A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Far-Red Fluorescent Senescence-Associated β-Galactosidase Probe for Identification and Enrichment of Senescent Tumor Cells by Flow Cytometry
In This Article
Summary
A protocol for fluorescent, flow cytometric quantification of senescent cancer cells induced by chemotherapy drugs in cell culture or in murine tumor models is presented. Optional procedures include co-immunostaining, sample fixation to facilitate large batch or time point analysis, and the enrichment of viable senescent cells by flow cytometric sorting.
Abstract
Cellular senescence is a state of proliferative arrest induced by biological damage that normally accrues over years in aging cells but may also emerge rapidly in tumor cells as a response to damage induced by various cancer treatments. Tumor cell senescence is generally considered undesirable, as senescent cells become resistant to death and block tumor remission while exacerbating tumor malignancy and treatment resistance. Therefore, the identification of senescent tumor cells is of ongoing interest to the cancer research community. Various senescence assays exist, many based on the activity of the well-known senescence marker, senescence-associated beta-galactosidase (SA-β-Gal).
Typically, the SA-β-Gal assay is performed using a chromogenic substrate (X-Gal) on fixed cells, with the slow and subjective enumeration of "blue" senescent cells by light microscopy. Improved assays using cell-permeant, fluorescent SA-β-Gal substrates, including C12-FDG (green) and DDAO-Galactoside (DDAOG; far-red), have enabled the analysis of living cells and allowed the use of high-throughput fluorescent analysis platforms, including flow cytometers. C12-FDG is a well-documented probe for SA-β-Gal, but its green fluorescent emission overlaps with intrinsic cellular autofluorescence (AF) that arises during senescence due to the accumulation of lipofuscin aggregates. By utilizing the far-red SA-β-Gal probe DDAOG, green cellular autofluorescence can be used as a secondary parameter to confirm senescence, adding reliability to the assay. The remaining fluorescence channels can be used for cell viability staining or optional fluorescent immunolabeling.
Using flow cytometry, we demonstrate the use of DDAOG and lipofuscin autofluorescence as a dual-parameter assay for the identification of senescent tumor cells. Quantitation of the percentage of viable senescent cells is performed. If desired, an optional immunolabeling step may be included to evaluate cell surface antigens of interest. Identified senescent cells can also be flow cytometrically sorted and collected for downstream analysis. Collected senescent cells can be immediately lysed (e.g., for immunoassays or 'omics analysis) or further cultured.
Introduction
Senescent cells normally accumulate in organisms over years during normal biological aging but may also develop rapidly in tumor cells as a response to damage induced by various cancer treatments, including radiation and chemotherapy. Though no longer proliferating, therapy-induced senescent (TIS) tumor cells may contribute to treatment resistance and drive recurrence1,2,3. Factors secreted by TIS cells can exacerbate tumor malignancy by promoting immune evasion or metastasis4,5. TIS cells develop complex, context-specific phenotypes, altered metabolic profiles, and unique immune responses6,7,8. Therefore, the identification and characterization of TIS tumor cells induced by various cancer treatment approaches is a topic of ongoing interest to the cancer research community.
To detect TIS tumor cells, conventional senescence assays are widely used, primarily based on detecting increased activity of the senescence marker enzyme, the lysosomal beta-galactosidase GLB19. Detection at a near-neutral (rather than acidic) lysosomal pH allows for specific detection of senescence-associated beta-galactosidase (SA-β-Gal)10. A standard SA-β-Gal assay that has been used for several decades uses X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), a blue chromogenic beta-galactosidase substrate, to detect SA-β-Gal in fixed cells by light microscopy11. The X-Gal assay allows the qualitative visual confirmation of TIS utilizing commonly available reagents and laboratory equipment. A basic transmitted light microscope is the only instrumentation required to evaluate the presence of the blue chromogen. However, the X-Gal staining procedure can lack sensitivity, sometimes requiring more than 24 h for color to develop. Staining is followed by low-throughput, subjective scoring of individual senescent cells based on counting the cells exhibiting some level of intensity of the blue chromogen under a light microscope. As X-Gal is cell-impermeable, this assay requires solvent-fixed cells, which cannot be recovered for downstream analysis. When working with limited samples from animals or patients, this can be a major drawback.
Improved SA-β-Gal assays using cell-permeant, fluorescent enzyme substrates, including C12-FDG (5-dodecanoylaminofluorescein Di-β-D-Galactopyranoside, green) and DDAOG (9H-(1,3-dichloro-9,9-dimethylacridin-2-one-7-yl) β-D-Galactopyranoside, far-red) have previously appeared in the literature12,13,14,15. The chemical probe structure and optical characteristics of DDAOG are shown in Supplementary Figure S1. These cell-permeant probes permit the analysis of living (rather than fixed) cells, and fluorescent rather than chromogenic probes facilitate the use of rapid high-throughput fluorescent analysis platforms, including high-content screening instruments and flow cytometers. Sorting flow cytometers enable the recovery of enriched populations of living senescent cells from cell cultures or tumors for downstream analysis (e.g., western blotting, ELISA, or 'omics). Fluorescence analysis also provides a quantitative signal, allowing for more accurate determination of the percentage of senescent cells within a given sample. Additional fluorescent probes, including viability probes and fluorophore-labeled antibodies, can readily be added for multiplexed analysis of targets beyond SA-β-Gal.
Similar to DDAOG, C12-FDG is a fluorescent probe for SA-β-Gal, but its green fluorescent emission overlaps with intrinsic cellular AF, which arises during senescence due to the accumulation of lipofuscin aggregates in cells16. By utilizing the far-red DDAOG probe, green cellular AF can be used as a secondary parameter to confirm senescence17. This improves assay reliability by using a second marker in addition to SA-β-Gal, which can often be unreliable as a single marker for senescence18. As the detection of endogenous AF in senescent cells is a label-free approach, it is a rapid and simple way to expand the specificity of our DDAOG-based assay.
In this protocol, we demonstrate the use of DDAOG and AF as a rapid, dual-parameter flow cytometry assay for the identification of viable TIS tumor cells from in vitro cultures or isolated from drug-treated tumors established in mice (Figure 1). The protocol uses fluorophores compatible with a wide range of standard commercial flow cytometry analyzers and sorters (Table 1). Quantitation of the percentage of viable senescent cells using standard flow cytometry analysis is enabled. If desired, an optional immunolabeling step may be performed to evaluate cell surface antigens of interest concurrently with senescence. Identified senescent cells can also be enriched using standard fluorescence-activated cell sorting (FACS) methodology.
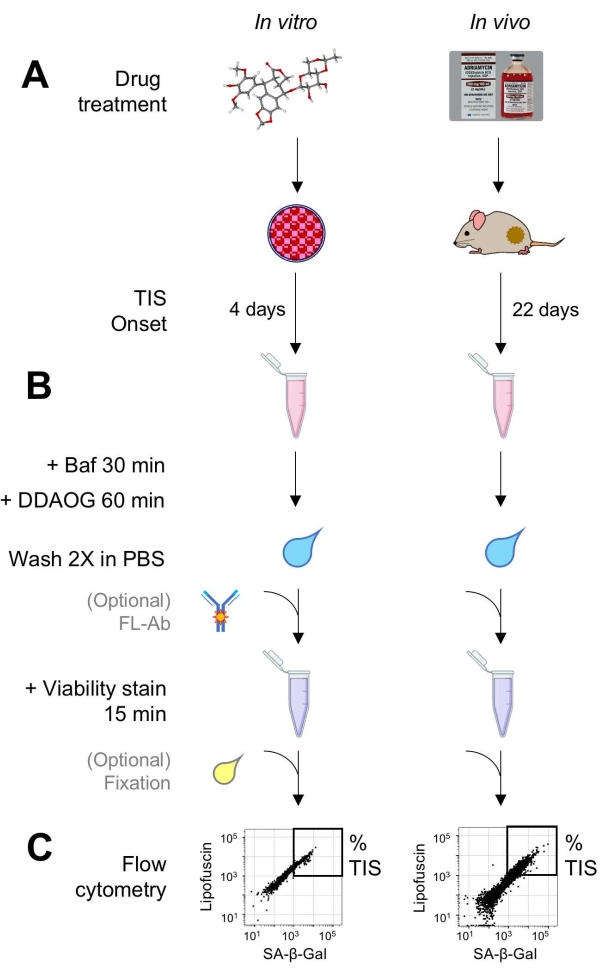
Figure 1: Experimental workflow. A schematic summarizing key points of the DDAOG assay. (A) A TIS-inducing drug is added to mammalian cultured cells or administered to tumor-bearing mice. Time is then allowed for the onset of TIS: for cells, 4 days following treatment; for mice, 22 days total, with three treatments every 5 days plus 7 days recovery. Cells are harvested or tumors are dissociated into suspension. (B) Samples are treated with Baf to adjust lysosomal pH for detection of SA-β-Gal for 30 min; then, DDAOG probe is added for 60 min to detect SA-β-Gal. Samples are washed 2x in PBS, and a viability stain is briefly added (15 min). Optionally, samples can be stained with fluorescent antibodies in open fluorescence channels and/or fixed for later analysis. (C) Samples are analyzed using a standard flow cytometer. Viable cells are visualized in dot plots showing red DDAOG (indicating SA-β-Gal) versus green autofluorescence (lipofuscin). A gate to determine the percentage of TIS cells is established based on untreated control samples (not shown). If a sorting cytometer (FACS) is used, TIS cells can be collected and placed back into culture for further in vitro assays or lysed and processed for molecular biology assays. Abbreviations: DDAO = 9H-(1,3-dichloro-9,9-dimethylacridin-2-one); DDAOG = DDAO-Galactoside; TIS = therapy-induced senescence; FL-Ab = fluorophore-conjugated antibody; Baf = Bafilomycin A1; SA-β-Gal = senescence-associated beta-galactosidase; PBS = phosphate-buffered saline; FACS = fluorescence-activated cell sorting. Please click here to view a larger version of this figure.
| Fluorophore | Detects | Ex/Em (nm) | Cytometer laser (nm) | Cytometer detector / bandpass filter (nm) |
| DDAOG | SA-β-Gal | 645/6601 | 640 | 670 / 30 |
| AF | Lipofuscin | < 600 | 488 | 525 / 50 |
| CV450 | Viability | 408/450 | 405 | 450 / 50 |
| PE | Antibody/surface marker | 565/578 | 561 | 582 / 15 |
Table 1: Fluorophores and cytometer optical specifications. Cytometer specifications used in this protocol are listed for an instrument with a total of 4 lasers and 15 emission detectors. DDAOG detected at 645/660 nm is the form of the probe cleaved by SA-β-Gal1. Uncleaved DDAOG can exhibit low level fluorescence at 460/610 nm but is removed by wash steps in the protocol. Abbreviations: DDAO = 9H-(1,3-dichloro-9,9-dimethylacridin-2-one); DDAOG = DDAO-Galactoside; AF = autofluorescence; PE = phycoerythrin; SA-β-Gal = senescence-associated beta-galactosidase.
Protocol
All animal work described was approved by the Institutional Animal Care and Use Committee at the University of Chicago.
1. Preparation and storage of stock solutions
NOTE: If cells will be flow-sorted, all solutions should be prepared using sterile techniques and filtered through a 0.22 µm filter device.
- Prepare a stock solution of DDAO-Galactoside at 5 mg/mL in DMSO. Aliquot at 50 µL per tube (or volume desired). Store at −20 °C in the dark for up to 1 year.
- Prepare a stock solution of Bafilomycin A1 at 1 mM in DMSO. Aliquot at 50 µL per tube (or volume desired). Store at −20 °C for up to 6 months.
- Prepare a stock solution of Calcein Violet 450 AM at 1 mM in DMSO. Aliquot at 50 µL per tube (or volume desired). Store at −20 °C in the dark for up to 1 year.
- For treatment of cultured cells in vitro, prepare 10 mM concentrated stock solutions of senescence-inducing agent(s) in the appropriate solvent, and sterilize using 0.2 µm syringe filters. Store at −20 °C or as directed by the manufacturer.
NOTE: For delivery of senescence-inducing chemotherapy agents in vivo (to mice with established tumors), the agents should be USP grade and diluted into saline from concentrated stock just prior to injection. - Prepare complete culture medium for the cell line(s) being used.
NOTE: For example, prepare medium for B16-F10 or A549 cells with DMEM 1x + 10% FBS + 1x glutamine substitute + 1x penicillin/streptomycin. Media must be kept sterile. Other cell line-specific media formulations can be used. Certain components such as glutathione may, in some cases, interfere with the onset of senescence. Empirical testing of various media formulations should be conducted if the onset of senescence is lower than expected or not observed with control chemotherapy agents. - Prepare staining and wash buffers.
- Prepare 1% bovine serum albumin (BSA) in 1x PBS for use in staining procedures. Dissolve 2 g of BSA into 200 mL of PBS and stir for 10 min, or until fully dissolved, at room temperature.
- Prepare 0.5% BSA in 1x PBS as a wash buffer. Dilute 100 mL of the 1% BSA prepared in step 1.6.1 into 100 mL of 1x PBS for 0.5% BSA.
- Store buffers at 4 °C for up to 1 month.
- Prepare 4% paraformaldehyde in 1x PBS. Use commercially available, sealed paraformaldehyde ampules (e.g., 16% v/v) for convenience and stability: 2.5 mL of 16% PFA + 7.5 mL of 1x PBS (= 10 mL of 4% PFA). Adjust the prepared volume depending on the total volume needed per experiment.
NOTE: Prepare as needed for cell fixation only; prepare fresh each time. - Prepare FACS sorting buffer: 1x PBS, 1 mM EDTA, 25 mM HEPES, 1% BSA (pH 7.2). Sterile-filter through a 0.22 µm filtration device and store at 4 °C for up to 1 month.
NOTE: Prepare as needed for FACS sorting only. Flow-sorting buffer formulations may vary across FACS instruments. The above formulation is compatible with the instrument used in this study (see Table of Materials). Consult the manufacturer's guidelines. - Prepare tumor dissociation solution: 20 µg/mL Liberase TL + 100 µg/mL DNAse I in RPMI-1640 media (without FBS or other supplements). Stock solutions are useful to keep on hand of Liberase TL (prepared and stored as directed by the manufacturer) and DNAse I (100 mg/mL in double-distilled water [ddH2O], stored at −20 °C).
NOTE: Prepare as needed if using tumors only; prepare fresh each time.
2. Induction of senescence by chemotherapy drugs in cultured cancer cells
NOTE: All cell manipulation steps in this section should be performed in a biosafety cabinet using sterile practices. This section is written for adherent cell types. Suspension cells may be used with appropriate modifications as noted.
- Grow cancer cell line(s) according to the standard protocol from the supplier or the laboratory that provided the specific cell line(s) used.
NOTE: Low passage cells (p < 10) are generally preferred as there will be lower levels of replicative senescence, i.e. lower background, in untreated cell samples. - One day prior to senescence induction by drugs, harvest cells with trypsin-EDTA 0.25% (or as recommended). Neutralize trypsin by adding an equal volume of complete culture medium, and transfer the cell suspension to a sterile conical tube.
NOTE: This step is not needed for suspension cells. - Count the cells using the standard hemacytometer method and record cells/mL. Plate cells at 1 × 103-10 × 103 cells/cm2 in standard 6-well plastic culture dishes.
NOTE: Optimal plating density is dependent on the proliferation rate of cells and should be user-determined. Cells should be in log phase growth at approximately 10%-20% confluency at the time of treatment (i.e., following 18-24 h incubation after plating). The starting density (cells/mL) of suspension cells should be user-determined. Six-well plates typically yield enough senescent cells per well for one standard flow cytometry analysis sample. If flow-sorting, a much larger surface area (e.g., multiple P150 plates) should be used to enable the recovery of sufficient numbers of senescent cells for downstream assays (≥1 × 106). - Incubate plated cells overnight (18-24 h) in a 37 °C incubator with 5% CO2 and a humidity pan.
- Treat the plated cells with senescence-inducing chemotherapy drug(s). Include at least one positive control, e.g., etoposide (ETO) or bleomycin (BLM). Prepare duplicate wells per drug. Include one set treated with vehicle-only as control.
NOTE: A dose curve for each experimental agent should be tested by the user to determine the optimal concentration for senescence induction in the cell line(s) being used. - Incubate cells for 4 days in a 37 °C incubator with 5% CO2 and a humidity pan to allow the onset of senescence. Examine daily for expected morphology changes using a light microscope.
NOTE: Incubation times from 3-5 days may be acceptable depending on the rate of senescence onset. Media can be changed and the agent reapplied (or not), as desired, to promote healthy growth conditions while achieving an acceptable percentage of senescent cells. - After the onset of senescence, harvest the cells by adding trypsin-EDTA 0.25% for 5 min at 37 °C. When cells are dissociated into suspension, neutralize trypsin with an equal volume of complete medium.
NOTE: This step is not needed for cells growing in suspension. If surface marker staining will be conducted, avoid the use of trypsin-EDTA as it can temporarily destroy surface antigens on cells. Instead, gently dissociate the monolayer using a sterile plastic cell scraper (or an alternate dissociation reagent designed to preserve surface antigens). - Count the cells in each sample using a hemacytometer. Calculate the cells/mL for each sample.
NOTE: Trypan blue can be added to evaluate the percentage of dead cells at this point (i.e., due to drug treatment), but cell death will also be determined with a fluorescent viability dye during DDAOG staining workflow. - Aliquot ≥0.5 × 106 cells per sample into 1.7 mL microcentrifuge tubes.
NOTE: The number of cells per sample should be standardized across all samples. - Centrifuge the tubes for 5 min at 1,000 × g in a microcentrifuge at 4 °C. Remove the supernatant.
NOTE: If a refrigerated microcentrifuge is unavailable, it may be acceptable to perform centrifugations at ambient temperature for certain resilient cell types. - Proceed to DDAOG staining in section 4.
3. Induction of senescence by chemotherapy drugs in tumors established in mice
NOTE: If tumor cells will be FACS-sorted, ensure sterility at each step by working in a biosafety cabinet and working with sterile instruments, procedures, and reagents.
- Create mouse tumor models by injecting cancer cells subcutaneously, according to standard methods (e.g., Appelbe et al.19).
NOTE: The number of cancer cells to be injected, the injection site, and the appropriate mouse strain should be optimized for each protocol. Here, B16-F10 cells were injected subcutaneously at 1 × 106 cells in 0.1 mL of saline (1 × 107 cells/mL).- Verify that the viability of cells using trypan blue is >90% before performing injections. Anesthetize the mice with isoflurane.
- Anesthetize 6-7-week-old female C57/BL6 mice with a mix of 3% isoflurane and air and maintain under these conditions in an induction chamber placed within a sterile biosafety cabinet. Confirm anesthesia by gently pinching the mouse's foot. Apply sterile veterinary ointment to both eyes to prevent corneal drying during the procedure. During the procedure, maintain mouse body temperature using a heating lamp.
- Working inside a sterile biosafety cabinet, remove the mouse from the induction chamber and place it in contact with a nose cone providing a 3% isoflurane supply. Shave the flank area at the injection site using a clean electric razor. Mix the prepared cell suspension briefly by manually inverting the tube just prior to injection, and inject the cell suspension subcutaneously into the shaved flank(s) using a sterile 0.5 mL syringe fitted with a sterile 27 G needle. Remove the mouse from the hood and transfer it to the recovery cage.
- In the recovery cage, monitor the vital signs of the mice continuously until they have regained sufficient consciousness to maintain sternal recumbency, demonstrate the righting reflex, and are able to safely move around in the cage. Do not leave mice unattended or return animals that have undergone tumor cell inoculation to the company of other animals until fully recovered. Monitor all inoculated mice daily for weight loss, reduced activity/mobility, and neurological symptoms; euthanize mice exhibiting severe symptoms in any category. For mice exhibiting pain symptoms following inoculation, administer buprenorphine (0.1-0.2 mg/kg) once subcutaneously.
NOTE: Mice exhibiting persistent pain after buprenorphine should be euthanized.
- Starting 5-7 days post cancer cell inoculation, measure tumor growth with calipers every 2-3 days. Initiate prosenescent treatment when the tumor has reached 50 mm3 ± 10 mm3 in volume.
NOTE: In this work, doses of USP grade doxorubicin hydrochloride (DOX) or PEGylated liposomal doxorubicin (PLD) at 10 mg/kg were administered in 0.9% sodium chloride injection (USP). Drugs were injected intraperitoneally 3x, once every 5 days, starting when tumors reached 50 mm3 ± 10 mm3. Mice recovered for 7 days following the final treatment to allow the onset of TIS in tumors. Senescence induction dosages and conditions for other treatments and/or tumor models should be optimized. - At 7 days after the final drug treatment, sacrifice the mice by CO2 overdose and cervical dislocation or other approved methods in compliance with laboratory animal work guidelines. Excise the tumors and collect them in sterile tubes or 6-well plates filled with sterile RPMI growth medium (to preserve viability during processing).
NOTE: If performing a histological examination (e.g., X-Gal or immunohistochemistry), tumors can be bisected here, with one half snap-frozen in O.C.T. embedding medium and cryosectioned using standard procedures for frozen tissue histology. The remaining tumor half should yield abundant material for dissociation and DDAOG staining. - Transfer one tumor to a P100 plastic dish containing 5 mL of RPMI medium. Mince the tumor into pieces using a scalpel.
- Transfer 5 mL of the suspension of tumor pieces containing suspended cells and debris into a 15 mL conical tube. Use the wider tip of a 25 mL serological pipet for transferring this suspension if large debris is present. Rinse the dish with an additional volume of sterile RPMI to collect materials. Cap and place the conical tube on ice.
- Repeat steps 3.45-3.5 for the remaining tumors. Use a separate plate and scalpel for each tumor to avoid cross-contamination, or rinse well with PBS in between tumors. Use 5 mL of fresh medium for mincing each tumor.
- Prepare the tumor dissociation solution: 20 µg/mL Liberase TL + 100 µg/mL DNAse I in RPMI-1640 media (without FBS).
NOTE: Many effective formulations for tumor dissociation solutions exist and can include a variety of enzymes and other components from different manufacturers. Optimal concentrations of components can vary greatly across tumor types. If red blood cells are highly present in the tumor, red blood cell lysis may be additionally conducted; if dead cells are an issue, a dead cell removal kit may be used. It is highly recommended for the user to independently determine optimal tumor dissociation conditions that provide high viability and low presence of contaminating cells, connective material, and debris. - Centrifuge all tumor samples in conical tubes for 5 min at 1,000 × g (4 °C). Remove the supernatant.
- Add 1-5 mL of tumor dissociation solution to each tumor sample, depending on the volume of tumor material. Ensure there is 1-2 mL in excess of the tumor material pellet in the tube. Vortex at a moderate speed to mix.
- Place the samples in a 37 °C incubator with rapid rotation for 45 min. Vortex briefly every 15 min.
- Filter each sample through a 100 µm cell strainer into a 50 mL conical tube. If the samples are too viscous to pass through the filter, add 10 mL of RPMI-1640 medium to dilute. Rinse the filters with RPMI medium to collect the residual cells.
- Use a hemacytometer to count the cells/mL for each sample.
- Aliquot two or more replicates of 5 × 106 cells per tumor sample.
- Centrifuge for 5 min at 1,000 × g (4 °C). Remove the supernatant.
- (Optional) Cryopreserve the tumor samples for later DDAOG staining if desired.
- Resuspend the dissociated tumor cell pellet in cryopreservation medium: 50% FBS, 40% RPMI-1640, 10% DMSO, prepared under sterile conditions at 5 × 106 cells/mL.
- Aliquot 1 mL of cell suspension into each cryovial.
- Freeze the cryovials for 24 h in an isopropanol cell freezing container at −80 °C; then, transfer to liquid nitrogen cryostorage for longer-term storage (>1 week).
- When staining is desired, thaw the cryovials on ice and proceed to DDAOG staining in section 4.
NOTE: Some tumors may not remain viable through cryopreservation, and resilience to this process should be evaluated by the user for the tumor model of interest.
- Proceed to section 4 for DDAOG staining.
4. DDAOG staining of SA-β-Gal in cell or tumor samples
- Dilute 1 mM Bafilomycin A1 stock at 1:1,000x into DMEM medium (without FBS) for a final concentration of 1 µM.
- Add prepared Baf-DMEM solution to the cell pellet samples (from step 2.11 or step 3.16) for a concentration of 1 × 106 cells/mL.
NOTE: For example, if using 0.5 × 106 cells per sample, add 0.5 mL of Baf-DMEM. For tumors, 5 × 106 cells can be stained in 5 mL of Baf-DMEM. - Incubate for 30 min at 37 °C (without CO2) on a rotator/shaker set at a slow speed.
NOTE: Avoid CO2 incubators for the staining process, which can acidify solutions and thereby interfere with Baf and DDAOG staining. - Without washing, add the DDAOG stock solution (5 mg/mL) at 1:500x (10 µg/mL final) to each sample. Pipette to mix. Replace on a rotator/shaker at 37 °C (without CO2) for 60 min. Protect from direct light.
- Centrifuge the tubes for 5 min at 1,000 x g at 4 °C. Remove the supernatant.
- Wash with 1 mL of ice-cold 0.5% BSA per tube and pipette to mix. Centrifuge the tubes for 5 min at 1,000 x g at 4 °C and remove the supernatant. Repeat this step 2x to thoroughly wash the cells. Remove the supernatant and proceed.
NOTE: It is important to perform the wash steps in step 4.6 to remove uncleaved DDAOG, which can exhibit undesired fluorescence emission (460/610 nm).
NOTE: If immunostaining for cell surface markers, proceed to section 5 below. - (Optional) Fixation and storage of DDAOG stained cells for later analysis
- Add 0.5 mL of ice-cold 4% paraformaldehyde dropwise to each washed sample. Pipette to mix.
- Incubate for 10 min at room temperature.
- Wash the cells 2x with 1 mL of PBS.
- Store the samples for up to 1 week at 4 °C prior to flow cytometry analysis.
NOTE: For fixed samples, skip step 4.8.
- Dilute Calcein Violet 450 AM stock (1 mM) at 1:1,000x into 1% BSA-PBS (1 µM final). Add 300 µL (for cultured cell samples) or 1,000 µL (for tumor samples) to the washed cell pellets from step 4.6. Incubate for 15 min on ice in the dark.
- Proceed to flow cytometry setup (section 6).
5. (Optional) Immunostaining for cell surface markers in combination with DDAOG
NOTE: As with any flow cytometry experiment, single-stained control samples with DDAOG only and fluorescent antibody only should be prepared to determine crosstalk (if any) across fluorescence channels. If crosstalk is observed, standard flow cytometry compensation should be performed20.
- Resuspend the cell pellets obtained in step 4.6 in 100 µL of staining buffer (1% BSA in 1x PBS).
- Add the Fc receptor blocking reagent appropriate for the cell species (mouse or human) at the manufacturer-recommended titration. Incubate for 10 min at 24 °C.
- Add fluorophore-conjugated antibodies at the titration recommended by the manufacturer (or determined by the user). Incubate for 20 min on ice, protected from light.
- Centrifuge the tubes for 5 min at 1,000 × g at 4 °C. Remove the supernatant.
- Wash with 1 mL of ice-cold wash buffer (0.5% BSA-PBS) per tube and pipette to mix. Centrifuge the tubes for 5 min at 1,000 × g at 4 °C and remove the supernatant. Repeat this step 2x to thoroughly wash the cells.
- Dilute 1 mM Calcein Violet 450 AM at 1:1,000x into 1% BSA-PBS. Add 300 µL to the washed cell pellets from step 5.5. Incubate for 15 min on ice in the dark.
- Proceed to flow cytometry analysis (sections 6-7).
6. Flow cytometer setup and data acquisition
- Transfer the cell samples to tubes compatible with the flow cytometry instrument. Place the tubes on ice and keep them protected from light.
NOTE: If aggregates are observed in the cell suspensions, pass the suspension through 70-100 µm cell strainers prior to analysis. Do not use 40 µm strainers because they can exclude some of the larger senescent cells. - In the referenced software (see the Table of Materials), open the following plots: 1) FSC-A vs SSC-A dot plot, 2) violet channel histogram, 3) far-red channel (e.g., APC-A) versus green channel (e.g., FITC-A) dot plot.
NOTE: Doublet exclusion plots and single-channel histograms can also be used but are not strictly required. - Initiate cytometer data acquisition.
- Place the vehicle-only control sample stained with DDAOG on the intake port. At a low intake speed, begin to acquire sample data.
- Adjust FSC and SSC voltages so that >90% of events are contained within the plot. If cells do not fit well on the plot, lower the area scaling setting to 0.33-0.5 units.
- Remove the vehicle-only sample without recording data.
- (Optional) Add one droplet of rainbow calibration microspheres to a cytometer tube with 1 mL of PBS. Place the tube on the cytometer intake port. Begin to acquire sample data.
- Adjust violet, green, and far-red channel voltages so that the top peak of the rainbow microsphere is in the range of 104-105 units of relative fluorescence in each channel and all peaks are well separated in each channel. Record 10,000 events. Remove the tube.
- Place the positive control sample (e.g., BLM, ETO) stained with DDAOG on the intake port. At a low speed, acquire sample data. Observe the events in FSC, SSC, violet, green, and far-red channels to ensure that over 90% of events are contained within all plots. Look for an increase in AF and DDAOG signal versus vehicle-only control.
- If using a sorting cytometer, initiate sorting at this step.
- For record-keeping purposes, record 10,000 cells for the control sample and each sorted sample.
- Sort the desired amount of cells (≥1 × 106 is typically suitable) into an instrument-appropriate collection tube with 3-5 mL of culture medium.
- After sorting, proceed to downstream culture or analysis.
- Skip to section 7 for the routine analysis of sorted samples.
- If using fluorescent antibodies, optimize channel voltages here using the unstained, single-stained, and double-stained samples prepared in section 5.
NOTE: For the calibrated flow cytometer used herein, optimal channel voltages typically fell between 250 and 600 (mid-range), but the optimal voltages and channel voltage ranges will vary across instruments. Avoid using voltages at very low or high ranges, which may suppress signal or amplify noise. - After completing steps 6.1-6.5 and making adjustments to the cytometer settings as necessary, record data for all the samples. Ensure that the settings remain uniform for all sample recordings. Record ≥10,000 events per cultured cell sample or ≥100,000 events per tumor cell sample.
NOTE: Although gating and analysis can be performed using data acquisition software (e.g., FACSDiva), a complete gating and analysis workflow to be conducted post acquisition using separate analysis software (FlowJo) is described in section 7 below. Post-acquisition analysis is preferred to reduce time at the cytometer workstation and take advantage of additional tools included in the dedicated analysis software. - Save sample data in .fcs file format. Export the files to a workstation computer equipped with flow cytometry analysis software (e.g., FlowJo). Proceed to section 7.
7. Flow cytometry data analysis
NOTE: The workflow presented uses FlowJo software. Alternative flow cytometry data analysis software may be used if the key steps described in this section are similarly followed.
- Using FlowJo software, open .fcs data files from step 6.7.
- Open the layout window.
- Drag and drop all samples into the layout window.
- Gate viable cells.
- First double-click on the sample data for the vehicle-only control to open its data window.
- Visualize the data as a violet channel histogram. Identify the viable cells stained by CV450 based on their brighter fluorescence than the dead cells.
- Draw a gate using the single-gate histogram tool to include viable cells only. Name the gate viable.
- Then, from the sample layout window, drag the viable gate onto the other cell samples to apply the gate uniformly.
- In the layout window, visualize all samples as violet channel (viability) histograms. Verify that viable cell gating is appropriate across samples before proceeding; if not, make adjustments as needed.
NOTE: Viability staining can exhibit variations across treatments or tumors.
- Gate senescent cells.
- Double-click on the gated viable cell data for the vehicle-only control to open its data window.
- Visualize the data as a dot plot for far-red channel (DDAOG) vs green channel (AF).
- Draw a gate using the rectangle gating tool to include <5% of cells that are DDAOG+ and AF+ (upper right quadrant). Name the gate senescent.
- Then, from the sample layout window, drag the senescent gate onto the viable subsets of the other cell samples to apply the gate uniformly.
- Into the layout window, drag and drop all viable cell subsets gated in section 7.4. Visualize all viable samples as far-red (e.g., APC-A) versus green channel (e.g., FITC-A) dot plots.
- Ensure that the senescent gate drawn in step 7.5.3 is visible on all plots and that the gate for the vehicle-only control exhibits ≤5%-10% senescent cells.
- Once the percentage of senescent cells has been determined using the steps above, present the resulting data using the FlowJo plots, summarized in a data table and/or statistically analyzed using standard software.
Results
Several experiments were performed to demonstrate the comparability of DDAOG to X-Gal and C12-FDG for the detection of senescence by SA-β-Gal. First, X-Gal was used to stain senescent B16-F10 melanoma cells induced by ETO (Figure 2A). An intense blue color developed in a subset of ETO-treated cells, while other cells exhibited less intense blue staining. Morphology was enlarged in most ETO-treated cells. Staining ETO-treated cells with fluorescent SA-β-Gal substrate C
Discussion
Over the last decade or so, flow cytometry has become a more common assay platform in cancer research due to the emerging popularity of tumor immunology, the development of lower-cost flow cytometers, and the improvement of shared instrumentation facilities at academic institutions. Multicolor assays are now standard, as most newer instruments are equipped with violet, blue-green, and red to far-red optical arrays. Thus, this DDAOG protocol is likely to be compatible with a wide array of flow cytometers. Of course, any f...
Disclosures
The authors have no conflicts of interest to declare for this study.
Acknowledgements
We thank the Cytometry and Antibody Core Facility at the University of Chicago for support on flow cytometry instrumentation. The Animal Research Center at the University of Chicago provided animal housing.
Materials
| Name | Company | Catalog Number | Comments |
| Bafilomycin A1 | Research Products International | B40500 | |
| Bleomycin sulfate | Cayman | 13877 | |
| Bovine serum albumin (BSA) | US Biological | A1380 | |
| Calcein Violet 450 AM viability dye | ThermoFisher Scientific | 65-0854-39 | eBioscience |
| DPP4 antibody, PE conjugate | Biolegend | 137803 | Clone H194-112 |
| Cell line: A549 human lung adenocarcinoma | American Type Culture Collection | CCL-185 | |
| Cell line: B16-F10 mouse melanoma | American Type Culture Collection | CRL-6475 | |
| Cell scraper | Corning | 3008 | |
| Cell strainers, 100 µm | Falcon | 352360 | |
| DDAO-Galactoside | Life Technologies | D6488 | |
| DMEM medium 1x | Life Technologies | 11960-069 | |
| DMSO | Sigma | D2438 | |
| DNAse I | Sigma | DN25 | |
| Doxorubicin, hydrochloride injection (USP) | Pfizer | NDC 0069-3032-20 | |
| Doxorubicin, PEGylated liposomal (USP) | Sun Pharmaceutical | NDC 47335-049-40 | |
| EDTA 0.5 M | Life Technologies | 15575-038 | |
| Etoposide | Cayman | 12092 | |
| FBS | Omega | FB-11 | |
| Fc receptor blocking reagent | Biolegend | 101320 | Anti-mouse CD16/32 |
| Flow cytometer (cell analyzer) | Becton Dickinson (BD) | Various | LSRFortessa |
| Flow cytometer (cell sorter) | Becton Dickinson (BD) | Various | FACSAria |
| GlutaMax 100x | Life Technologies | 35050061 | |
| HEPES 1 M | Lonza | BW17737 | |
| Liberase TL | Sigma | 5401020001 | Roche |
| Paraformaldehyde 16% | Electron Microscopy Sciences | 15710 | |
| Penicillin/Streptomycin 100x | Life Technologies | 15140122 | |
| Phosphate buffered saline (PBS) 1x | Corning | MT21031CV | Dulbecco's PBS (without calcium and magnesium) |
| Rainbow calibration particles, ultra kit | SpheroTech | UCRP-38-2K | 3.5-3.9 µm, 2E6/mL |
| RPMI-1640 medium 1x | Life Technologies | 11875-119 | |
| Sodium chloride 0.9% (USP) | Baxter Healthcare Corporation | 2B1324 | |
| Software for cytometer data acquisition, "FACSDiva" | Becton Dickinson (BD) | n/a | Contact BD for license |
| Software for cytometer data analysis, "FlowJo" | TreeStar | n/a | Contact TreeStar for license |
| Trypsin-EDTA 0.25% | Life Technologies | 25200-114 |
References
- Saleh, T., Tyutyunyk-Massey, L., Gewirtz, D. A. Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Cancer Research. 79 (6), 1044-1046 (2019).
- Wang, B., Kohli, J., Demaria, M. Senescent cells in cancer therapy: friends or foes. Trends in Cancer. 6 (10), 838-857 (2020).
- Prasanna, P. G., et al. Therapy-induced senescence: Opportunities to improve anticancer therapy. Journal of the National Cancer Institute. 113 (10), 1285-1298 (2021).
- Velarde, M. C., Demaria, M., Campisi, J. Senescent cells and their secretory phenotype as targets for cancer therapy. Interdisciplinary Topics in Gerontology and Geriatrics. 38, 17-27 (2013).
- Ou, H. L., et al. Cellular senescence in cancer: from mechanisms to detection. Molecular Oncology. 15 (10), 2634-2671 (2021).
- Hernandez-Segura, A., Nehme, J., Demaria, M. Hallmarks of cellular senescence. Trends in Cell Biology. 28 (6), 436-453 (2018).
- Bojko, A., Czarnecka-Herok, J., Charzynska, A., Dabrowski, M., Sikora, E. Diversity of the senescence phenotype of cancer cells treated with chemotherapeutic agents. Cells. 8 (12), 1501 (2019).
- Mikuła-Pietrasik, J., Niklas, A., Uruski, P., Tykarski, A., Książek, K. Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells. Cellular and Molecular Life Sciences. 77 (2), 213-229 (2020).
- Lee, B. Y., et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging cell. 5 (2), 187-195 (2006).
- Itahana, K., Itahana, Y., Dimri, G. P. Colorimetric detection of senescence-associated β galactosidase. Methods in Molecular Biology. 965, 143-156 (2013).
- Dimri, G. P., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America. 92 (20), 9363-9367 (1995).
- Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J., Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nature Protocols. 4 (12), 1798-1806 (2009).
- Noppe, G., et al. Rapid flow cytometric method for measuring senescence associated beta-galactosidase activity in human fibroblasts. Cytometry A. 75 (11), 910-916 (2009).
- Tung, C. -. H., et al. In vivo imaging of β-galactosidase activity using far red fluorescent switch. Cancer Research. 64 (5), 1579-1583 (2004).
- Gong, H., et al. beta-Galactosidase activity assay using far-red-shifted fluorescent substrate DDAOG. Analytical Biochemistry. 386 (1), 59-64 (2009).
- Terman, A., Brunk, U. T. Lipofuscin: Mechanisms of formation and increase with age. APMIS. 106 (2), 265-276 (1998).
- Georgakopoulou, E. A., et al. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging. 5 (1), 37-50 (2013).
- Wang, B., Demaria, M. The quest to define and target cellular senescence in cancer. Cancer Research. 81 (24), 6087-6089 (2021).
- Appelbe, O. K., Zhang, Q., Pelizzari, C. A., Weichselbaum, R. R., Kron, S. J. Image-guided radiotherapy targets macromolecules through altering the tumor microenvironment. Molecular Pharmaceutics. 13 (10), 3457-3467 (2016).
- Maciorowski, Z., Chattopadhyay, P. K., Jain, P. Basic multicolor flow cytometry. Current Protocols in Immunology. 117, 1-38 (2017).
- Fan, Y., Cheng, J., Zeng, H., Shao, L. Senescent cell depletion through targeting BCL-family proteins and mitochondria. Frontiers in Physiology. 11, 593630 (2020).
- Kim, K. M., et al. Identification of senescent cell surface targetable protein DPP4. Genes and Development. 31 (15), 1529-1534 (2017).
- Flor, A. C., Kron, S. J. Lipid-derived reactive aldehydes link oxidative stress to cell senescence. Cell Death Discovery. 7 (9), 2366 (2016).
- Jochems, F., et al. The Cancer SENESCopedia: A delineation of cancer cell senescence. Cell reports. 36 (4), 109441 (2021).
- Fallah, M., et al. Doxorubicin and liposomal doxorubicin induce senescence by enhancing nuclear factor kappa B and mitochondrial membrane potential. Life Sciences. 232, 116677 (2019).
- Kasper, M., Barth, K. Bleomycin and its role in inducing apoptosis and senescence in lung cells - modulating effects of caveolin-1. Current Cancer Drug Targets. 9 (3), 341-353 (2009).
- Muthuramalingam, K., Cho, M., Kim, Y. Cellular senescence and EMT crosstalk in bleomycin-induced pathogenesis of pulmonary fibrosis-an in vitro analysis. Cell Biology International. 44 (2), 477-487 (2020).
- Flor, A. C., Wolfgeher, D., Wu, D., Kron, S. J. A signature of enhanced lipid metabolism, lipid peroxidation and aldehyde stress in therapy-induced senescence. Cell Death Discovery. 3, 17075 (2017).
- Burd, C. E., et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 152 (1-2), 340-351 (2013).
- Liu, J. Y., et al. Cells exhibiting strong p16 (INK4a) promoter activation in vivo display features of senescence. Proceedings of the National Academy of Sciences of the United States of America. 116 (7), 2603-2611 (2019).
- Wang, L., Lankhorst, L., Bernards, R. Exploiting senescence for the treatment of cancer. Nature Reviews Cancer. 22 (6), 340-355 (2022).
- Baek, K. -. H., Ryeom, S. Detection of oncogene-induced senescence in vivo. Methods in Molecular Biology. 1534, 185-198 (2017).
- González-Gualda, E., Baker, A. G., Fruk, L., Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. The FEBS Journal. 288 (1), 56-80 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved