopic patterns are displayed. RSDs of all analytes from six technical repeats are within 4%. With the measured concentration and the volume of extracts, the amount of analytes can be calculated. With the cell counts and cell volume, or protein content, absolute cellular concentration (µM) or amount normalized by protein content (pmol/mg protein) are commonly the final outcomes of such an analysis.
According to an earlier study, two batches of diverged HCT116 cells have a variation of InsP8 levels, HCT116UCL cells contain 6-fold higher levels of InsP8 than HCT116NIH cells29. With the CE-MS method, 1,5-(PP)2-InsP4 in HCT116NIH could be easily quantified (Figure 3), and HCT116UCL cells contain 7-fold higher levels of InsP8 than in HCT116NIH. In addition, significant accumulation of 1,5-(PP)2-InsP4 in HCT116UCL cells is paralleled by a significantly increased 1-PP-InsP5, which is now quantitatively shown in Figure 3.
PP-InsPs levels increase by inhibiting their dephosphorylation using sodium fluoride. CE-ESI-MS analysis of NaF-treated HCT116NIH cells demonstrated the -5-PP-InsP5 elevation along with a reduction in InsP6 and an appearance of 5-PP-Ins(1,3,4,6)P4 (Figure 4). Besides, the elevation of InsP8 levels is noticeable, while 1-PP-InsP5 decreases to some degree. 1-PP-InsP5 is not completely absent in NaF-treated HCT116NIH, but mostly either under the limit of detection or quantitation.
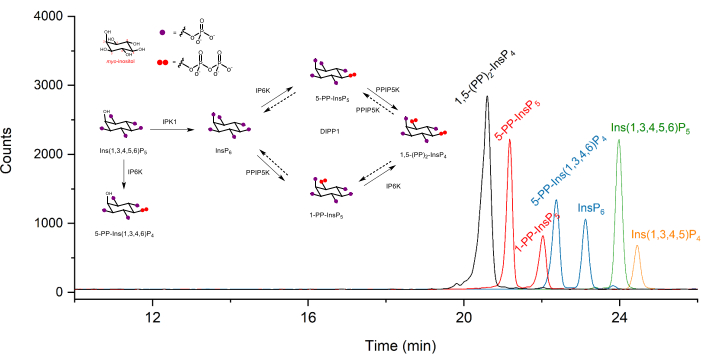
Figure 1: Typical extracted ion electropherograms (EIEs) of inositol (pyro)phosphate standards in CE-ESI-MS analysis using the described protocol. The concentration of each analyte is 2 µM. Injected sample volume is ca. 10 nL with an injection at 50 mbar for 10 s. Inserts show the metabolism of inositol pyrophosphates in mammals. IPPK: inositol pentakisphosphate 2-kinase, IP6K: inositol hexakisphosphate kinase, PPIP5K: diphosphoinositol pentakisphosphate kinase, DIPP1: diphosphoinositolpolyphosphate phosphohydrolase 1. Please click here to view a larger version of this figure.
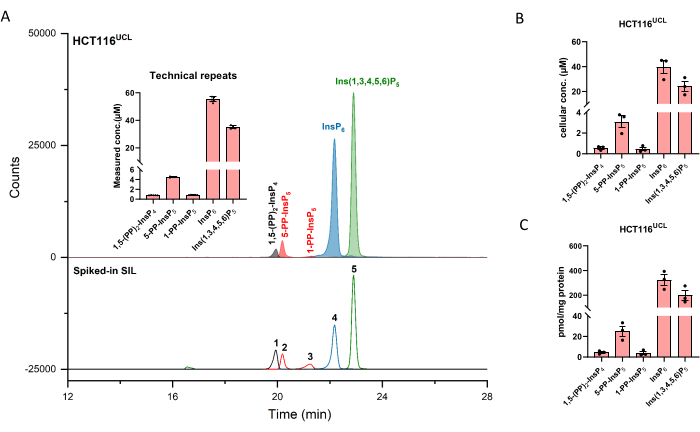
Figure 2: Representative InsP profile of HCT116UCL cells. (A) EIEs of the main inositol (pyro)phosphates in HCT116NIH and spiked SIL ISs 2 µM [13C6]1,5-(PP)2-InsP4 (1), 4 µM [13C6]5-PP-InsP5 (2), 4 µM [13C6]1-PP-InsP5 (3), 20 µM [13C6]InsP6 (4), and 20 µM [13C6]Ins(1,3,4,5,6)P5 (5). Inserts show six technical repeats of InsP analysis by CE-ESI-MS, data are presented as means ± SD. (B) Cellular concentration of PP-InsPs and InsPs in human cell lines HCT116UCL and (C) PP-InsPs and InsPs amount normalized by protein content. Data are means ± SEM from three independent experiments. Please click here to view a larger version of this figure.
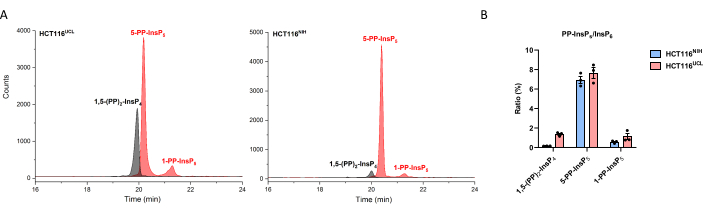
Figure 3: Variation in InsP8 levels between two diverged HCT116 cells. (A)EIEs of inositol pyrophosphate inHCT116UCL and HCT116NIH. InsP8 in HCT116UCL is markedly more abundant than in HCT116NIH. (B) Ratio of inositol pyrophosphate to InsP6 (%) in both HCT116 cells. HCT116UCL cells contain 7-fold higher levels of InsP8 as compared to in HCT116NIH, while the 5-PP-InsP5 levels are equal. Data are means ± SEM from three independent experiments. Please click here to view a larger version of this figure.
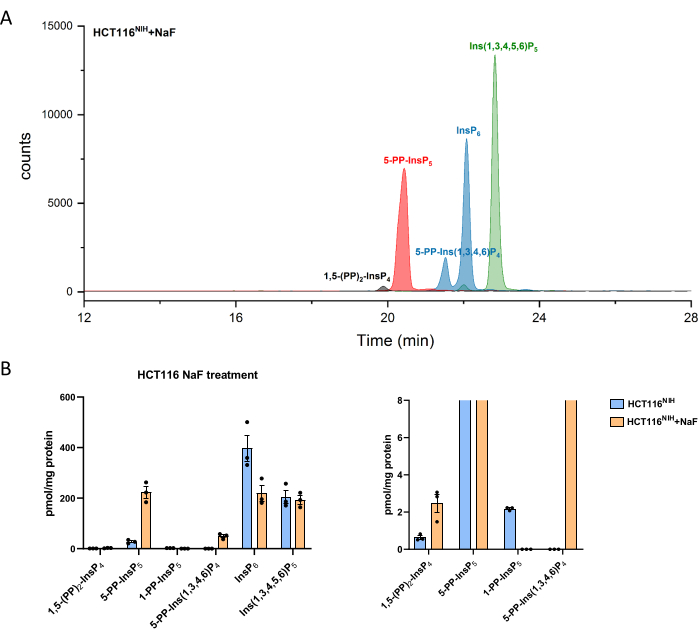
Figure 4: Inositol (pyro)phosphate levels in HCT116NIH cells, with NaF treatment. (A) EIEs of inositol (pyro)phosphate in HCT116NIH with sodium fluoride treatment (NaF, 10 mM). Levels of inositol pyrophosphate including 1,5-(PP)2-InsP4, 5-PP-InsP5, and 5-PP-Ins(1,3,4,6)P4 increase via blocking their dephosphorylation using NaF. (B) Inositol (pyro)phosphate levels (amounts are normalized by protein content) in untreated and NaF-treated HCT116NIH cells. Data are means ± SEM from three independent experiments. Please click here to view a larger version of this figure.
Table 1: CE-ESI-MS parameter settings. Source parameter and iFunnel parameters are optimized by Source and iFunnel Optimizer. MSM parameter settings for inositol (pyro)phosphates are optimized by MassHunter Optimizer. Please click here to download this Table.
Table 2: Theoretical and experimental regression equation. Concentration of [13C6]5-PP-InsP5 [13C6]InsP6, and [13C6]Ins (1, 3, 4, 5, 6)P5 is 4 µM, 20 µM, and 20 µM, respectively. For regression equation, 5-PP-InsP5 concentration is at 0.04 µM, 0.1 µM, 0.2 µM, 0.4 µM, 1 µM, 2 µM, 4 µM, 8 µM, 16 µM, 24 µM. InsP6 and Ins (1, 3, 4, 5, 6)P5 concentration is at 0.2 µM, 0.5 µM, 1 µM, 2 µM, 5 µM, 10 µM, 20 µM, 40 µM, 80 µM, 120 µM. x is concentration, y is (Area InsP)12C/(Area InsP)13C. Please click here to download this Table.
Discussion
Presented here is a practical and sensitive method for the quantitation of highly charged inositol pyrophosphates in mammalian cells. Combining this analysis approach with current state-of-the-art InsP extraction with perchloric acid followed by enrichment with TiO2, CE-ESI-MS analysis has unprecedented advantages. With regards to its throughput, sensitivity, stability, absolute quantitation, isomer identification, and matrix in-dependence, this method stands out compared to other approaches. This protocol is applicable to mammalian cells, but indeed this strategy succeeds in many different samples (e.g., yeast, plants, parasites, mouse tissues, etc.).
The applied extraction protocol fully recovers PP-InsPs and InsP6 from mammalian cell extracts16,19. It will also extract many other anionic metabolites, particularly phosphate-containing species, e.g., sugar phosphates and nucleotides. Evaluation of the recovery and decomposition for the user's analytes with this protocol would be necessary.
Generally, the CE-ESI-MS system runs smoothly and can accommodate around 200 samples every week using this protocol. Unlike HPLC, though, CE has been regarded as a method for experts and specialized persons for a long time, which restricted its market and limited its application. Thus, a CE-ESI-MS device is usually absent in analytical faculties. People who want to carry out CE-ESI-MS analysis probably lack CE experience and will spend more time troubleshooting. Here, the critical steps are highlighted. First and foremost is the quality of the capillary cut. The sensitivity and stability of ESI spray mostly rely on a first-class capillary cut. Secondly, the capillary outlet end should be exactly 0.1 mm out of the sprayer tip. The sprayer needle and the CE capillary should be in the axial direction. The quality of the ESI spray is critical for quantitation; technical runs should be performed to evaluate the repeatability.
With the described protocol, the limit of quantitation (LOQ) for PP-InsPs is 40 nM with an injection at 50 mbar for 10 s (10 nL). There are several approaches to further increase the method sensitivity. Firstly, an injection at 100 mbar for 20 s (40 nL) will still result in a good peak shape and sufficient resolution for regioisomers 5-PP-InsP5 and 1-PP-InsP5. Secondly, InsP extracts can be dissolved in a smaller amount of water. Thirdly, the dwell time could be increased when using less MRM transitions for quantitation. In addition, a CE-MS ion source using ultra-low sheath liquid flow would significantly increase the sensitivity.
The CE running buffer with pH 9 provides the best resolution between InsP6-InsP8. When increasing pH to 9.7, the resolution among InsP3-InsP6 will significantly improve. Due to the excellent resolution, a shorter capillary length of 72 cm is recommended for further increasing the throughput. Besides, a higher CE cassette temperature at 40 °C decreases the viscosity of the aqueous electrophoretic buffer and accelerates their movement under EOF. According to different research demands, modifications of this method can further facilitate InsPs and PP-InsPs analysis. Therefore, the described CE-ESI-MS protocols have the potential of opening novel research avenues into this multifaceted family of signaling molecules.
Disclosures
The authors declare no competing interests.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 864246, to HJJ). DQ acknowledges the financial support from the Brigitte-Schlieben-Lange-Programm. AS is supported by the MRC program grant MR/T028904/1.
Materials
| Name | Company | Catalog Number | Comments |
| Materials | |||
| 1.5 mL microcentrifuge tubes | Greiner Bio-One | 616201 | - |
| 15 cm tissue culture dishes | Thermo Fisher | 168381 | - |
| 2.0 mL microcentrifuge tubes | Greiner Bio-One | 623201 | - |
| 50 mL centrifuge tubes | Greiner Bio-One | 227261 | - |
| 96-well plates | Thermo Fisher | 260836 | for the DC protein assay |
| CE fused silica capillary | CS Chromatographie | 105180 | 50 µM i.d. 360 µM o.d. |
| Pipette tips | Starlab | I1054-0001, S1111-6701, S1113-1700, S1111-3700 | 10 mL, 1000 µL, 200 µL, 10 µL pipette tips |
| Serological pipets | TPP | 94550, 94525, 94010, 94005 | 50 mL, 25, mL, 10 mL, 5 mL serological pipettes |
| T75 flasks | TPP | 90076 | - |
| Chemicals and Reagents | |||
| NaOH | AppliChem | A6829,0500 | sodium hydroxide pellets for molecular biology, for preparation of cell lysis buffer |
| 0.25% trypsin-EDTA | Gibco | 25200056 | - |
| Ammonium acetate | Thermo Fisher | 1677373 | HPLC grade |
| BSA | Thermo Fisher | 23209 | albumin standard (2.0 mg/mL) for standard curve preparation |
| DC protein assay | Biorad | 5000116 | DC protein assay reagents package |
| DMEM | Gibco | 41966029 | high glucose, pyruvate |
| FBS | Gibco | 10270106, 10500064 (heat inactivated) | 10270106 for HCT116UCL, 10500064 for HCT116NIH |
| Isopropanol | Carl Roth | AE73.2 | 99.95% LC-MS grade |
| NH4OH, 10% | Carl Roth | 6756.1 | for preparation of 3% NH4OH |
| PBS | Gibco | 10010015 | - |
| Perchloric acid, 70% | Carl Roth | 9216.1 | for preparation of 1 M perchloric acid |
| SDS | SERVA | 20760.02 | for preparation of cell lysis buffer |
| Sodium fluoride | Sigma Aldrich | S7920 | - |
| TiO2 beads | GL Sciences | 5020-75000 | 5 µm particle size |
| Trypan blue solution | Gibco | 15250061 | trypan blue stain (0.4%) |
| Ultrapure (Type 1) water | Milli-Q | ZRQSVP3WW | model: Direct-Q 3 UV Water Purification System |
| Equipment | |||
| Analytical balance | Mettler Toledo | 30105893 | model: XPE26; for weighing of beads (5-6 mg per sample) |
| Automated cell counter | Logos Biosystems | L40002 | model: LUNA-II Automated Cell Counter |
| Benchtop centrifuge | Hettich | 1401 | model: UNIVERSAL 320 |
| Benchtop centrifuge with cooling | VWR | 521-1647P | model: Microstar 17R |
| CE system | Agilent | G7100A | - |
| CE/MS Adapter Kit | Agilent | G1603A | - |
| CE/MS Sprayer Kit | Agilent | G1607A | - |
| Cell counting slides | Logos Biosystems | L12001 | LUNA Cell Counting Slides |
| Centrifugal evaporator | Eppendorf | 5305000304 | model: Concentrator plus complete system |
| ESI source | Agilent | AJS ESI | - |
| Super Support Film | Nisshin EM Co. Ltd, Tokyo | 647 | |
| Humidified incubator | Binder | 9040-0088 | model: CB E6.1, for cultivation of mammalian cells |
| Ice box | - | - | should provide enough space for samples, dishes, etc. |
| Isocratic LC system | Agilent | G7110B 1260 Iso Pump | model: Infinity II Quaternary system |
| MSD | Agilent | G6495C | triple quadrupole |
| Multiplate reader | Tecan | 30086375 | model: SPARK 10 M |
| Pipette filler | Thermo Fisher | 10072332 | for serological pipettes |
| Pipettes | Brand | 705884, 705880, 705878, 705872, 705870 | various pipettes |
| Rotator | Labnet | H5500 | model: Mini LabRoller Rotator |
| Shortix capillary column cutter | SGT | S0020 | - |
| Test tube shaker (vortex mixer) | Carl Roth | HXH6.1 | model: Rotilabo-Mini Vortex |
| Tilt table | Labnet | S0600 | model: EDURO MiniMix Nutating Mixer |
| Water bath | Thermo Fisher | FSGPD05 | model: Isotemp GPD 05 |
| Software | |||
| MassHunter Workstation | Agilent | Version 10.1 | - |
| MassHunter Workstation LC/MS Data Acquisition | Agilent | Version 10.1 | - |
| MassHunter Workstation Optimizer | Agilent | Version 10.1 | - |
| MassHunter Workstation Qualitative Analysis | Agilent | Version 10.0 | - |
| QQQ Quantitaion Analysis | Agilent | Version 10.1 | - |
References
- Stephens, L., et al. The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s). The Journal of Biological Chemistry. 268 (6), 4009-4015 (1993).
- Menniti, F. S., Miller, R. N., Putney, J. W. Jr, Shears, S. B. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. The Journal of Biological Chemistry. 268 (6), 3850-3856 (1993).
- Shears, S. B. Inositol pyrophosphates: Why so many phosphates. Advances in Biological Regulation. 57, 203-216 (2015).
- Irvine, R. F., Schell, M. J. Back in the water: the return of the inositol phosphates. Nature Reviews Molecular Cell Biology. 2 (5), 327-338 (2001).
- Szijgyarto, Z., Garedew, A., Azevedo, C., Saiardi, A. Influence of inositol pyrophosphates on cellular energy dynamics. Science. 334 (6057), 802-805 (2011).
- Wild, R., et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science. 352 (6288), 986-990 (2016).
- Chakraborty, A., et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 143 (6), 897-910 (2010).
- Bittner, T., et al. Photolysis of caged inositol pyrophosphate InsP8 directly modulates intracellular Ca2+ oscillations and controls C2AB domain localization. Journal of the American Chemical Society. 142 (24), (2020).
- Hauke, S., et al. Photolysis of cell-permeant caged inositol pyrophosphates controls oscillations of cytosolic calcium in a β-cell line. Chemical Science. 10 (9), 2687-2692 (2019).
- Saiardi, A., Sciambi, C., McCaffery, J. M., Wendland, B., Snyder, S. H. Inositol pyrophosphates regulate endocytic trafficking. Proceedings of the National Academy of Sciences of the United States of America. 99 (22), 14206-14211 (2002).
- Koldobskiy, M. A., et al. p53-mediated apoptosis requires inositol hexakisphosphate kinase-2. Proceedings of the National Academy of Sciences of the United States of America. 107 (49), 20947(2010).
- Rao, F., et al. Inositol hexakisphosphate kinase-1 mediates assembly/disassembly of the CRL4-signalosome complex to regulate DNA repair and cell death. Proceedings of the National Academy of Sciences. 111 (45), 16005(2014).
- Williams, S. P., Gillaspy, G. E., Perera, I. Y. Biosynthesis and possible functions of inositol pyrophosphates in plants. Frontiers in Plant Science. 6, 67(2015).
- Wilson, M. S. C., Livermore, T. M., Saiardi, A. Inositol pyrophosphates: between signalling and metabolism. The Biochemical Journal. 452 (3), 369-379 (2013).
- Harmel, R. K., et al. Harnessing 13C-labeled myo-inositol to interrogate inositol phosphate messengers by NMR. Chemical Science. 10 (20), 5267-5274 (2019).
- Qiu, D., et al. Analysis of inositol phosphate metabolism by capillary electrophoresis electrospray ionization mass spectrometry. Nature Communications. 11 (1), 6035(2020).
- Azevedo, C., Saiardi, A. Extraction and analysis of soluble inositol polyphosphates from yeast. Nature Protocols. 1 (5), 2416-2422 (2006).
- Wilson, M. S. C., Saiardi, A. Importance of radioactive labelling to elucidate inositol polyphosphate signalling. Phosphate Labeling and Sensing in Chemical Biology. , 67-87 (2017).
- Wilson, M. S. C., Bulley, S. J., Pisani, F., Irvine, R. F., Saiardi, A. A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine. Open Biology. 5 (3), 150014(2015).
- Losito, O., Szijgyarto, Z., Resnick, A. C., Saiardi, A. Inositol pyrophosphates and their unique metabolic complexity: analysis by gel electrophoresis. PloS One. 4 (5), 5580(2009).
- Dong, J., et al. Inositol pyrophosphate InsP8 acts as an intracellular phosphate signal in arabidopsis. Molecular Plant. 12 (11), 1463-1473 (2019).
- Riemer, E., et al. ITPK1 is an InsP6/ADP phosphotransferase that controls systemic phosphate homeostasis in Arabidopsis. bioRxiv. , (2020).
- Whitfield, H., et al. An ATP-responsive metabolic cassette comprised of inositol tris/tetrakisphosphate kinase 1 (ITPK1) and inositol pentakisphosphate 2-kinase (IPK1) buffers diphosphosphoinositol phosphate levels. The Biochemical Journal. 477 (14), 2621-2638 (2020).
- Ito, M., et al. Hydrophilic interaction liquid chromatography-tandem mass spectrometry for the quantitative analysis of mammalian-derived inositol poly/pyrophosphates. Journal of chromatography. A. 1573, 87-97 (2018).
- Mantilla, B. S., Amaral, L. D. D., Jessen, H. J., Docampo, R. the inositol pyrophosphate biosynthetic pathway of Trypanosoma cruzi. ACS Chemical Biology. 16 (2), 283-292 (2021).
- Wilson, M. S. C., Saiardi, A. Inositol phosphates purification using titanium dioxide beads. Bio-Protocol. 8 (15), 2959(2018).
- Puschmann, R., Harmel, R. K., Fiedler, D. Scalable chemoenzymatic synthesis of inositol pyrophosphates. Biochemistry. 58 (38), 3927-3932 (2019).
- Gu, C., et al. KO of 5-InsP7 kinase activity transforms the HCT116 colon cancer cell line into a hypermetabolic, growth-inhibited phenotype. Proceedings of the National Academy of Sciences of the United States of America. 114 (45), 11968-11973 (2017).
- Gu, C., Wilson, M. S. C., Jessen, H. J., Saiardi, A., Shears, S. B. Inositol Pyrophosphate Profiling of Two HCT116 Cell Lines Uncovers Variation in InsP8 Levels. PloS One. 11 (10), 0165286(2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved