A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
An Experimental Approach to Investigating Effects of Artificial Light at Night on Free-Ranging Animals: Implementation, Results, and Directions for Future Research
In This Article
Summary
Artificial light at night (ALAN) has wide-reaching biological effects. This article describes a system for manipulating ALAN inside nest boxes while monitoring behavior, consisting of LED lights coupled to a battery, timer, and audio-capable infrared video camera. Researchers could employ this system to explore many outstanding questions regarding the effects of ALAN on organisms.
Abstract
Animals have evolved with natural patterns of light and darkness. However, artificial light is being increasingly introduced into the environment from human infrastructure and recreational activity. Artificial light at night (ALAN) has the potential to have widespread effects on animal behavior, physiology, and fitness, which can translate into broader-scale effects on populations and communities. Understanding the effects of ALAN on free-ranging animals is non-trivial due to challenges such as measuring levels of light encountered by mobile organisms and separating the effects of ALAN from those of other anthropogenic disturbance factors. Here we describe an approach that allows us to isolate the effects of artificial light exposure on individual animals by experimentally manipulating light levels inside nest boxes. To this end, a system can be used consisting of light-emitting diode (LED) light(s) adhered to a plate and connected to a battery and timer system. The setup allows exposure of individuals inside nest boxes to varying intensities and durations of ALAN while simultaneously obtaining video recordings, which also include audio. The system has been used in studies on free-ranging great tits (Parus major) and blue tits (Cyanistes caeruleus) to gain insight into how ALAN affects sleep and activity patterns in adults and physiology and telomere dynamics in developing nestlings. The system, or an adaptation thereof, could be used to answer many other intriguing research questions, such as how ALAN interacts with other disturbance factors and affects bioenergetic balance. Furthermore, similar systems could be installed in or near the nest boxes, nests or burrows of a variety of species to manipulate levels of ALAN, evaluate biological responses, and work towards building an interspecific perspective. Especially when combined with other advanced approaches for monitoring the behavior and movement of free-living animals, this approach promises to yield ongoing contributions to our understanding of the biological implications of ALAN.
Introduction
Animals have evolved with the natural patterns of light and darkness that define day and night. Thus, circadian rhythms in hormonal systems orchestrate rest and activity patterns and allow animals to maximize fitness1,2,3. For instance, the circadian rhythm in glucocorticoid hormones, with a peak at the onset of daily activity, primes vertebrates to behave appropriately across the 24-h period via effects on glucose metabolism and responsiveness to environmental stressors4. Similarly, the pineal hormone melatonin, which is released in response to darkness, is integrally involved in governing patterns of circadian rhythmicity and also has antioxidant properties5,6. Entrainment of many aspects of circadian rhythmicity, such as melatonin release, is affected by the photoreception of levels of light in the environment. Thus, the introduction of artificial light into the environment to support human activity, recreation, and infrastructure has the potential to have wide-reaching effects on the behavior, physiology and fitness of free-ranging animals7,8. Indeed, diverse effects of exposure to artificial light at night (ALAN) have been documented9,10, and ALAN has been highlighted as a priority for global change research in the 21st century10.
Measuring the effects of ALAN on free-ranging animals poses non-trivial challenges for a number of reasons. First, mobile animals moving through the environment constantly experience different levels of light. Thus, how does one quantify the level of light that individual animals are exposed to? Even if levels of light on the territory of the animal can be quantified, the animal may employ avoidance strategies that affect patterns of exposure, thus demanding simultaneous tracking of animal location and light levels. Indeed, in most field studies, the mean and variation in light exposure levels are unknown11. Second, exposure to ALAN is often correlated with exposure to other anthropogenic disturbance factors, such as noise pollution, chemical exposure, and habitat degradation. For instance, animals occupying habitats along the margins of roadways will be exposed to light from street lamps, noise from vehicular traffic, and air pollution from vehicular emissions. How then does one effectively isolate the effects of ALAN from the effects of confounding variables? Rigorous field experiments that enable good measurements of both light exposure levels and response variables are essential to evaluating the severity of the biological effects of ALAN, and to developing effective mitigation strategies11.
This article describes an experimental approach that, although not without its limitations (see discussion section), helps assuage, if not eliminate the difficulties identified above. The approach entails experimentally manipulating ALAN levels inside the nest boxes of a free-living, diurnal bird species, the great tit (Parus major), using a system of light-emitting diode (LED) lights and an infrared (IR) camera installed within nest boxes. The setup enables simultaneous acquisition of video recordings, including audio, which allows researchers to assess effects on behaviors and vocalizations. Great tits utilize nest boxes for breeding, and sleep in the nest boxes between November and March. Females also sleep inside the nest boxes during the breeding season12. The system has also been used to a lesser extent to study effects of ALAN on blue tits (Cyanistes caeruleus). The first difficulty, involving knowing light levels encountered by the animal, is mitigated in that, given that an individual is willing to enter the nest box (or is already in the nest box in the case of immobile nestlings), light levels can be precisely determined by the researcher. The second difficulty, involving correlations to confounding variables, can be controlled by using nest boxes in similar environments, and/or measuring the levels of confounding variables near nest boxes. In addition, in cavity-nesting birds, adopting an experimental approach is powerful because nest boxes or natural cavities can shield nestlings and adults from ALAN13, which may explain why some correlative studies find little effect of ALAN (or anthropogenic noise)14, whereas experimental studies more often find clear effects (see below). Moreover, a repeated measures experimental design can be adopted in which individuals serve as their own control, which further increases statistical power, and the probability of detecting meaningful biological effects. The sections below: (1) explain the details of the design and implementation of the system, (2) summarize the important results that have been thus far derived using the system, and (3) propose future research directions that could be pursued, both in tits and other animals.
Access restricted. Please log in or start a trial to view this content.
Protocol
All applications of this system to animal experiments were approved by the University of Antwerp's ethical committee and conducted in accordance with Belgian and Flemish laws. Methodology adhered to the ASAB/ABS guidelines for the use of animals in behavioral research. The Belgian Royal Institute for Natural Sciences (Koninklijk Belgisch Instituut voor Natuurwetenschappen; KBIN) provided licenses for all researchers and personnel.
1. Creating the experimental system
- Obtain broad-spectrum LED(s) to use in creating ALAN. Take LED light(s) from a LED headlight. Use either a single LED light or multiple (e.g., 4) broad-spectrum LED lights for more diffuse lighting (Figure 1).
NOTE: As a modification, LEDs with different spectral properties (e.g., red versus blue) could be used but would have to be obtained from a different source (see the Supplementary material of Grunst et al. 201915 for the spectral properties of the LEDs used in past studies using this system). - Design a system to mount the LEDs along with an IR camera to allow for behavioral monitoring. Researchers can accomplish this end in a number of ways.
- Option 1. Insert a single broad-spectrum LED into the nest box separately in a plastic tube adjacent to an IR camera mounted with adhesive on a plastic or metal plate that fits within the nest box (Figure 1A, B).
- Option 2. Mount an IR camera in a central position on a plastic or metal plate and then mount LED lights in fixed positions on the plate surrounding the IR camera (Fig. 1C).
- Design a means to connect the system to a power source (battery) and timer.
- Use a knife or drill to make groves in the side of the nest box through which wire connectors can extend to connect the system to a Fe-battery (12 V; 120 Wh) and homemade timer (12 V).
- Design a dark green wooden enclosure that matches the nest box in coloration, length, and width (e.g., nest boxes used in past studies had the dimensions: 120 mm x 155 mm x 250 mm ), and with one side opening via a hinge to house the battery, recorder for the video, and timer system for the LEDs (Figure 2; Supplementary Figure 1 and Supplementary Figure 2).
- Design a means through which to adjust ALAN intensity.
- Obtain a resistor (value contingent on battery voltage and illumination) and connect it in series with the LED(s).
- Design "dummy" boxes with the same dimensions as the enclosures that house the timer and battery for use in habituating birds to the system (i.e., as in Figure 2A, but without the internal electronics).
NOTE: Section 2 and section 3 discuss the step-by-step methods used to study the effects of ALAN on the focal organism.
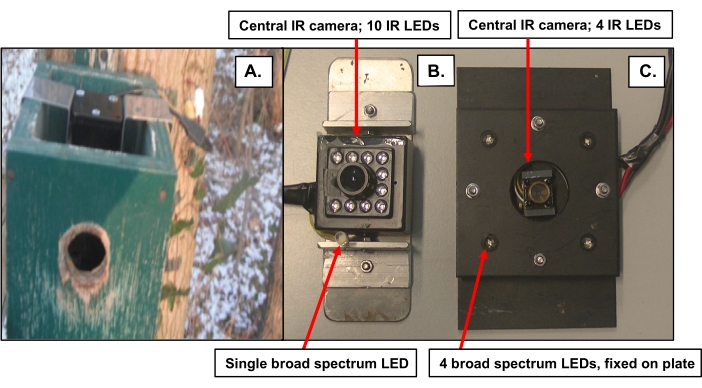
Figure 1: Two systems consisting of IR cameras and LED light(s) used to manipulate ALAN inside nest boxes. (A) Top view of the nest box with plate holding the older system in place. (B) Older system with 1 broad-spectrum LED to manipulate ALAN and central camera with 10 IR LEDs (c) Newer system with 4 broad-spectrum LEDs and central IR camera with 4 IR LEDs. Please click here to view a larger version of this figure.
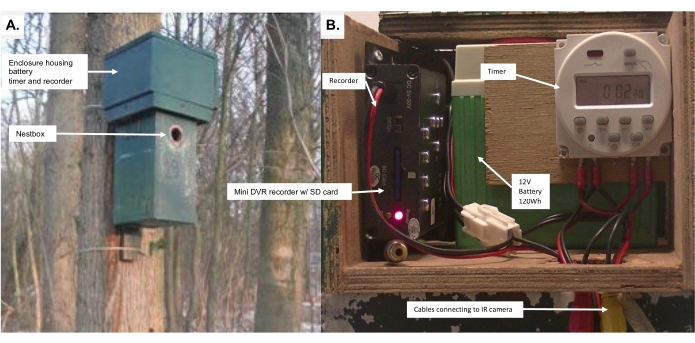
Figure 2: The homemade battery and timer unit used to manipulate ALAN and video-record behavior. (A) The unit is enclosed within a wooden box that is mounted on top of the nest box. (B) View of the electronics inside the unit. Connectors extend from inside the nest box up into the wooden enclosure to connect the electronics to the IR camera and broad-spectrum LEDs. Please click here to view a larger version of this figure.
2. Planning the experiment and adjusting ALAN intensity and timing
- Determine the desired light intensity to which to expose animals.
- Carefully consider which experimental light intensity to use so as to produce meaningful results that answer the research question. In general, this will mean selecting an ecologically relevant light intensity, which free-ranging animals are likely to encounter (see Table 1 for guidance).
- Adjust the LED lights to the desired light intensity (e.g., 1-3 lux, as used in past studies; Table 1 and Table 2).
- Prior to placement in the field, place the system on a nest box taken into the laboratory to calibrate the light intensity. Connect the LEDs to the power source, as described further below (Protocol section 3).
- Adjust the light emitted by the LEDs to the desired intensity (lux) by placing a light meter at the level of the bird within the nest box (~8 cm from the bottom) and simultaneously adjusting the resistor in series with the LEDs.
NOTE: It is possible to achieve very low light intensities (e.g., rural sky glow levels; 0.01 lux).
- Determine the timeframe over which to expose animals to ALAN.
- Determine the length and timing of exposure across the night. For instance, one can expose animals to ALAN across the entire night, for only part of the night, or leave a period of darkness in the middle of the night to reduce the degree of perturbation.
- In cases that an animal must enter the nest box (or a specific area) to be exposed to the ALAN, also consider whether the light should be turned on before or after the entry event is likely to occur.
- Set the timer to control the period of light exposure during the night.
- Set the timer connected to the broad spectrum LEDs so that the light turns on and off at specified periods (e.g., on at least 2 h before sunset; off 2 h after sunrise).
NOTE: The IR camera allows the behavior of the animal to be recorded simultaneously for the duration of the light exposure and will be on as long as it is connected to a charged battery.
- Set the timer connected to the broad spectrum LEDs so that the light turns on and off at specified periods (e.g., on at least 2 h before sunset; off 2 h after sunrise).
- Determine the appropriate experimental design to use for the target research question(s).
NOTE: For some questions, a repeated measures experimental design will be the most powerful option (e.g., How does exposure to ALAN affect sleep behavior?). For others, paired control and experimental groups will be needed (e.g., How does exposure to ALAN affect telomere loss in developing nestlings?).
| Source/exposure level | Intensity (lux) |
| Full sunlight | 103000 |
| Full moonlight | 0.05–1 |
| Urban Sky glow | 0.2–0.5 |
| Exposure of free-living European blackbirds | 0.2 (0.07–2.2) |
| Past experimental studies using the system | 1–3 |
| LED street lights | ~10 |
| Low pressure sodium street lights | ~10 |
| High pressure sodium | ~10 |
| Florescent lighting | 300 |
| Metal halide | 400–2000 |
Table 1: Characteristic light intensities in the environment3,9, exposure levels of free-ranging birds41, and intensities used in past studies using this system (references in Table 2).
3. Implementing the exposure to ALAN
- Habituate the animals to the experimental setup.
- If possible within the context of the experiment, habituate animals to the setup by placing dummy boxes on the top of the nest boxes at least 1 day prior to the experiment to minimize the effects of novelty aversion.
- Survey the focal individuals.
- Fit animals in the study population with passive integrative transponder (PIT) tags to allow for identification within nest boxes without disturbing the birds.
- In experiments involving the effect of ALAN on sleep behavior, visit the nest boxes on the night before the experiment and scan the boxes with a radio-frequency identification (RFID) reader to determine which birds are roosting inside.
- In experiments during the breeding season involving exposure of developing nestlings to ALAN, consistently monitor (e.g., every other day) nest boxes, and check for nest contents and adult identity. Carefully select nest boxes containing broods with certain characteristics (i.e., modal brood size, both parents present and feeding) for use in the experiment.
- Select and implement the experiment.
- For experiments involving sleep behavior, implement a repeated measures design by first recording individuals sleeping under conditions of darkness for at least one night to record undisturbed sleep in the absence of ALAN (control treatment) following steps 3.3.2-3.3.21.
- To this end, make sure to synchronize the time on the IR cameras with the local time prior to taking them into the field.
- Insert an SD card into the SD slot in the mini DVR recorder adjacent to the battery (Figure 2B; Supplementary Figure 2). Check to make sure that the SD card is empty, and if not, erase the data it contains.
- At least 2 h prior to the onset of darkness, remove the dummy box from on top of the nest box.
- Open the nest box lid.
- Place the plate containing the IR camera inside the nest box with the camera objective oriented downward.
- Extend the electronic connectors out of the grove in the nest box.
- Close the nest box lid.
- Place the enclosure containing the battery, recorder, and timer on top of the nest box.
- Connect the battery power connectors. Connect the red connector from the recorder to the white connector from the camera (audio), the yellow connector from the recorder to the yellow connector from the camera (video), and the black connector from the battery to the red connector from the camera (power) (Supplementary Figure 1 and Supplementary Figure 2).
- Push the record button to initiate the camera recording.
NOTE: The timer will not be set and/or the power will not be connected to the timer controlling the LEDs so that no ALAN will be produced on control nights. - Check with a small tft screen to ensure the recording has started and that the image is correct. A port to connect the tft screen is located below the recorder (Supplementary Figure 2).
- Approximately 1 h after dark, return to the nest box and check the identity of the bird sleeping inside by moving a RFID transponder reader around the bottom and sides of the nest box and recording the unique identification number communicated from the PIT tag.
- On the morning following the control recording, at least 2 h after sunrise, return to the nest box and collect the battery system and IR camera.
- Again, place a dummy box on top of the nest box.
- In the laboratory or office, charge the battery and remove and download the SD card from the recorder to collect the behavioral data.
NOTE: Batteries have a life span of ~30 h in cold conditions to enable recording for the entire night, but need to be fully recharged between consecutive nights of recording. - After successfully downloading the data, erase the data from the SD card and then reinsert it into the mini DVR recorder.
- On the subsequent night, implement the light exposure treatment (e.g., 1-3 lux, as used in past experiments using the system; Table 1 and Table 2).
- Set the timer system for the desired time period of light exposure.
- Follow the same steps (3.3.2-3.3.17) described above for the control recording, but also connect the timer to the power and the LEDs to the timer (Supplementary Figure 1 and Supplementary Figure 2).
- If desired, repeat the control recording (of sleep behavior under conditions of darkness, i.e., absence of ALAN) on night three.
- For experiments involving exposure of nestlings to ALAN, use control and experimental broods as described in steps 3.3.23-3.3.25.
- Place dummy boxes (lacking electronics) on top of the nest boxes of control broods and handle both control and experimental nestlings in equivalent ways.
- Implement the experimental ALAN exposure for experimental boxes. During the experimental period, mount the LED system and IR camera within the nest box, as described above, and set the timer to control the desired period of light exposure.
- Recharge the batteries. For experiments involving multiple nights of light exposure and video recording, collect the systems each morning to recharge the batteries during the day and then replace the system in the evening.
- Collect data on the response variable(s) of interest.
- If behavior within the nest box is the variable of interest, the IR camera will allow simultaneously documenting behavior (e.g., sleep behavior; Figure 3).
- Collect any other data of interest via additional monitoring methods, with sampling occurring at variable points in time (e.g., blood samples taken before and after light exposure15).
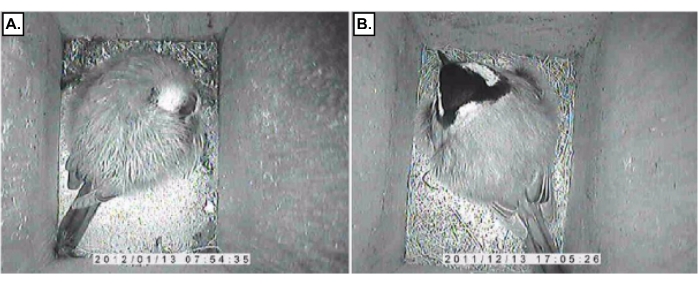
Figure 3: Infrared image of a great tit inside a nest box exposed to ALAN. (A) Sleeping and (B) Alert great tit Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Results
The peer-reviewed research articles published using this system are summarized in Table 2. Several other manuscripts are in progress. These studies address three major suites of research questions. First, the system has been used to study the effects of light exposure on sleep behavior and activity levels in adults. To this end, a repeated measures experimental design was employed, in which the same individual was first recorded sleeping under natural conditions and subsequently recorded sleeping in a li...
Access restricted. Please log in or start a trial to view this content.
Discussion
This nest box-based system of LED lights and a paired IR camera has allowed researchers to assess a range of intriguing questions regarding the biological effects of ALAN. Moreover, there are many more research directions that can be pursued with the system. In addition, expanding the use of the system to other species could help foster an understanding of interspecific differences in sensitivity to ALAN. Below some non-exhaustive possibilities for future research are presented in the hope that this paper will help motiv...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
Our research program involving the biological effects of ALAN on birds has received funding from the FWO Flanders (to M.E. and R.P., project ID: G.0A36.15N), the University of Antwerp and the European Commission (to M.L.G, Marie Skłodowska-Curie fellowship ID: 799667). We acknowledge the intellectual and technical support of members of the Behavioral Ecology and Ecophysiology Research group at the University of Antwerp, especially Peter Scheys and Thomas Raap.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Broad spectrum; 15 mm x 5 mm; LED headlight | RANEX; Gilze; Nederlands | 6000.217 | A similar model could also be used |
| Battery | BYD | R1210A-C | Fe-battery 12 V 120 Wh ( lithium iron phosphate battery) |
| Dark green paint | Optional. To color nest boxes/electronic enclosures | ||
| Electrical tape | For electronics | ||
| Homemade timer system | Amazon | YP109A 12V | A similar model could also be used |
| Infrared camera | Koberts-Goods, Melsungen, DE | 205-IR-L | Mini camera; a similar model could also be used |
| Light level meter | ISO-Tech ILM; Corby; UK | 1335 | To calibrate light intensity |
| Mini DVR video recorder | Pakatak, Essex, UK | MD-101 | Surveillance DVR Recorder Mini SD Car DVR with 32 GB |
| Passive integrated transponder (PIT) tags | Eccel Technology Ltd, Aylesbury, UK | EM4102 | 125 Kh; Provides unique electronic ID |
| Radio frequency identification (RFID) Reader | Trovan, Aalten, Netherlands | GR-250 | To scan PIT tags and determine bird identity |
| Resistor | RS Components | Value depending on voltage battery and illumination | |
| SD card | SanDisk | 64 GB or larger | |
| SongMeter | Wildlife Acoustics; Maynard, MA | Optional. Provides a means of monitoring vocalizations outside of nest boxes | |
| TFT Color LED Portable Test Monitor | Walmart | Allows verification that the camera is on and recording the image correctly | |
| Wood | To construct nest boxes/electronic encolsures |
References
- Gwinner, E., Brandstätter, R. Complex bird clocks. Philosophical Transactions of the Royal Society of London B. 356 (1415), 1801-1810 (2001).
- Dominoni, D., Helm, B., Lehmann, M., Dowse, H. B., Partecke, J. Clocks for the city: circadian differences between forest and city songbirds. Proceedings of the Royal Society of London B. 280 (1763), 20130593(2013).
- Ouyang, J. Q., Davies, S., Dominoni, D. Hormonally mediated effects of artificial light at night on behavior and fitness: linking endocrine mechanisms with function. Journal of Experimental Biology. 221, (2018).
- Mohawk, J., Pargament, J., Lee, T. Circadian dependence of corticosterone release to light exposure. in the rat. Physiology and Behavior. 92 (5), 800-806 (2007).
- Reiter, R., Tan, D., Osuna, C., Gitto, E. Actions of melatonin in the reduction of oxidative stress: a review. Journal of Biomedical Science. 7 (6), 444-458 (2000).
- Jones, T., Durrant, J., Michaelides, E., Green, M. P. Melatonin: a possible link between the presence of artificial light at night and reductions in biological fitness. Philosophical Transactions of the Royal Society of London B. 370 (1667), 20140122(2020).
- Fonken, L. K., Nelson, R. J. The effects of light at night on circadian clocks and metabolism. Endocrine Reviews. 35 (4), 648-670 (2014).
- Falcón, J., et al. Exposure to artificial light at night and the consequences for flora, fauna, and ecosystems. Frontiers in Neuroscience. 14, 602796(2020).
- Gaston, K. J., Bennie, J., Davies, T. W., Hopkins, J. The ecological impacts of nighttime light pollution: a mechanistic approach. Biological Reviews. 88 (4), 912-927 (2013).
- Davies, T. W., Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Global Change Biology. 24 (3), 872-882 (2017).
- Raap, T., Pinxten, R., Eens, M. Rigorous field experiments are essential to understand the genuine severity of light pollution and to identify possible solutions. Global Change Biology. 23 (12), 5024-5026 (2017).
- Raap, T., Sun, J. C., Pinxten, R., Eens, M. Disruptive effects of light pollution on sleep in free-living birds: season and/or light intensity-dependent effects. Behavioral Processes. 144, 13-19 (2017).
- Raap, T., Pinxten, R., Eens, M. Cavities shield birds from effects of artificial light at night on sleep. Journal of Experimental Zoology A. 329 (8-9), 449-456 (2018).
- Casasole, G., et al. Neither artificial light at night, anthropogenic noise nor distance from roads are associated with oxidative status of nestlings in an urban population of songbirds. Comparative Biochemistry and Physiology A. 210, 14-21 (2017).
- Grunst, M. L., Raap, T., Grunst, A. S., Pinxten, R., Eens, M. Artificial light at night does not affect not telomere shortening in a developing free-living songbird: a field experiment. Science of the Total Environment. 662, 266-275 (2019).
- Raap, T., Pinxten, R., Eens, M. Light pollution disrupts sleep in free-living animals. Scientific Reports. 5, 13557(2015).
- Raap, T., Pinxten, R., Eens, M. Artificial light at night disrupts sleep in female great tits (Parus major) during the nestling period, and is followed by a sleep rebound. Environmental Pollution. 215, 125-134 (2016).
- Raap, T., Thys, B., Grunst, A. S., Grunst, M. L., Pinxten, R., Eens, M. Personality and artificial light at night in a semi-urban songbird population: no evidence for personality-dependent sampling bias, avoidance or disruptive effects on sleep behaviour. Environmental Pollution. 243 (2), 1317-1324 (2018).
- Raap, T., et al. Artificial light at night affects body mass but not oxidative status in free-living nestling songbirds: an experimental study. Scientific Reports. 6, 35626(2016).
- Grunst, M. L., et al. Early-life exposure to artificial light at night elevates physiological stress in free-living songbirds. Environmental Pollution. 259, 113895(2020).
- Raap, T., Casasole, G., Pinxten, R., Eens, M. Early life exposure to artificial light at night affect the physiological condition: an experimental study on the ecophysiology of free-living nestling songbirds. Environmental Pollution. 218, 909-914 (2016).
- Raap, T., Pinxten, R., Eens, M. Artificial light at night causes an unexpected increase in oxalate in developing male songbirds. Conservation Physiology. 6 (1), 005(2018).
- Sun, J., Raap, T., Pinxten, R., Eens, M. Artificial light at night affects sleep behaviour differently in two closely related songbird species. Environmental Pollution. 231 (1), 882-889 (2017).
- Ziegler, A. -K., et al. Exposure to artificial light at night alters innate immune response in wild great tit nestlings. Journal of Expimental Biology. 224 (10), (2021).
- Dominoni, D. M., Teo, D., Branston, C. J., Jakhar, A., Albalawi, B. F. A., Feather Evans, N. P. but not plasma, glucocorticoid response to artificial light at night differs between urban and forest blue tit nestlings. Integrative and Comparative Biology. 16 (3), 1111-1121 (2021).
- Levy, K., Wegrzyn, Y., Efronny, R., Barnea, A., Ayali, A. Lifelong exposure to artificial light at night impats stridulation and locomotion activity patterns in the cricket Gryllus bimaculatus. Proceedings of the Royal Society of London B. 288 (1959), 20211626(2021).
- Dominoni, D., Smit, J. A. H., Visser, M. E., Halfwerk, W. Multisensory pollution: artificial light at night and anthropogenic noise have interactive effects on activity patterns of great tits (Parus major). Environmental Pollution. 256, 113314(2020).
- Ouyang, J. Q., de Jong, M., Hau, M., Visser, M. E., van Grunsven, R. H. A., Spoelstra, K. Stressful colours: Corticosterone concentrations in a free-living songbird vary with the spectral composition of experimental illumination. Biology Letters. 11 (8), 20150517(2015).
- Van Dis, N. E., Spoelstra, K., Visser, M. E., Dominoni, D. M. Colour of artificial light at night affects incubation behaviour in the great tit, Parus major. Frontiers in Ecology and Evolution. 9, 697(2021).
- Welbers, A. A. M. H., et al. Artificial light at night reduces daily energy expenditure in breeding great tits (Parus major). Frontiers in Ecology and Evolution. 5, 55(2017).
- Lighton, J. R. B. Measuring metabolic rates: A manual for scientists. , Oxford University Press, Oxford Scholarship Online. (2008).
- Butler, P. J., Green, J. A., Boyd, I. L., Speakman, J. R. Measuring metabolic rate in the field: The pros and cons of the doubly labeled water and heart rate methods. Functional Ecology. 18 (2), 168-183 (2004).
- Elliott, H., Le Vaillant, M., Kato, A., Speakman, J. R., Ropert-Coudert, Y. Accelerometry predicts daily energy expenditure in a bird with high activity levels. Biology Letters. 9, 20120919(2013).
- Pettersen, A. K., White, C. R., Marshall, D. J. Metabolic rate covaries with fitness and pace of the life history in the field. Proceedings of the Royal Society of London B. 283 (1831), 20160323(2016).
- Grunst, A. S., Grunst, M. L., Pinxten, R., Bervoets, L., Eens, M. Sources of individual variation in problem-solving performance in urban great tits (Parus major): Exploring effects of metal pollution, urban disturbance and personality. Science of the Total Environment. 749, 141436(2020).
- Croston, R., Kozlovsky, D. Y., Branch, C. L., Parchman, T. L., Bridge, E. S., Pravosudoy, V. V. Individual variation in spatial memory performance in wild mountain chickadees from different elevations. Animal Behaviour. 111, 225-234 (2016).
- Iserbyt, A., Griffioen, M., Borremans, B., Eens, M., Müller, W. How to quantify animal activity from radio-frequency identification (RFID) recordings. Ecology and Evolution. 8 (20), 10166-10174 (2018).
- Naef-Daenzer, B., Fruh, D., Stalder, M., Wetli, P., Weise, E. Miniaturization (0.2 g) and evaluation of attachment techniques of telemetry transmitters. Journal of Experimental Biology. 208 (21), 4063-4068 (2005).
- Van Hasselt, S. J., Rusche, M., Vyssotski, A. L., Verhulst, S., Rattenborg, N. C., Meerlo, P. Sleep time in European starlings is strongly affected by night length and moon phase. Current Biology. 30 (9), 1664-1671 (2020).
- Eberle, M., Kappeler, P. M. Family insurance: kin selection and cooperative breeding in a solitary primate (Microcebus murinus). Behavioral Ecology Sociobiology. 60 (4), 582-588 (2006).
- Dominoni, D. M., Quetting, M., Partecke, J. Artificial light at night advances avian reproductive physiology. Proceedings of the Royal Society of London B. 280, 20123017(2013).
- De Jong, M., Ouyang, J. Q., van Grunsven, R. H. A., Visser, M. E., Spoelstra, K. Do wild great tits avoid exposure to light at night. Plos ONE. 11 (6), 0157357(2016).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved