A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Measurement of the Compressibility of Cell and Nucleus Based on Acoustofluidic Microdevice
In This Article
Summary
Here a protocol is presented to build a fast and non-destructive system for measuring cell or nucleus compressibility based on acoustofluidic microdevice. Changes in mechanical properties of tumor cells after epithelial-mesenchymal transition or ionizing radiation were investigated, demonstrating the application prospect of this method in scientific research and clinical practice.
Abstract
Cell mechanics play an important role in tumor metastasis, malignant transformation of cells, and radiosensitivity. During these processes, studying the mechanical properties of the cells is often challenging. Conventional measurement methods based on contact such as compression or stretching are prone to cause cell damage, affecting measurement accuracy and subsequent cell culture. Measurements in adherent state can also affect accuracy, especially after irradiation since ionizing radiation will flatten cells and enhance adhesion. Here, a cell mechanics measurement system based on acoustofluidic method has been developed. The cell compressibility can be obtained by recording the cell motion trajectory under the action of the acoustic force, which can realize fast and non-destructive measurement in suspended state. This paper reports in detail the protocols for chip design, sample preparation, trajectory recording, parameter extraction and analysis. The compressibility of different types of tumor cells was measured based on this method. Measurement of the compressibility of nucleus was also achieved by adjusting the resonance frequency of the piezoelectric ceramic and the width of the microchannel. Combined with the molecular level verification of immunofluorescence experiments, the cell compressibility before and after drug-induced epithelial to mesenchymal transition (EMT) were compared. Further, the change of cell compressibility after X-ray irradiation with different doses was revealed. The cell mechanics measurement method proposed in this paper is universal and flexible and has broad application prospects in scientific research and clinical practice.
Introduction
Cell mechanical properties play an important role in tumor metastasis, malignant transformation of cells, and radiosensitivity1,2. To gain an in-depth understanding of the role of cell mechanical properties in the above process, accurate measurement of cellular mechanics is critical, and the measurement should not cause damage to the cells for subsequent culture and analysis. The measurement process should be as fast as possible, otherwise cell viability may be affected if cells are removed from the cultivation environment for a long time.
Existing cell mechanics measurement methods face some limitations. Some methods, such as magnetic twisting cytometry, magnetic tweezers and particle-tracking microrheology, cause cell damage due to the introduction of particles into cells3,4,5. Methods that measure by contact with cells, such as atomic force microscope (AFM), micropipette aspiration, micro-constriction, and parallel-plate technique, are also prone to cell damage and the throughput is difficult to increase6,7,8. In addition, ionizing radiation will flatten cells and increase their adhesion9; it is therefore necessary to measure whole cell mechanics in suspension.
In response to the above challenges, a cell mechanics measurement system based on acoustofluidic method10,11,12,13,14 has been developed. The channel width is matched to the acoustic half wavelength, thus creating a standing wave node at the midline of the microchannel. Under the action of acoustic radiation force, the cells or standard beads can move to the acoustic pressure node. Since the physical properties of the standard beads (size, density, and compressibility) are known, the acoustic energy density can be determined. Then, the cell compressibility can be obtained by recording the motion trajectories of cells in the acoustic field. Non-destructive high-throughput measurement of cells in suspension state can be achieved. This paper will introduce the design of the microfluidic chip, the establishment of the system and the measurement steps. Measurement of various types of tumor cells has been carried out to verify the accuracy of the method. The application scope of this method had been extended to subcellular structures (such as nucleus) by adjusting the resonance frequency of the piezoelectric ceramic and the width of the microchannel. In addition, the changes in cell compressibility after drug-induced EMT or X-ray irradiation with different doses were investigated. The results demonstrate the broad applicability of this method as a powerful tool for studying the correlation between biochemical changes and cellular mechanical properties.
Protocol
1. Fabricating and assembly of the acoustofluidic microdevice
- Fabrication of the microfluidic chip.
- Design a single-channel chip with only one inlet and outlet as shown in Figure 1. For measuring cells, keep the rectangular cross-section of the microchannel at 740 µm wide and 100 µm deep. For measuring cell nucleus, change the width and depth of the microchannel to 250 µm and 100 µm, respectively.
- Prepare the microchannel on silicon wafer via reactive ion etching. Seal the top of the microchannel with a piece of transparent heat-resistant glass by anodic bonding15. Wash the chips with an ultrasonic cleaner for 10 min. Dry them in a drying oven at 50 °C for later use.
- Fabricate polydimethylsiloxane (PDMS) blocks.
- Add 30 mL of pre-polymer to a 100 mm (in diameter) glass dish. Add 3 mL of curing agent to the pre-polymer with a syringe.
NOTE: The volume ratio of the curing agent and pre-polymer is 1:10. - Vigorously mix the PDMS pre-polymer and curing agent with a glass rod for about 10 min. Look for small and uniformly separated air bubbles in the solution, which indicate that the PDMS pre-polymer and curing agent are well mixed.
- Place the glass dish in a vacuum desiccator and evacuate for 15-25 s. Repeat this process until there are no air bubbles in the mixture.
- Place the glass dish in a drying oven set at 50 °C for 1 h to allow the mixture to cure. After the incubation, use a scalpel to cut the PDMS into blocks of suitable size about 1.2 cm long and 1 cm wide.
NOTE: The length of the PDMS block is consistent with the width of the chip, and the width is selected to ensure that there is enough space in the middle for the piezoelectric ceramic when two PDMS blocks are adhered on the chip.
- Add 30 mL of pre-polymer to a 100 mm (in diameter) glass dish. Add 3 mL of curing agent to the pre-polymer with a syringe.
- Bind the PDMS block to the chip.
- Punch holes in the PDMS block for inlet and outlet ports with a 1 mm diameter hollow needle. Put the PDMS blocks and chip (back side up) in a plasma cleaner for 1 min.
- Align the holes on the PDMS blocks with the chip inlet and outlet. Gently press the PDMS blocks to the chip for 15 s. This should cause bonding to occur between the PDMS blocks and the surface of the chip.
- Connect the polytetrafluoroethylene (PTFE) catheter to the chip (Figure 2B).
- Cut two pieces of PTFE catheter with an inner diameter of 0.8 mm and a length of 10 cm. Bend a stainless-steel needle with an inner diameter of 0.7 mm and a length of 1.5 cm by 90° into an L shape. Connect it to one end of the catheter. Prepare two such catheters with needles.
- Insert the stainless-steel needles into the holes of the PDMS blocks. For the inlet, connect a 19 G dispensing needle to the other end of the catheter as a connector for a syringe.
- After completing the above steps, inject deionized water to test the tightness of the overall channel. Impervious to water means a good seal.
- Piezoelectric ceramic assembly (Figure 2C)
- Use a diamond wire cutter to cut piezoelectric ceramic sheets with a diameter of 2 cm into four strips with a width of 5 mm.
- Ensure that the resonant frequency of the piezoelectric ceramic matches the width of the chip microchannel. For the 740 µm and 250 µm wide microchannel, use piezoelectric ceramics with resonance frequencies of 1 MHz and 3 MHz, respectively.
- Weld wires on both sides of the piezoelectric ceramic at one end.
- Glue the piezoelectric ceramic to the middle of the back of the chip with cyanoacrylate glue.
- To spread the glue evenly, place a drop of glue on the piezoelectric ceramic, smooth the glue with a toothpick and remove the excess glue. Then, quickly press it on the chip and continue to press for about 1 min. Ensure that the piezoelectric ceramic and the chip are firmly bonded and evenly contacted.
- Mount the microdevice (Figure 2D).
- Cut a piece of PDMS (about 1.5 cm long and 1 cm wide) as the base of the microdevice. Using double-sided tape, stick one side of the base to the inlet and outlet PDMS blocks, and the other side to a transparent glass slide. Fix the whole microdevice to the microscope stage to keep the chip in one focal plane.
2. Sample preparation
- Preparation of polystyrene standard particle solutions.
- Add 0.05 mL of polystyrene particle (6 µm in diameter) solution (2.1 x 108 particle/mL) to 10 mL of phosphate buffered saline (PBS) and mix well.
NOTE: In order to reduce the measurement error caused by the change of the acoustic energy density, the polystyrene particle solution was mixed with the sample solution in each experiment as calibration.
- Add 0.05 mL of polystyrene particle (6 µm in diameter) solution (2.1 x 108 particle/mL) to 10 mL of phosphate buffered saline (PBS) and mix well.
- Preparation of cell suspensions.
- Wash the adherent cells (e.g., MCF7, MDA-MB-231, HCT116) at 90% confluency (~5 x 105 cells) with PBS. Add 500 µL 0.25% trypsin (1x) for 1-2 min at room temperature (25 °C). Remove the trypsin, add 1 mL complete medium and form a cell suspension by pipetting.
- Centrifuge the cell suspension at 100 x g for 5 min. Remove the supernatant and resuspend in 0.5-1 mL of PBS in order to obtain a cell suspension. Cells were counted with a hemocytometer and the concentration was about 3-5 x 105 cells/mL.
- Preparation of cell nucleus suspension
- Carry out step 2.2. Then, remove the supernatant and add 200 µL of cytoplasmic protein extraction reagent A (supplemented with 1% PMSF) per 20 µL cell pellet (approximately 5 million cells) and mix well.
- Vortex the above mixture at 220 x g for 5 s, and then place on ice bath for 10 min. After incubation, add 10 µL of cytoplasmic protein extraction reagent B to the solution.
- Vortex at 220 x g for 5 s. Place on ice bath for 1 min and vortex again at 220 x g for 5 s. Then, finally centrifuge at 1,000 x g for 5 min at 4 °C.
NOTE: The volume ratio of cytoplasmic protein extraction reagents A and B is 20:1. - Remove the supernatant and resuspend the pellet in 1 mL of PBS. Then, centrifuge at 1,000 x g at 4 °C for 4 min. Remove the supernatant and resuspend in 100 µL of PBS as cell nucleus suspension.
- Add trypan blue to the above cell nucleus suspension and stain at room temperature (25 °C) for 4 min. The volume ratio of trypan blue solution to nucleus suspension is 1:1. Count the number of nuclei under the inverted microscope with a 10x objective.
NOTE: To clearly identify the cell nuclei under the microscope, trypan blue staining is required. Trypan blue solution needs to be in a 37 °C water bath for 10 min before use for effective staining. - Dilute the above cell nucleus suspension with PBS buffer to a concentration of 2-3 x 105 nucleus/mL. Filter the cell nucleus suspension through a 70 µm sieve.
3. Measuring the compressibility of cell and nucleus
- Set up the measurement system (Figure 3)
- Turn on the light source of the microscope and open the camera software. Use the 4x objective to find the middle position of the microchannel, i.e., the position of the piezoelectric ceramic.
- Connect the wires and weld them to the positive and negative terminals of the signal generator output on the piezoelectric ceramic, respectively.
- Place the syringe on the microinjection pump and connect it to the inlet catheter. Place a small container at the end of the outlet catheter to hold the fluid flowing out of the microchannel.
- Determine measurement parameters
- Aspirate the polystyrene particle solution with the syringe and inject it into the chip microchannel. Avoid air bubbles in the chip microchannel to ensure accurate measurement. Ensure that particles are evenly distributed in the chip microchannel.
NOTE: Measurement can be conducted without flow or syringe pump. If needed, the flow rate of the microinjection pump should be set to a proper value. Here, the range of flow rate is 0-20 µL/h. - Set the output of the signal generator to a sine signal with a frequency of 1 MHz (3 MHz for cell nucleus measurement) and peak-to-peak voltage (Vpp) of 10 V.
- Fine tune the frequency of the signal until it's observed that the particles move toward the midline of the microchannel and remain in forward motion along the midline after reaching the midline (Figure 4).
NOTE: The speed of the particles moving toward the midline is determined by the voltage amplitude, which can be adjusted between 5 Vpp and 20 Vpp.
- Aspirate the polystyrene particle solution with the syringe and inject it into the chip microchannel. Avoid air bubbles in the chip microchannel to ensure accurate measurement. Ensure that particles are evenly distributed in the chip microchannel.
- Measure cells and nuclei
- Mix 1 mL cell or nucleus suspension with the standard particle solution at the ratio of 1:1 and inject it into the microchannel with a syringe.
- Start recording with CCD camera when the cells or nuclei enter the field of view. Then, turn on the signal generator. Stop recording when the cells or nuclei reach the midline.
- Rinse the microchannel with deionized water, 75% alcohol, and deionized water in sequence for later use.
4. Data processing
- Map particle or cell trajectories.
- Import the captured video into ImageJ software: File > Open> Select Folder. Click on the ellipse shape in the toolbar of the ImageJ software to pick a cell of interest and its adjacent particle (Figure 5).
- As shown in Figure 5, preset measurement parameters in ImageJ software as Analyze→ Set Measurement →Area, Centroid, Display Label.
- Taking the frame where the target cell or particle undergoes longitudinal displacement as the start frame; record the pixel position and size of the cell or particle in each frame until it reaches the midline of the microchannel. Export the data as a spreadsheet file and repeat the step until trajectories for all cells of interest are obtained.
- Coordinate transformation and correction.
- Record the pixel coordinates of the four corners of the microchannel in this field of view as (0, y1), (0, y2), (x3, y3), (x4, y4). Here x3 = x4.
- For each measurement point (xi, yi), calculate the new coordinate (xi', yi') after rotation correction using the following formulas:
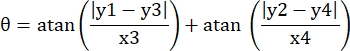
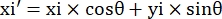

- Convert pixel coordinates to real size coordinates. The actual coordinates can be obtained by multiplying pixel coordinates by the ratio. The ratio was the actual width of the microchannel divided by the pixel width (H) of the microchannel.
- Transform and correct the pixel coordinates of the cells and particles obtained in step 4.1 to the final motion trajectory data. All coordinates minus the coordinates of the lower-left corner, i.e., (0, y2). The frame rate of the video is 40 frames per second, so multiply the number of frames corresponding to each coordinate by 0.025 s to obtain the time of particle movement, thereby obtaining the change of the position in the y direction with time.
- Calculate the acoustic energy density (Figure 6A,B).
- The motion of the cell or particle in the Y direction is driven by the acoustic force Fac and hydrodynamic force FD. Calculate the motion trajectory using the following formulas:
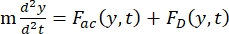 (1)
(1)
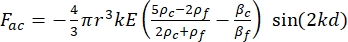 (2)
(2)
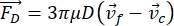 (3)
(3)
where r and D are the radius and diameter of the cell or particle, ρ and β are the density and compressibility, ν is the velocity vector. The subscripts c and f denote the cell and fluid, respectively. d is the distance from the nearest acoustic pressure node, μ is the dynamic viscosity of the fluid, k is the wave number, and E is the acoustic energy density.
NOTE: The density of MCF7, HCT116, A549 and cell nuclei was 1068 kg/m3, 1077 kg/m3, 1073 kg/m3, and 1155 kg/m3, respectively 12,16,17. - According to the formulas described in step 4.3.1, use the MATLAB software to obtain the numerical solution for the standard particle trajectory under the acoustic field with finite difference method.
- Within the preset acoustic field range, change the acoustic energy density and fit the numerical solution (obtained in step 4.3.2) and the measured motion trajectory (obtained in step 4.2) for the standard particle. Select the best fitting result according to the fitting mean square error. The acoustic energy density obtained here is used as a parameter for subsequent calculation of cell compressibility.
- The motion of the cell or particle in the Y direction is driven by the acoustic force Fac and hydrodynamic force FD. Calculate the motion trajectory using the following formulas:
- Calculate the cell compressibility (Figure 6C,D).
- Set the acoustic energy density to the value obtained in step 4.3.3.
- According to the formulas described in step 4.3.1, use the MATLAB software to obtain the numerical solution for the cell trajectory under the acoustic field with finite difference method.
- Similar to step 4.3.3, within the preset compressibility range, change the compressibility and fit the numerical solution (obtained in step 4.4.2) and the measured motion trajectory for the cell (obtained in step 4.2). Use the compressibility coefficient corresponding to the best fitting result as the measured cell compressibility.
Results
Here, the work presented a protocol for the construction of a fast and non-destructive cell compressibility measuring system based on acoustofluidic microdevice and demonstrated its advantages for measuring cell and nucleus under different situations. Figure 1 shows the schematic of the microfluidic channel. The components and assembly of the acoustofluidic microdevice are shown in Figure 2. Figure 3 shows the setup of the measureme...
Discussion
Commonly used cell mechanics measurement methods are AFM, micropipette aspiration, microfluidics methods, parallel-plate technique, optical tweezers, optical stretcher, and acoustic methods20. Microfluidics methods can work with three approaches: micro-constriction, extensional flow, and shear flow. Among them, optical stretcher, optical tweezers, acoustic methods, extensional flow, and shear flow approaches are non-contact measurements. In contrast to contact measurements, non-contact measurement...
Disclosures
The authors have no competing financial interests or other conflicts of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant numbers 12075330 and U1932165) and the Natural Science Foundation of Guangdong Province, China (Grant number 2020A1515010270).
Materials
| Name | Company | Catalog Number | Comments |
| 0.25% trypsin(1x) | GIBCO | 15050-065 | |
| 502 glue | Evo-bond | cyanoacrylate glue | |
| A549 | ATCC | CCL-185 | lung adenocarcinoma |
| Cytonucleoprotein and cytoplasmic protein extraction kit | Beyotime | P0027 | Contains cytoplasmic protein extraction reagents A and B |
| Dulbecco’s modified Eagle medium (DMEM) | corning | 10-013-CVRC | |
| Fetal Bovine Srum(FBS) | AUSGENEX | FBS500-S | |
| HCT116 | ATCC | CCL247 | colorectal carcinoma |
| Heat-resistant glass | Pyrex | ||
| Leibovitz’s L-15 medium | GIBCO | 11415-064 | |
| MCF-7 | ATCC | HTB-22 | breast Adenocarcinoma |
| MDA-MB-231 | ATCC | HTB-26 | breast Adenocarcinoma |
| Minimum Essential Medium (MEM) | corning | 10-010-CV | |
| Penicillin-Streptomycin | GIBCO | 15140-122 | |
| Phosphate buffer | corning | 21-040-cvc | |
| PMSF | Beyotime | ST506 | 100mM |
| Polybead Polystyrene Red Dyed Microsphere | polysciences | 15714 | The diameter of microshpere is 6.00µm |
| propidium iodide(PI) | Sigma-Aldrich | P4170 | |
| SYLGARD 184Silicone ELASTOMER | Dow-Corning | 1673921 | Contains prepolymers and curing agents |
| Trypan Blue | Beyotime | C0011 |
References
- Wirtz, D., Konstantopoulos, K., Searson, P. C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nature Reviews. Cancer. 11 (7), 512-522 (2011).
- Frame, F. M., et al. HDAC inhibitor confers radiosensitivity to prostate stem-like cells. British Journal of Cancer. 109 (12), 3023-3033 (2013).
- Tseng, Y., Kole, T. P., Wirtz, D. Micromechanical mapping of live cells by multiple-particle-tracking microrheology. Biophysical Journal. 83 (6), 3162-3176 (2002).
- Möller, W., Brown, D. M., Kreyling, W. G., Stone, V. Ultrafine particles cause cytoskeletal dysfunctions in macrophages: role of intracellular calcium. Particle and Fibre Toxicology. 2, 7 (2005).
- Wang, X., et al. A three-dimensional magnetic tweezer system for intraembryonic navigation and measurement. IEEE Transactions on Robotics. 34 (1), 240-247 (2018).
- Machida, S., et al. Direct manipulation of intracellular stress fibres using a hook-shaped AFM probe. Nanotechnology. 21 (38), 385102 (2010).
- Bufi, N., et al. Human primary immune cells exhibit distinct mechanical properties that are modified by inflammation. Biophysical Journal. 108 (9), 2181-2190 (2015).
- Hogan, B., Babataheri, A., Hwang, Y., Barakat, A. I., Husson, J. Characterizing cell adhesion by using micropipette aspiration. Biophysical Journal. 109 (2), 209-219 (2015).
- Jung, J. -. W., et al. Ionising radiation induces changes associated with epithelial-mesenchymal transdifferentiation and increased cell motility of A549 lung epithelial cells. European Journal of Cancer. 43 (7), 1214-1224 (2007).
- Hartono, D., et al. On-chip measurements of cell compressibility via acoustic radiation. Lab-on-a-Chip. 11 (23), 4072-4080 (2011).
- Sitters, G., et al. Acoustic force spectroscopy. Nature Methods. 12 (1), 47-50 (2015).
- Augustsson, P., Karlsen, J. T., Su, H. -. W., Bruus, H., Voldman, J. Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping. Nature Communications. 7 (1), 11556 (2016).
- Cushing, K. W., et al. Ultrasound characterization of microbead and cell suspensions by speed of sound measurements of neutrally buoyant samples. Analytical Chemistry. 89 (17), 8917-8923 (2017).
- Riaud, A., Wang, W., Thai, A. L. P., Taly, V. Mechanical characterization of cells and microspheres sorted by acoustophoresis with in-line resistive pulse sensing. Physical Review Applied. 13 (3), 034058 (2020).
- Petersson, F., Aberg, L., Swärd-Nilsson, A. -. M., Free Laurell, T. flow acoustophoresis: microfluidic-based mode of particle and cell separation. Analytical Chemistry. 79 (14), 5117-5123 (2007).
- Griwatz, C., Brandt, B., Assmann, G., Zänker, K. S. An immunological enrichment method for epithelial cells from peripheral blood. Journal of Immunological Methods. 183 (2), 251-265 (1995).
- Katholnig, K., Poglitsch, M., Hengstschläger, M., Weichhart, T. Lysis gradient centrifugation: a flexible method for the isolation of nuclei from primary cells. Methods in Molecular Biology. 1228, 15-23 (2015).
- Fu, Q., Zhang, Y., Huang, T., Liang, Y., Liu, Y. Measurement of cell compressibility changes during epithelial-mesenchymal transition based on acoustofluidic microdevice. Biomicrofluidics. 15 (6), 064101 (2021).
- Zhang, Y., et al. Ionizing radiation-induced DNA damage responses affect cell compressibility. Biochemical and Biophysical Research Communications. 603, 116-122 (2022).
- Hao, Y., et al. Mechanical properties of single cells: Measurement methods and applications. Biotechnology Advances. 45, 107648 (2020).
- Yousafzai, M., et al. Effect of neighboring cells on cell stiffness measured by optical tweezers indentation. Journal of Biomedical Optics. 21 (5), 057004 (2016).
- Wei, M. -. T., et al. A comparative study of living cell micromechanical properties by oscillatory optical tweezers. Optics Express. 16 (12), 8594-8603 (2008).
- Khan, Z. S., Vanapalli, S. A. Probing the mechanical properties of brain cancer cells using a microfluidic cell squeezer device. Biomicrofluidics. 7 (1), 011806 (2013).
- Hirawa, S., Masudo, T., Okada, T. Acoustic recognition of counterions in ion-exchange resins. Analytical Chemistry. 79 (7), 3003-3007 (2007).
- Joosse, S. A., Gorges, T. M., Biology Pantel, K. detection, and clinical implications of circulating tumor cells. EMBO Molecular Medicine. 7 (1), 1-11 (2015).
- Martin, O. A., Anderson, R. L., Narayan, K., MacManus, M. P. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis. Nature Reviews Clinical Oncology. 14 (1), 32-44 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved