Method Article
A Fluorescence-Based Assay of Membrane Potential for High-Throughput Functional Study of Two Endogenous Ion Channels in Two Epithelial Cell Lines
In This Article
Summary
This protocol describes a method for the study of electrogenic membrane proteins by measuring changes in membrane potential. This assay provides a platform for the functional readout of multiple ion channels endogenously expressed in epithelial cell lines.
Abstract
Fluorescence-based studies are suitable for high-throughput plate reader assays of cells in culture. They have been commonly employed for drug discovery campaigns targeting recombinant ion channel proteins overexpressed in cells such as HEK-293 cells. However, there is increasing emphasis on the use of tissue-relevant cell lines for studying the effects of small molecule interventions. The following protocol describes the adaptation of a fluorescence-based membrane potential assay for the study of ion channels endogenously expressed in epithelial cell lines. The membrane potential assay details a high-throughput assay for chloride channel activity of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in two commonly studied epithelial cell lines, Caco-2 and Calu-3. In addition, this paper describes a novel application of this system to measure the activity of the Epithelial Sodium Channel (ENaC) in a high-throughput format in the same epithelial cell lines. Together, these fluorescence-based assays provide a robust and flexible platform for studying small molecule modulators, targeting two epithelial channels in a relevant cellular context.
Introduction
Fluorescence-based, high-throughput activity assays of recombinant channel proteins have been highly effective in identifying small-molecule modulators that have the potential to become future therapies1,2,3,4. One common method of screening small molecule libraries for ion channel modulators is through measuring changes in membrane potential. Typically, these screens are conducted using recombinant channels expressed heterologously in cell lines such as HEK-293 cells5,6,7.
However, there is a need for validation of "hits" in relevant cell types. We propose that a secondary screen in relevant cell lines that endogenously express the channels of interest is desirable. Such a secondary screen will identify those modulators most likely to be effective in final validation assays that rely on less accessible primary human tissues.
There is also the need for assay systems that have the flexibility to report the activity of multiple ion channel targets in the same tissue type. For example, there is substantive published literature that supports the concept that CFTR and ENaC functionally interact8,9,10. Although several well-studied epithelial cell lines are known to express both CFTR and ENaC, to date, the assay conditions for studying both in a high-throughput manner have not been described.
This fluorescence-based membrane potential assay is versatile, as it employs an exogenous chemical probe to measure the function of CFTR and ENaC, bypassing the need for genetically encoded probes11. As proof of principle, two commonly used epithelial cell lines are studied as examples to highlight the critical steps necessary for measuring channel activity of both CFTR and ENaC.
Protocol
1. Cell plating and differentiation
- Culture Calu-3 and Caco-2 cells in EMEM containing 20% fetal bovine serum (FBS) and 1% penicillin-streptomycin (pen/strep) in a T-75 flask.
- Once the cells reach 80-100% confluency, aspirate the medium from the T-75 flask.
- Gently wash the cells once with 10 mL of phosphate-buffered saline (PBS).
- Add 2 mL of prewarmed 0.25% Trypsin/0.1% EDTA to the cell monolayer and place the T-75 flask at 37 °C for approximately 5 min to allow the cells to lift from the culturing flask surface and dissociate into a single-cell suspension.
- Once the cells are lifted from the flask surface, neutralize the reaction with 8 mL of culture medium (step 1.1) for a total of 10 mL of cell suspension.
NOTE: Mechanically dissociate cell clumps into a single-cell suspension with a 10 mL serological pipette. - Plate the cells onto a 96-well plate (~140,000-150,000 cells/well) or a 384-well plate (~45,000-50,000 cells/well) at 30-40% confluency.
- To plate one full 96-well plate, add 1.5 mL of the cell suspension to 18.5 mL of the culture medium in a 50 mL conical tube. After mixing, add 200 µL of the cell suspension to each well.
- To plate one full 384-well plate, add 1 mL of the cell suspension to 17 mL of the culture medium in a 50 mL conical tube. After mixing, add 50 µL of the cell suspension to each well.
NOTE: Ensure there are no clumps of cells, and single cells are distributed evenly across each well.
- Change the medium every 2 days after plating. If the cells do not reach confluency at the same time between different wells, discard the plate and repeat step 1.5.
- Once seeded, wait for the cells to reach 100% confluency within 3-5 days. Change the medium 24 h prior to the functional studies.
2. CFTR functional assay buffer solution preparation
- Prepare the sodium-free, chloride-free buffer solution with the reagents listed in Table 1.
- Add the reagents to tissue culture grade, double-distilled H2O (ddH2O). Allow the reagents to dissolve (in a closed container to avoid evaporation) by stirring overnight at room temperature.
- Once the solution is stable, measure the pH. If the pH of the solution exceeds 7.4, add 1 M N-methyl-D-glucamine (NMDG) solution dropwise to adjust the pH to 7.4.
NOTE: Do not adjust the pH of the solution with HCl because the solution must remain chloride-free. - Allow the solution to mix for another 30 min and measure the osmolarity of the solution.
- Reduce the osmolarity of buffer solution to a physiological range of 300 ± 5 mOsm/kg.
- Filter and store the buffer solution in sterile bottles.
NOTE: The solution can be stored at 4 °C for up to 6 months. If stored for longer than 6 months, check the pH and osmolarity at room temperature before use.
3. CFTR functional assay
- Dissolve the membrane potential dye in the sodium-free, chloride-free buffer solution (0.5 mg of dye/1 mL of buffer solution) and warm it to 37 °C.
NOTE: Avoid exposing the membrane potential dye powder to light. - Remove the culture medium from Calu-3 or Caco-2 cell monolayers and add the dye-containing solution to each well (195 µL per well for a 96-well plate, 95 µL per well for a 384-well plate).
NOTE: Up to 112 µL can be added per well of 384-well plates. - Allow the cells to load the dye for 35 min at 37 °C and 5% CO2.
- Move the cells, loaded with dye solution, to the fluorescence plate reader. Take fluorescence measurements from the bottom of the plate, with excitation = 530 ± 5 nm, emission = 560 ± 5 nm
- Begin by taking continuous baseline readings for 5 min at 30 s intervals.
- Add 5 µL of a low-concentration solution of forskolin or DMSO to each well for a final concentration of 1 µM forskolin (1x). Take a stimulation reading for 20 min with measurements at 15 s intervals.
- For the 96-well plate format, prepare the low-concentration (40 µM forskolin (1:25 dilution)) solution of forskolin by diluting 2 µL of 1 mM forskolin (1,000x) stock solution or DMSO in 48 µL of sodium-free, chloride-free buffer solution.
NOTE: The low-concentration 40 µM forskolin is followed by a second dilution (1:40 dilution) in the next step for a final concentration of 1 µM forskolin. - For the 384-well plate format, prepare the low-concentration (20 µM forskolin (1:50 dilution)) solution by diluting 1 µL of 1 mM (1,000x) forskolin stock solution or DMSO in 49 µL of sodium-free, chloride-free buffer solution.
NOTE: The low-concentration 20 µM forskolin is followed by a second dilution (1:20 dilution) in the next step for a final concentration of 1 µM forskolin.
- For the 96-well plate format, prepare the low-concentration (40 µM forskolin (1:25 dilution)) solution of forskolin by diluting 2 µL of 1 mM forskolin (1,000x) stock solution or DMSO in 48 µL of sodium-free, chloride-free buffer solution.
- Add 5 µL of low-concentration solution of CFTRInh172 for a final concentration of 10 µM CFTRInh172. Take an inhibition reading for 15 min with measurements at 30 s intervals.
- For the 96-well plate format, prepare the CFTRInh172 low-concentration (400 µM CFTRInh172 (1:25 dilution)) solution by diluting 2 µL of a 10 mM CFTRInh172 (1,000x) stock solution in 48 µL of sodium-free, chloride-free buffer solution.
NOTE: The low-concentration 400 µM CFTRInh172 is followed by a second dilution (1:40 dilution) in the next step for a final concentration of10 µM CFTRInh172. - For the 384-well plate format, prepare the CFTRInh172 low-concentration (200 µM CFTRInh172 (1:50 dilution)) solution by diluting 1 µL of 10 mM CFTRInh172 (1,000x) stock solution in 49 µL of sodium-free, chloride-free buffer solution.
NOTE: The low-concentration 200 µM CFTRInh172 is followed by a second dilution (1:20 dilution) in the next step for a final concentration of10 µM CFTRInh172.
- For the 96-well plate format, prepare the CFTRInh172 low-concentration (400 µM CFTRInh172 (1:25 dilution)) solution by diluting 2 µL of a 10 mM CFTRInh172 (1,000x) stock solution in 48 µL of sodium-free, chloride-free buffer solution.
- Quantify the fluorescence intensity measurements of each well, and export the values as a spreadsheet in column format containing individual wells. To calculate forskolin-induced changes, divide the RFU (Relative Fluorescence Units) measurements from each well of the 96- or 384-well plate by the last fluorescence intensity measurement of the baseline reading and plot them.
- Alternatively, quantify CFTRInh172-mediated inhibition response to better confirm the specificity of CFTR function12, as acute treatment with forskolin may result in membrane depolarization through the activity of other chloride channels, such as SLC26A912.
- Measure the peak responses as the maximum fluorescence intensity measurement from baseline during forskolin stimulation. Use this measurement or area under the curve to quantify the CFTR response.
4. ENaC functional assay buffer solution preparation
- Prepare the high-sodium, chloride-free buffer solution with the reagents listed in Table 2.
- Once the solution is stable, measure the pH. If the pH of the solution is below 7.4, add 1 N NaOH solution dropwise to adjust the pH to 7.4.
NOTE: Do not adjust the pH of the solution with HCl because the solution must remain chloride-free. - Reduce the osmolarity of buffer solution to a physiological range of 300 ± 5 mOsm/kg.
- Filter and store the buffer solution in sterile bottles at 4 °C for up to 6 months.
NOTE: If storage is longer than 6 months, check the pH and osmolarity at room temperature before use.
5. ENaC functional assay
- Dissolve the membrane potential dye in the high-sodium, chloride-free buffer solution (0.5 mg of dye/1 mL of buffer solution) and warm it to 37 °C.
NOTE: Avoid exposing the membrane potential dye powder to light. - Remove the culture medium from Calu-3 or Caco-2 cell monolayers and add the dye-containing solution to each well (195 µL per well for 96-well plates, 95 µL per well for 384-well plates).
NOTE: Avoid exposing the dye solution to light. - Allow the cells to load dye for 35 min at 37 °C and 5% CO2.
- Move the cells, loaded with dye solution, to the fluorescence plate reader. Read fluorescence measurements from the bottom of the plate, with excitation = 530 ± 5 nm, emission = 560 ± 5 nm.
- Begin by taking continuous baseline readings for 5 min at 30 s intervals.
- To inhibit ENaC function, add 5 µL of low-concentration solution amiloride or low-concentration solution DMSO dissolved in the high sodium, zero chloride buffer solution for a final concentration of 10 µM amiloride.
- For the 96-well plate format, prepare the low-concentration (400 µM amiloride (1:25 dilution)) solution of amiloride by diluting 2 µL of 10 mM amiloride (1,000x) stock solution in 48 µL of high-sodium, chloride-free buffer solution.
NOTE: The low-concentration 400 µM amiloride is followed by a second dilution (1:40 dilution) in the next step for a final concentration of 10 µM amiloride. - For the 384-well plate format, prepare the low-concentration (200 µM amiloride (1:50 dilution)) solution of amiloride by adding 1 µL of 10 mM amiloride (1,000x) stock solution in 49 µL of high-sodium, chloride-free buffer solution.
NOTE: The low-concentration 200 µM amiloride is followed by a second dilution (1:20 dilution) in the next step for a final concentration of 10 µM amiloride.
- For the 96-well plate format, prepare the low-concentration (400 µM amiloride (1:25 dilution)) solution of amiloride by diluting 2 µL of 10 mM amiloride (1,000x) stock solution in 48 µL of high-sodium, chloride-free buffer solution.
- Take an inhibition reading for 60 min with measurements at 45 s intervals.
- Repeat step 3.7 for data analysis.
- Measure the amiloride response as the percent inhibition from baseline to the point of plateau, approximately 15-20 min after amiloride addition. Use this difference to quantify the ENaC response.
Results
To measure CFTR channel activity, well-differentiated, adhered cells are incubated in a zero-chloride extracellular solution containing the membrane potential-sensitive dye. CFTR function is consistently detected as membrane depolarization after forskolin stimulation. Chloride efflux is detected as a sharp increase in fluorescence compared to the vehicle control. The fluorescence signal is sustained until the addition of CFTR inhibitor (CFTRInh172), leading to a rapid decline in the fluorescence intensity, as seen in Caco-2 cells (Figure 1A), and is reproducible in both Caco-2 and Calu-3 cells (Figure 1B). The assessment of CFTR activity as the difference in the maximal change in fluorescence after forskolin or the DMSO (vehicle). Individual points ranged between ± 3 standard deviations from the mean forskolin stimulation (black points) or DMSO control (grey points), which corresponded to a Z' factor of 0.586. This excellent score indicated the reproducibility of this assay (Figure 1C). In case there is a minor drift in the baseline, the impact of the drift can be minimized by extrapolating the baseline throughout the trace after activation up to the point of inhibitor addition. The response can be quantified as the area under the curve, with the lower limit defined by the extrapolated line.
ENaC activity can also be measured in confluent airway and colonic epithelial cells grown in 96-well plates. In contrast to the assay for CFTR channel activity, these cells are submerged in a high-sodium-containing solution, enabling sodium absorption through ENaC. With the acute addition of amiloride, sodium uptake is blocked, and cells experience relative membrane hyperpolarization. This is shown in Caco-2 cells at both low (10 µM) (Figure 2A) and high concentrations (50 µM) of amiloride (Figure 2A,B) and in Calu-3 cells with a high concentration of amiloride (50 µM) (Figure 2B). The ENaC assay was expanded to a 384-well plate format and demonstrated great reproducibility in ENaC inhibition. Similarly, the individual points ranged between ± 3 standard deviations from the mean amiloride inhibition (black points) or DMSO control (grey points). The ENaC response in Calu-3 cells reported a Z' factor of 0.629 with acute treatment using amiloride (50 µM) (Figure 2C), which indicates that these parameters support high-throughput screening.
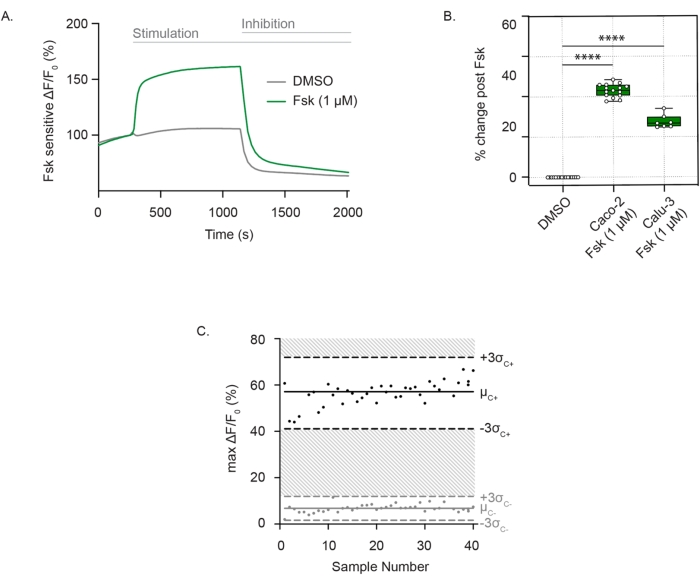
Figure 1: CFTR channel measurement as changes in membrane depolarization in Caco-2 and Calu-3 cells. (A) Representative trace of CFTR stimulation with forskolin (1 µM)and DMSO control in Caco-2 cells. (B) Box and whisker plot of forskolin stimulation in Caco-2 and Calu-3 cells (**** P < 0.0001, n > 3 biological replicates, n > 3 technical replicates). Biological replicates are independent cell line passages; technical replicates refer to independent wells of the multiwell plates. Box plot depicts the median value; bounds depict IQR, with the whiskers defining the minima and maxima values. (C) Bland-Altman plot of reproducible CFTR response with forskolin stimulation compared to DMSO control in Caco-2 cells. Black points represent maximal absolute changes in fluorescence in response to forskolin stimulation. Grey points represent changes in fluorescence in response to DMSO control. Abbreviations: CFTR = Cystic Fibrosis Transmembrane Conductance Regulator; DMSO = dimethyl sulfoxide; IQR = interquartile range; Fsk = forskolin; ΔF/F0 = change in fluorescence. Please click here to view a larger version of this figure.
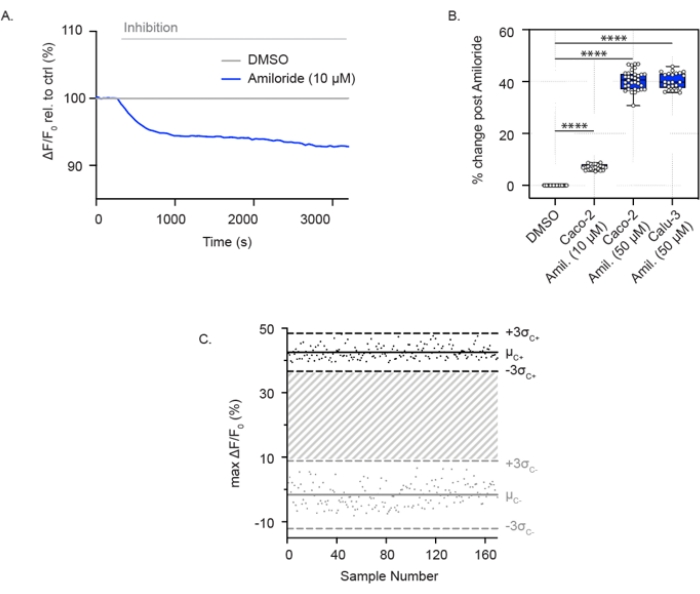
Figure 2: ENaC inhibition measurements through membrane hyperpolarization in Caco-2 and Calu-3 cells. (A) Representative trace of ENaC inhibition with amiloride (10 µM)relative to DMSO control in Caco-2 cells. (B) Box and whisker plot of amiloride inhibition in Caco-2 and Calu-3 cells (**** P < 0.0001, n > 3 biological replicates, n > 3 technical replicates). (C) Bland-Altman plot of reproducible ENaC response with amiloride inhibition compared to DMSO control in Calu-3 cells. Black points represent maximum absolute changes in fluorescence in response to amiloride inhibition. Grey points represent changes in fluorescence in response to DMSO control. Abbreviations: ENaC = epithelial sodium channel; DMSO = dimethyl sulfoxide; Amil. = amiloride; ΔF/F0 = change in fluorescence. Please click here to view a larger version of this figure.
| Name | Concentration |
| NMDG (N-Methyl-D-glucamine) | 150 mM |
| Gluconic Acid Lactone | 150 mM |
| Potassium Gluconate | 3 mM |
| HEPES | 10 mM |
Table 1: Reagents and their corresponding concentrations for CFTR functional assay with zero sodium and chloride. Abbreviation: CFTR = Cystic Fibrosis Transmembrane Conductance Regulator.
| Name | Concentration |
| Sodium Gluconate | 150 mM |
| Potassium Gluconate | 3 mM |
| HEPES | 10 mM |
Table 2: Reagents and their corresponding concentrations for ENaC functional assay buffer with high sodium, zero chloride. Abbreviation: ENaC = Epithelial sodium (Na) Channel.
Discussion
Here, we have described fluorescence-based methods for measuring CFTR and ENaC activity in the epithelial colorectal cancer cell line, Caco-2, and the human epithelial lung cancer cell line, Calu-3. These membrane potential assays in epithelial cell lines can be used to confirm the efficacy of small molecule modulator compounds, previously identified in heterologous expression systems, prior to final in vitro validation in primary patient-derived epithelial cultures.
It is imperative to confirm that the above measurements are appropriately attributed to the channel of interest. For example, the measurement ascribed to CFTR should show dependence on CFTR protein expression and the electrochemical driving force of chloride, which is modulated by forskolin and the inhibitor CFTRInh-172. Similarly, the membrane potential changes caused by 10 µM amiloride can be attributed to ENaC, if they are reliant on ENaC protein expression and an inward sodium driving force. Although we have confirmed these properties of ENaC in MDCK cells following transfection with all three subunits of ENaC2, we have not yet formally confirmed that the amiloride response measured in Caco-2 and Calu-3 cells is conferred by ENaC. This is particularly important, as amiloride can modulate the function of the sodium-proton exchanger NHE3 in addition to ENaC13. Conceivably, the amiloride-induced hyperpolarization observed in this assay could partially reflect the indirect effects of intracellular acidification on other channels. To confirm that the amiloride-induced hyperpolarization measured using this membrane potential-sensitive dye is conferred by ENaC in the above cell lines and more complex cell systems, such as iPSC-derived tissues, we are confirming that this activity is lost by knocking out an obligate ENaC subunit (beta) in these lines.
The success of these assays requires attention to several factors. First, the epithelial cultures must be confluent and well differentiated. For the measurement of CFTR and ENaC function, approximately 145,000 cells should be plated per well in 96-well plates or 45,000 cells per well in 384-well plates. Once the epithelial cells reach confluency (within 3-4 days from plating), allow 2-5 days of differentiation. This timing was determined previously based on maximal CFTR protein expression by immunoblotting14. Similarly, optimal timing was determined for functional ENaC expression in both cell lines, Calu-3 and Caco-2.
Nonconfluent monolayers or cell overgrowth leads to low reproducibility across technical replicates. In cases where cells in individual wells do not reach confluency at the same time, these plates should be discarded. Additionally, unstable baseline readings or lack of stimulation responses may be an indication of poor cell quality, which must be excluded from analysis. Reduced responses to the CFTR activator, forskolin, or the ENaC inhibitor, amiloride, could potentially reflect the use of excessively passaged cells15. Although not an issue for Calu-3 and Caco-2 cells, other cells or tissues may not adhere well to the plates and may require precoating of the wells. For example, in previous studies, 2D cholangiocyte cultures were attached to the plates using collagen coating16. Alternatively, the adherence of 2D intestinal tissues was increased using Poly-L-Lysine2.
Further, in testing the modulation of ion channels by small molecules using a fluorescence-based assay, it is critical that potential artifacts conferred by fluorescent properties of the small molecules be addressed. To confirm the specificity of functional responses, membrane potential assays can be further validated using conventional electrophysiological methods, such as Ussing studies17.
After confirming that the response detected by membrane potential-sensitive dyes is specifically conferred by the channel of interest, these assays have tremendous potential to validate promising modulators of epithelial ion channels in their relevant cellular context. This platform can bridge the gap between high-throughput modulator screens in heterologous expression systems and time-consuming, bioelectric measurements in difficult-to-access primary tissues.
Disclosures
The authors declare no competing interests.
Acknowledgements
The authors would like to thank Christopher Fladd and SPARC BioCentre at the Hospital for Sick Children for their help in conducting the membrane potential assays measuring CFTR and ENaC. This work was supported by the CFIT Program with funding provided by CF Canada and the Sick Kids Foundation. This work was funded by the Government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-148). This study was funded by the Government of Ontario. S.X. was supported by the Canadian Association of Gastroenterology (CAG) Ph.D. Scholarship.
Materials
| Name | Company | Catalog Number | Comments |
| Amiloride | Spectrum Chemical | TCI-A2599-5G | Dissolved in DMSO and stored at -20 °C |
| CFTRInh-172 | CF Foundation Therapeutics | Dissolved in DMSO and stored at -20 °C | |
| EMEM, 1xs | Wisent | 320-005-CL | |
| Fetal Bovine Serum (FBS) | Wisent | 080-450 | |
| FLIPR Membrane Potential Dye | Molecular Devices | R8042 | Stored at 4 °C |
| Forskolin | Sigma-Aldrich | F3917 | Dissolved in DMSO and stored at -20 °C |
| Gluconic acid lactone or (D-(+)-Gluconic acid δ-lactone) | Sigma-Aldrich | G4750 | Stored at room temperature |
| HEPES | Bioshop | HEP001.5 | Stored at room temperature |
| Human Bronchial Adenocarcinoma Cell Line (Calu-3) | ATCC | HTB-55 | |
| Human Epithelial Colorectal Adenocarcinoma Cell Line (Caco-2) | ATCC | HTB-37 | |
| N-Methyl-D-glucamine (NMDG | Sigma-Aldrich | M2004 | Stored at room temperature |
| Penicillin-Streptomycin Solution | Wisent | 450-200-EL | |
| Phosphate-Buffered Saline (PBS) | Wisent | 311-010-CL | |
| Potassium Gluconate | Sigma-Aldrich | P1847 | Stored at room temperature |
| Sodium Gluconate | Sigma-Aldrich | G9005 | Stored at room temperature |
References
- Ahmadi, S., et al. Phenotypic profiling of CFTR modulators in patient-derived respiratory epithelia. NPJ Genomic Medicine. 2, 12 (2017).
- Xia, S., et al. High-throughput functional analysis of CFTR and other apically localized proteins in iPSC-derived human intestinal organoids. Cells. 10 (12), 3419 (2021).
- Jiang, J. X., et al. A new platform for high-throughput therapy testing on iPSC-derived lung progenitor cells from cystic fibrosis patients. Stem Cell Reports. 16 (11), 2825-2837 (2021).
- Hu, J., et al. Human Embryonic Kidney 293 Cells: a vehicle for biopharmaceutical manufacturing, structural biology, and electrophysiology. Cells, Tissues, Organs. 205 (1), 1-8 (2018).
- Hadida, S., et al. Discovery of N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (VX-770, ivacaftor), a potent and orally bioavailable CFTR potentiator. Journal of Medicinal Chemistry. 57 (23), 9776-9795 (2014).
- Chen, M. X., et al. Validation and optimization of novel high-throughput assays for human epithelial sodium channels. Journal of Biomolecular Screening. 20 (2), 242-253 (2015).
- Maitra, R., Sivashanmugam, P., Warner, K. A rapid membrane potential assay to monitor CFTR function and inhibition. Journal of Biomolecular Screening. 18 (9), 1132-1137 (2013).
- Mall, M. A. ENaC inhibition in cystic fibrosis: potential role in the new era of CFTR modulator therapies. European Respiratory Journal. 56 (6), 2000946 (2020).
- Moore, P. J., Tarran, R. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease. Expert Opinion on Therapeutic Targets. 22 (8), 687-701 (2018).
- Stutts, M. J., et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 269 (5225), 847-850 (1995).
- Merkert, S., et al. High-throughput screening for modulators of CFTR activity based on genetically engineered cystic fibrosis disease-specific iPSCs. Stem Cell Reports. 12 (6), 1389-1403 (2019).
- Bertrand, C. A., et al. The CFTR trafficking mutation F508del inhibits the constitutive activity of SLC26A9. American Journal of Physiology. Lung Cellular and Molecular Physiology. 312 (6), 912-925 (2017).
- Frelin, C., et al. Amiloride and its analogs as tools to inhibit Na+ transport via the Na+ channel the Na+/H+ antiport and the Na+/Ca2+ exchanger. Biochimie. 70 (9), 1285-1290 (1988).
- Di Paola, M., et al. SLC6A14 is a genetic modifier of cystic fibrosis that regulates Pseudomonas aeruginosa attachment to human bronchial epithelial cells. mBio. 8 (6), 02073 (2017).
- Schiavi, S. C., et al. Biosynthetic and growth abnormalities are associated with high-level expression of CFTR in heterologous cells. American Journal of Physiology. 270, 341-351 (1996).
- Ogawa, M., et al. Generation of functional ciliated cholangiocytes from human pluripotent stem cells. Nature Communications. 12 (1), 6504 (2021).
- Lu, C., Pribanic, S., Debonneville, A., Jiang, C., Rotin, D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic. 8 (9), 1246-1264 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved