A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Three-Dimensional Culture of Vascularized Thermogenic Adipose Tissue from Microvascular Fragments
In This Article
Summary
Here, we present a detailed protocol outlining the use of microvascular fragments isolated from rodent or human fat tissue as a straightforward approach to engineer functional, vascularized beige adipose tissue.
Abstract
Engineering thermogenic adipose tissue (e.g., beige or brown adipose tissues) has been investigated as a potential therapy for metabolic diseases or for the design of personalized microtissues for health screening and drug testing. Current strategies are often quite complex and fail to accurately fully depict the multicellular and functional properties of thermogenic adipose tissue. Microvascular fragments, small intact microvessels comprised of arteriole, venules, and capillaries isolated from adipose tissue, serve as a single autologous source of cells that enable vascularization and adipose tissue formation. This article describes methods for optimizing culture conditions to enable the generation of three-dimensional, vascularized, and functional thermogenic adipose tissues from microvascular fragments, including protocols for isolating microvascular fragments from adipose tissue and culture conditions. Additionally, best practices are discussed, as are techniques for characterizing the engineered tissues, and sample results from both rodent and human microvascular fragments are provided. This approach has the potential to be utilized for the understanding and development of treatments for obesity and metabolic disease.
Introduction
The goal of this protocol is to describe an approach for developing vascularized beige adipose tissue from a single, potentially autologous source, microvascular fragment (MVF). Brown and beige adipose tissues have been demonstrated to display beneficial properties related to metabolic regulation; however, the small volume of these adipose tissue depots in adults limits the potential impact on systemic metabolism, particularly in diseased conditions such as obesity or type 2 diabetes1,2,3,4,5,6,7. There is significant interest in brown/beige fat as a therapeutic target for preventing the harmful metabolic effects linked with obesity and its comorbidities8,9,10,11,12.
MVFs are vessel structures that can be directly isolated from adipose tissue, cultured, and maintained in a three-dimensional configuration for extended periods of time13,14,15. Previous work from our group, and others, have begun to exploit the multicellular and multipotent capacity of MVFs, specifically as it relates to adipose tissue formation16,17,18. As a buildup of this work, we recently demonstrated that MVFs derived from rodent models of healthy and type 2 diabetes19 and from human subjects (adults over 50 years of age)20 contained cells capable of being induced to form thermogenic, or beige, adipose tissue.
Herein is an innovative approach from which a single source MVF is utilized, not only capable of creating beige adipose tissue but also its associated and critical vascular component21. The use of this technique could be of great value for studies looking for a straightforward tissue-engineered approach for thermogenic adipose tissue formation. Unlike other methods aspiring to engineer beige adipose tissue22,23,24,25,26,27,28, the process described in this study does not require using multiple cell types or complex induction regimens. Vascularized beige and white fat models can be created with MVFs originating from rodent and human sources, demonstrating great translation potential. The end product of this protocol is an engineered beige thermogenic fat tissue with a structure and metabolic function comparable to brown adipose tissue. Overall, this protocol presents the idea that an easily accessible and possibly autologous source MVF may be a worthwhile therapeutic intervention and tool for studying metabolic disorders.
Protocol
This study was conducted in compliance with the Animal Welfare Act and the Implementing Animal Welfare Regulations in accordance with the principles of the Guide for the Care and Use of Laboratory Animals. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas at San Antonio.
NOTE: For the steps described below, male Lewis Rats are utilized. Slight protocol adjustments must be made for a female, as well as mouse microvascular fragment (MVF) collection29. For protocols using human MVFs (h-MVFs), the only steps required are the resuspension of h-MVFs following the manufacturer's protocol, growth media preparation (1.3), formation of fibrin hydrogels (5), and culturing (6). For an overview of the protocol, please see Figure 1.
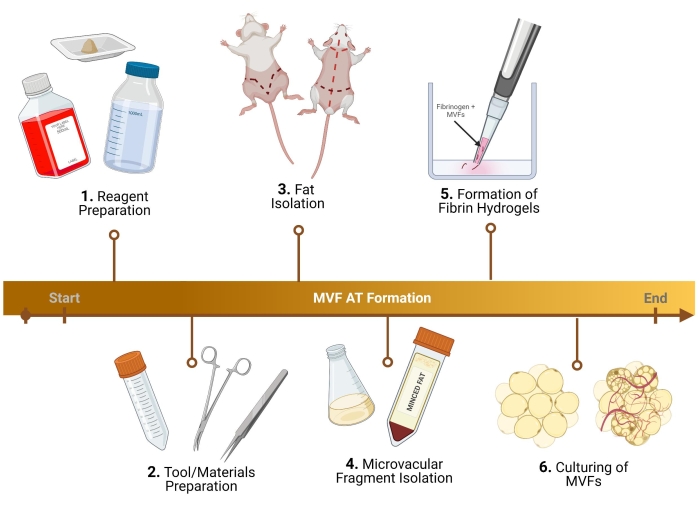
Figure 1: Experimental outline. Breakdown of six key steps, prior to analysis, for the formation of thermogenic adipose tissue using MVF. Please click here to view a larger version of this figure.
1. Reagent preparation
NOTE: Reagents below correspond to one rat, weighed and made inside a biohood.
- Prepare bovine serum albumin (BSA) in PBS.
- Prepare 10 mg/mL (1.0%) BSA in PBS to be diluted for wash steps (1 mg/mL, 0.1%) and digestion (4 mg/mL, 0.4%).
- Prepare different concentrations of BSA, as mentioned in step 1.1.1, following steps 1.1.3-1.1.5.
- 10 mg/mL BSA in PBS (1% BSA in PBS)
- Add 500 mg of BSA and 500 mL of PBS to a 50 mL conical tube and vortex the solution to dissolve the BSA.
- If excessive bubbles form, centrifuge the solution at 350 x g for 2 min. Filter sterilize the solution with a 0.22 µm nylon net filter.
- 1 mg/mL BSA in PBS (0.1% BSA in PBS)
- Dilute 15 mL of 10 mg/mL BSA in PBS 1:10 with PBS by adding 15 mL of 10 mg/mL BSA in PBS to 135 mL of PBS in a 500 mL sterile bottle and gently shake the bottle to ensure a homogenous mixture.
- 4 mg/mL BSA in PBS (0.4% BSA in PBS)
- Dilute 35 mL of 10 mg/mL BSA in PBS 1:2.5 with PBS by adding 35 mL of 10 mg/mL BSA in PBS + 57.5 mL of PBS in a 100 mL sterile bottle and gently shake bottle to ensure a homogenous mixture.
- Prepare collagenase in BSA for the digestion of minced fat pads.
- Prepare 6 mg/mL collagenase.
- In a 50 mL conical tube, weigh 72 mg of collagenase (label "for Epi").
- In three 50 mL conical tubes, weigh 144 mg of collagenase (label "for Ing 1", "for Ing 2", and "for SubQ") each.
- Store the weighed out collagenase at 4 ˚C until use.
NOTE: Do not add BSA/PBS until right before digestion. - Add 12 mL of 4 mg/mL BSA in PBS to the tube containing 72 mg of collagenase.
- Add 24 mL of 4 mg/mL BSA in PBS to the tubes containing 144 mg of collagenase.
- Shake the tubes to ensure a homogenous solution and filter sterilize the solution with a 0.22 µm nylon net filter.
- Prepare 6 mg/mL collagenase.
- Prepare growth media supplemented with Aminocaproic Acid (ACA) to feed/differentiate the isolated MVF.
- Growth media (GM): Supplement DMEM with 20% FBS, 1% penicillin-streptomycin (pen strep), 0.2% mycoplasma prophylactic, and 1 mg/mL ACA.
- Prepare white adipogenic media (WAM).
- WAM induction: Supplement DMEM/F12 with 20% FBS, 1% pen strep, 0.2% mycoplasma prophylactic, 10 µg/mL insulin, 10 µm of forskolin, 1 µm of dexamethasone, and 1 mg/mL ACA.
NOTE: For h-MVFs, in addition, add 125 µM of indomethacin. - WAM maintenance: Supplement DMEM/F12 with 20% FBS, 1% pen strep, 0.2% mycoplasma prophylactic, 5 µg/mL insulin, and 1 mg/mL ACA.
NOTE: For h-MVFs, change the insulin concentration to 10 µg/mL.
- WAM induction: Supplement DMEM/F12 with 20% FBS, 1% pen strep, 0.2% mycoplasma prophylactic, 10 µg/mL insulin, 10 µm of forskolin, 1 µm of dexamethasone, and 1 mg/mL ACA.
- Prepare beige adipogenic media (BAM).
- BAM induction: Supplement DMEM/F12 with 20% FBS, 1% pen strep, 0.2% mycoplasma prophylactic, 10 µg/mL insulin, 10 µm of forskolin, 1 µm of dexamethasone, 1 µm of rosiglitazone, 20 nM 3,3′,5-Triiodo-L-thyronine (T3), and 1 mg/mL ACA.
NOTE: For h-MVFs, change the concentration of T3 to 120 nM. - BAM maintenance: Supplement DMEM/F12 with 20% FBS, 1% pen strep, 0.2% mycoplasma prophylactic, 5 µg/ml insulin, 10 µm of forskolin, 1 µm of rosiglitazone, 20 nM T3, and 1 mg/mL ACA.
NOTE: For h-MVFs, change the insulin concentration to 10 µg/mL and the T3 concentration to 120 nM.
- BAM induction: Supplement DMEM/F12 with 20% FBS, 1% pen strep, 0.2% mycoplasma prophylactic, 10 µg/mL insulin, 10 µm of forskolin, 1 µm of dexamethasone, 1 µm of rosiglitazone, 20 nM 3,3′,5-Triiodo-L-thyronine (T3), and 1 mg/mL ACA.
- Prepare thrombin (only need to be made if the stock aliquoted solution is not available) to make the clotting agent to be used in fibrin gels.
- 10 U/mL thrombin in ddH2O:
- Resuspend thrombin powder using 1-5 mL of ddH2O in the vial from the manufacturer and transfer the resuspension to a 250 mL beaker.
- Bring up the solution to 100 mL and pipet the solution up and down numerous times to ensure a homogenous mixture. Aliquot the solution into 15 mL conical tubes (~10 mL/tube) and store the aliquots in a -20 ˚C freezer.
NOTE: To use, thaw the thrombin at room temperature (RT).
- 10 U/mL thrombin in ddH2O:
2. Tool/materials preparation
NOTE: All instrumentation should be autoclaved/sterilized prior to use.
- Epididymal/inguinal/subcutaneous fat isolation (surgery to be done in a defined surgical area)
- Ensure the availability of disposable underpads, two scissors, one hemostat (optional), two forceps, and four 50 mL conical tubes with 10-15 mL of 1 mg/mL BSA (0.1%) in PBS.
- Microvascular fragment isolation
- For mincing (to be done in a biohood), ensure the availability of three uncoated sterile 100 mm Petri dishes, one pair of forceps, and one pair of curved scissors.
- For digestion and isolation (to be done in a biohood), ensure the availability of one pair of scissors, one pair of forceps, eight uncoated sterile 100 mm Petri dishes, three uncoated sterile 35 mm Petri dishes, collagenase weighed and at 4 ˚C, four 250 mL flasks, one plastic holder with a hole at the center, four 500 µM screens (cut into 3 in rounded squares), four 37 µM screens (cut into 3 in rounded squares), and 11 50 mL conical tubes.
- Fibrin resuspension
- For fibrin hydrogels (to be done in a biohood), ensure that fibrinogen, thrombin, and growth media (made during reagent preparation) are available.
3. Fat isolation protocol
- Preliminary steps
- Fill a beaker with ethanol to wash and sanitize the surgical instruments before using them. Next, prepare the table for handling the rat and have a vacuum ready to aspirate the fur generated during the shaving step.
- Prepare a bucket with ice where the previously prepared 50 mL conical tubes, each containing 10 mL of 1 mg/mL BSA, will be placed. Label the tubes accordingly.
NOTE: It might be necessary to have two separate conical tubes for the inguinal fat, since two sides need to be extracted and the amount of fat gathered from this region tends to be the largest compared to epididymal and posterior subcutaneous. - Start with a euthanized rat in the defined surgical area with the required surgical tools. Generally, CO2 is used to euthanize the rats following IACUC protocols.
- Using hair clippers, shave the rat around the area of interest. Specifically, shave between the groins and half of the abdomen for epididymal and inguinal fat isolation (use the smaller shaver for the fur in this region). Shave the entire back to get the posterior subcutaneous fat located between the scapulas (it is best to use a larger shaver as the fur is thicker on the backside of the rat).
- Aseptically prepare the rat by spraying it down with 70% ethanol. Generally, it is recommended to clean the area that is going to be cut twice.
- Isolation of different fat depots
- Inguinal (subcutaneous)
- In the supine position, lift the skin below the penis with a pair of scissors. Begin the incision with scissors, starting in the center and cutting laterally, forming a "V" shape and looping to the rat's backside to access the entire fat depot. Remember to cut superficially so that the fat below is intact. During the cutting step, ensure the separation of the skin from the fat by cutting the interconnected fascia tissue (Figure 2A).
- Once the fascia is appropriately cut, ensure that the two sides of the inguinal fat are visible (the inguinal fat extends from the groin area toward the back). Next, remove the fat from the two sides in two separate conical tubes containing 10 mL of 1 mg/mL BSA (Figure 2B).
- Epididymal (visceral)
- Harvest the epididymal fat by cutting through the abdominal skin and carefully through the thin layer surrounding the testicles (Figure 2C).
- With forceps, gently pull the fat tissue out and cut it out using scissors. Avoid dissecting any major visible blood vessels (should the testis and epididymis be harvested, remove them during the cleaning step in the biohood).
- Carefully place the removed fat into a 50 mL conical tube with 10 mL of 1 mg/mL BSA in PBS.
NOTE: The removal of the epididymal fat should be done after the inguinal fat has been removed. The epididymal fat is generally smaller in volume and located below the inguinal fat, below the abdominal skin surrounding the testis and epididymis.
- Posterior subcutaneous
- Turn the rat prone (dorsal side up) and, using large scissors, cut the skin of the back (the skin in this region is thick) all the way up to the scalp, taking care not to cut too deep, just below the skin (Figure 2D).
- Cut the fascia connecting the skin to the tissue; the fat is located in the interscapular region. Make a note to differentiate/separate the subcutaneous fat from the brown fat. The brown fat is closer to the spine (Figure 2E).
- Isolate and place the fat in the corresponding 50 mL conical tube(s) with 10 mL of 1 mg/mL BSA in PBS.
- Inguinal (subcutaneous)
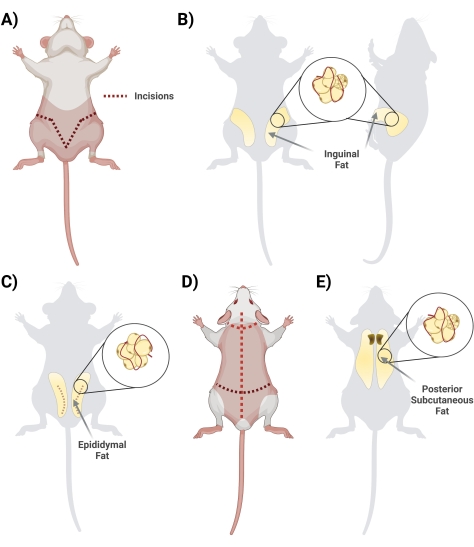
Figure 2: Isolation of different adipose tissue depots. (A) Initial incisions needed for excision of the inguinal adipose tissue. (B) Location of the inguinal fat depot. (C) Location of the epididymal fat depot, noting incision of the outer skin needed for access. (D) Additional incisions needed once the mice are placed prone to access additional fat. (E) Location of the posterior subcutaneous fat depot. Please click here to view a larger version of this figure.
4. Microvascular fragment isolation protocol
- Place 50 mL conical tube(s) containing excised fat from the rat into biohood.
- Using forceps, place the fat in a standard 100 mm Petri dish (with ~0.5 mL of 1 mg/mL BSA in PBS to keep the tissue hydrated).
- Clean and remove any visible blood vessels and muscle/extraneous tissue from fat.
- Mince the fat with scissors for ~10 min (mince enough so that it can be transferred with a 10 mL pipet).
- Check for lumps by adding ~0.5 mL of 1 mg/mL BSA in PBS; continue mincing if necessary.
- Transfer the minced fat to a sterile 250 mL flask with a 10 mL pipet.
NOTE: Note the volume using a pipet. - Add enough BSA (1 mg/mL) so the final volume is 20 mL.
- Add 4 mg/mL BSA in PBS to collagenase (collagenase final concentration: 6 mg/mL) (i. e., 12 mL for the 72 mg of collagenase or 24 mL for the 144 mg of collagenase).
NOTE: Do not add until the mincing is complete as it is time sensitive. - Gently shake the conical tube to ensure a homogenous solution and filter sterilize the solution with a 0.22 µm nylon net filter.
- For the epididymal fat, digest for ~8-10 min; for the inguinal and posterior subcutaneous fat, digest for ~15-20 min in a 37 °C water bath, shaking the flask in a circular motion throughout (stop at the moment the fat gets to where there are only a few clumps remaining).
- Transfer the digested fat into a 50 mL conical tube (there should be ~30 mL/tube), and label the tube as "digested fat".
- Spin the tube at 400 x g for 4 min; post spinning, the pellet must be red (Figure 3A).
- During the spin, place the sterile 37 µM screen and 500 µM screens in a sterile Petri dish with 5 mL of 1 mg/mL BSA in PBS to presoak before use.
- After the spin, decant the supernatant into a 50 mL conical tube labeled as "waste". Perform the decantation gently to remove the superficial fat and to not disturb the pellet made of MVFs.
- Add 10 mL of 1 mg/mL BSA in PBS to the tube containing the pellet ("digested fat"). Triturate (pipet up and down) the pellet 2x.
- Avoid being too rough on the pellet in order not to disrupt the fragments.
- Place the 500 µM screen in a new Petri dish above the plastic screen holder (Figure 3B).
- Pipet 10 mL from the "digested pellet" tube over a 500 µM screen using concentric circles (Figure 3C).
- Wash the filter with an additional 5 mL of 1 mg/mL BSA in PBS. The desired cells will filter through into the Petri dish; therefore, discard the 500 µM screen but save the filtered liquid inside the Petri dish.
- Place the 37 µM screen in a new Petri dish above the plastic screen holder.
- Change out the pipet to eliminate any clumps before using the 37 µM screen.
- Pipet the liquid obtained from the first filtration over the 37 µM screen using concentric circles.
- Wash the filter with an additional 5 mL of 1 mg/mL BSA in PBS. The desired cells will remain in the filter, therefore discard the filtered liquid, but save the 37 µM screen.
- Slide the 37 µM screen into a new Petri dish containing 5 mL of 1 mg/mL BSA in PBS.
- Shake the dish by tapping it against a conical holder to dislodge the fragments. Do not shake too vigorously, as the liquid/cells may spill out of the Petri dish.
- Rinse the filter with an additional 5 mL of 1 mg/mL BSA in PBS. The desired cells will remain in liquid solution in the Petri dish. Save the 37 µM screen and the dislodged fragment containing liquid inside the Petri dish for the following steps.
- Transfer the BSA + fragment containing liquid to a sterile 50 mL conical tube.
- Repeat rinsing of the 37 µM screen several more times (each time with ~5 mL of 1 mg/mL BSA in PBS) and add to the conical tube. Repeat until the total volume collected is ~15-20 mL. Ultimately, the desired cells will be collected from the liquid solution in the Petri dish and placed in a conical tube; at this point, discard the 37 µM screen after the final rinse but save the dislodged fragments containing liquid inside the 50 mL conical tube.
- Clip the end off of a 200 µL pipet tip using scissors. Gently shake the 50 mL tube, remove two samples of 20 µL, and put them in a clean 35 mm Petri dish.
- Count the number of fragments in each sample in the Petri dish to obtain the total number of MVFs isolated.
Total Fragments =
- Spin the remaining dislodged fragment containing liquid in a 50 mL conical tube at 400 x g for 4 min to collect the MVF.
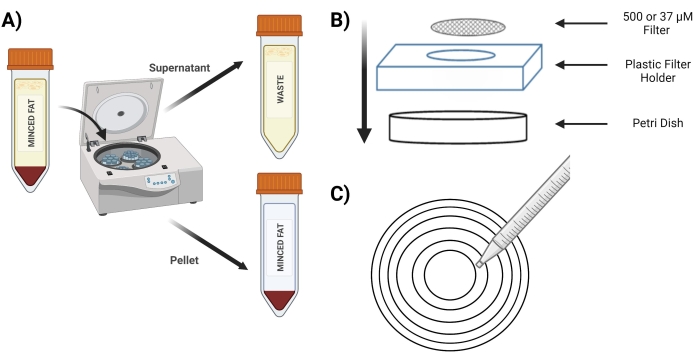
Figure 3: Isolation of MVFs. (A) Post digestion of the adipose tissue, depiction of the separation of MVFs containing pellet and supernatant following a spin-down. (B) Layout of supplies for filtration and entrapment of MVFs. (C) Illustration of the concentric circle method for filtration/washing steps. Please click here to view a larger version of this figure.
5. Formation of fibrin hydrogels
- Example calculations:
NOTE: Below are the calculations for MVFs seeded at ~15,000-20,000 MVF/mL and the fibrinogen:thrombin gel ratio at 2:5, with fibrinogen used at a 20 mg/mL concentration.- For making five 250 µL gels, a total volume of 1,250 µL is required. Always account for pipetting errors, therefore, make enough for 1.5 mL of gels.
- Calculate the volume of fibrinogen required as follows:
 , X1 = 428.57 µL of fibrinogen
, X1 = 428.57 µL of fibrinogen - Calculate the volume of thrombin required as follows:
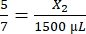 , X2 = 1,071.43 µL of thrombin
, X2 = 1,071.43 µL of thrombin - For the required volume of 428.57 µL, make 500 µL of fibrinogen as follows:
20 mg/mL * 0.5 mL = 10 mg of fibrinogen. Resuspend this in 500 µL of DMEM - To obtain each gel, calculate the volume of fibrinogen and thrombin as follows:
- Fibrinogen:
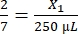 , X1 = 71.43 µL of fibrinogen (in DMEM) + MVF
, X1 = 71.43 µL of fibrinogen (in DMEM) + MVF - Thrombin:
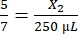 , X2 = 178.57 µL of thrombin
, X2 = 178.57 µL of thrombin
- Fibrinogen:
- Calculate the volume of fibrinogen required as follows:
- For making five 250 µL gels, a total volume of 1,250 µL is required. Always account for pipetting errors, therefore, make enough for 1.5 mL of gels.
- MVF fibrin gel casting
- Decant most of the liquid in the spun-down fragment in the 50 mL conical tube. Use a pipette to remove the small volume of liquid that catches on the rim of the conical tube.
- Add thrombin into wells where gels will be made (Figure 4A).
- Clip the end of a 200 µL pipet tip and resuspend the MVFs gently using fibrinogen to obtain a final density of ~15,000-20,000 MVF/mL once in the gel.
- Clip the end of a 200 µL pipet tip and pipet MVF+Fibrinogen into the thrombin solution gently. Quickly pipet up and down to ensure a homogenous mixture. Repeat until all gels are made (Figure 4B).
- Place the well plate(s) in an incubator (37 °C, 5% CO2) for ~15 min to allow for gel crosslinking (Figure 4C).
- Add 100-150 µL of growth media to each well.
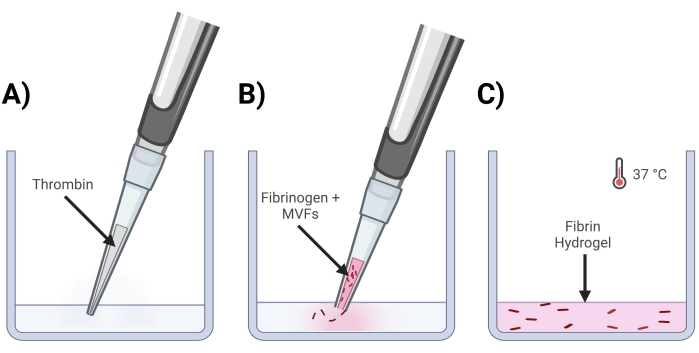
Figure 4: Formation of MVF fibrin gels. (A) A 5/7-part thrombin mixture gets pipetted into the corresponding well. (B) Next, with a clipped pipet tip (to not disturb MVFs), a 2/7-part fibrinogen+MVF mixture is pipetted into the well and gently mixed. (C) Lastly, all completed gels are placed into an incubator at 37 °C, allowing hydrogel to solidify fully before the media is placed on top. Please click here to view a larger version of this figure.
6. Culturing conditions of MVFs
- For culturing non-vascularized white and beige adipose tissue (+ GM Control), use the timelines19 shown in Figure 5.
- For culturing of vascularized white and beige adipose tissue (+ GM Control), use the timelines19 shown in Figure 6.
- Hydrogels should be maintained in an incubator (37 °C, 5% CO2) for the duration of culture for the study, with the media being changed every other day. For fixation and handling of samples for analysis, refer to previously published works16,19,20.

Figure 5: Timing for non-vascularized adipose tissue formation. This figure has been modified from Acosta et al.19. Please click here to view a larger version of this figure.
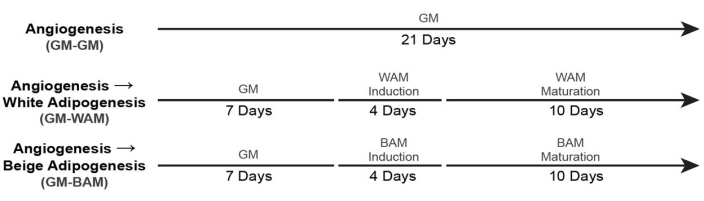
Figure 6: Timing for vascularized adipose tissue formation. This figure has been modified from Acosta et al.19. Please click here to view a larger version of this figure.
Results
There are a few key phenotypic morphological characteristics of beige/brown adipose tissue: it is multilocular/contains small lipid droplets, possesses a large number of mitochondria (the reason for its characteristically "brownish" appearance in vivo), correspondingly has a high oxygen consumption rate/mitochondrial bioenergetics, is highly vascularized, has increased lipolysis/insulin-stimulated glucose uptake, and, most notoriously, expresses high levels of uncoupling protein 1 (UCP1), a mitochondrial...
Discussion
The field of brown/beige adipose tissue engineering is largely immature22,23,24,25,26,27,28, with the bulk of adipose models being developed for white adipose tissue8,22,31. Engineered brown/beige m...
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Dr. Acosta is supported by the National Institutes of Health grants CA148724 and TL1TR002647. Dr. Gonzalez Porras is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, under Award Number F32-0DK122754. This work was supported, in part, by the National Institutes of Health (5SC1DK122578) and the University of Texas at San Antonio Department of Biomedical Engineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Figures were partially created with Biorender.com.
Materials
| Name | Company | Catalog Number | Comments |
| Aminocaproic Acid | Sigma Aldrich | A2504-100G | Added in DMEM at the concentration of 1 mg/mL |
| Blunt-Tipped Scissors | Fisher scientific | 12-000-172 | Sterilize in autoclave |
| Bovin Serum Albumin (BSA) | Millipore | 126575-10GM | Diluted in PBS to 4 mg/mL and 1 mg/mL |
| Collagenase Type 1 | Fisher scientific | NC9633623 | Diluted to 6 mg/mL in BSA 4 mg/mL, Digestion of minced fat |
| Dexamethasone | Thermo Scientific | AC230302500 | Diluted in ethanol at a 2 mg/ml stock concentration |
| Disposable underpads | Fisher scientific | 23-666-062 | For fluid absorption during surgery |
| Dissecting Scissors | Fisher scientific | 08-951-5 | Sterilize in autoclave |
| Dulbecco′s Modified Eagle′s Medium (DMEM) | Fisher scientific | 11885092 | |
| Dulbecco′s Modified Eagle′s Medium/Nutrient Mixture F-12 Ham (DMEM/F12) | Sigma Aldrich | D8062 | |
| Fetal Bovine Serum | Fisher scientific | 16140089 | Added in DMEM to 20% v/v. |
| Fibrinogen | Sigma Aldrich | F8630-25G | Solubilized in DMEM at the concentration of 20 mg/mL, Protein found in blood plasma and main component of hydrogel |
| Flask, 250 mL | Fisher scientific | FB500250 | Allows for digestion of fat using a large surface area |
| Forceps | Fisher scientific | 50-264-21 | Sterilize in autoclave, For handling of tissue and filters |
| Forskolin | Sigma Aldrich | F6886 | Diluted in ethanol at a 10 mM stock concentration |
| Human MVF | Advanced Solutions Life Scienes, LLC | https://www.advancedsolutions.com/microvessels | Human MVFs (hMVFs) isolated from three different patients (52-, 54-, and 56-year old females) were used in the current study. |
| Indomethacine | Sigma Aldrich | I7378 | Diluted in ethanol at a 12.5 mM stock concentration |
| Insulin from porcine pancreas | Sigma Aldrich | I5523 | Diluted in 0.01 N HCl at a 5 mg/ml stock concentration |
| MycoZap | Fisher scientific | NC9023832 | Added in DMEM to 0.2% w/v, Mycoplasma Prophylactic |
| Pennycilin/Streptomycin (10,000 U/mL) | Fisher scientific | 15140122 | Added in DMEM to 1% v/v. |
| Petri dishes, polystyrene (100 mm x 15 mm). | Fisher scientific | 351029 | 3 for removal of blood vessels and mincing, 8 (lid) for presoaking of screens & 8 (dish) for use when filtering with 500 or 37 µM screens |
| Petri dishes, polystyrene (35 mm x 10 mm). | Fisher scientific | 50-202-036 | For counting fragments |
| Phosphate Buffer Saline (PBS) | Fisher scientific | 14-190-250 | Diluted to 1x with sterile deionized water. |
| Rat Clippers (Andwin Mini Arco Pet Trimmer) | Fisher scientific | NC0854141 | |
| Rosiglitazone | Fisher scientific | R0106200MG | Diluted in DMSO at a 10 mM stock concentration |
| Scissors | Fine Science Tools | 14059-11 | 1 for initial incision, 1 for epididymal incision, 1 for tip clipping |
| Screen 37 µM | Carolina Biological Supply Company | 652222R | Cut into 3" rounded squares and sterilized in ethylene oxide, Fragment entrapment and removal of very small fragments/single cells and debris |
| Screen 500 µM | Carolina Biological Supply Company | 652222F | Cut into 3" rounded squares and sterilized in ethylene oxide, Removes larger fragments/debris |
| Serrated Hemostat | Fisher scientific | 12-000-171 | Sterilize in autoclave, For clamping of skin before incision |
| Steriflip Filter 0.22 μm | Millipore | SE1M179M6 | |
| Thrombin | Fisher scientific | 6051601KU | Diluted in deionzed water to 10 U/mL, Used as a clotting agent turning fibrinogen to fibrin |
| Thyroid hormone (T3) | Sigma Aldrich | T2877 | Diluted in 1N NaOH at a 0.02 mM stock concentration |
| Zucker diabetic fatty (ZDF) rats - obese (FA/FA) or lean (FA/+) male | Charles River | https://www.criver.com/products-services/find-model/zdf-rat-lean-fa?region=3611 https://www.criver.com/products-services/find-model/zdf-rat-obese?region=3611 | Obtained from Charles River (Wilmington, MA). Rats were acquired at 4 weeks of age and fed Purina 5008 until euthanasia (15-19 weeks of age). Glucose levels (blood from the lateral saphenous vein) were greater than 300 mg/dL in all FA/FA rats used in the study. All animals were housed in a temperature-controlled environment with a 12-h light-dark cycle and fed ad libitum. |
References
- Cohen, P., Spiegelman, B. M. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes. 64 (7), 2346-2351 (2015).
- Liu, X., et al. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology. 156 (7), 2461-2469 (2015).
- Tharp, K. M., Stahl, A. Bioengineering beige adipose tissue therapeutics. Frontiers in Endocrinology. 6, 164 (2015).
- Barquissau, V., et al. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Molecular Metabolism. 5 (5), 352-365 (2016).
- Kim, S. H., Plutzky, J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes & Metabolism Journal. 40 (1), 12-21 (2016).
- Lizcano, F., Vargas, D. Biology of beige adipocyte and possible therapy for type 2 diabetes and obesity. International Journal of Endocrinology. 2016, 9542061 (2016).
- Mulya, A., Kirwan, J. P. Brown and beige adipose tissue: therapy for obesity and its comorbidities. Endocrinology and Metabolism Clinics of North America. 45 (3), 605-621 (2016).
- Murphy, C. S., Liaw, L., Reagan, M. R. In vitro tissue-engineered adipose constructs for modeling disease. BMC Biomedical Engineering. 1, 27 (2019).
- Srivastava, S., Veech, R. L. Brown and brite: The fat soldiers in the anti-obesity fight. Frontiers in Physiology. 10, 38 (2019).
- Samuelson, I., Vidal-Puig, A. Studying brown adipose tissue in a human in vitro context. Frontiers in Endocrinology. 11, 629 (2020).
- Wang, C. -. H., et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Science Translational Medicine. 12 (558), (2020).
- Kaisanlahti, A., Glumoff, T. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. Journal of Physiology and Biochemistry. 75 (1), 1-10 (2019).
- Sato, N., et al. Development of capillary networks from rat microvascular fragments in vitro: the role of myofibroblastic cells. Microvascular Research. 33 (2), 194-210 (1987).
- Laschke, M. W., Später, T., Menger, M. D. Microvascular fragments: More than just natural vascularization units. Trends in Biotechnology. 39 (1), 24-33 (2021).
- Hoying, J. B., Boswell, C. A., Williams, S. K. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cellular & Developmental Biology-Animal. 32 (7), 409-419 (1996).
- Acosta, F. M., Stojkova, K., Brey, E. M., Rathbone, C. R. A straightforward approach to engineer vascularized adipose tissue using microvascular fragments. Tissue Engineering. Part A. 26 (15-16), 905-914 (2020).
- Acosta, F. M., et al. Adipogenic differentiation alters properties of vascularized tissue-engineered skeletal muscle. Tissue Engineering. Part A. 28 (1-2), 54-68 (2021).
- Strobel, H. A., Gerton, T., Hoying, J. B. Vascularized adipocyte organoid model using isolated human microvessel fragments. Biofabrication. 13 (3), 035022 (2021).
- Acosta, F. M., et al. Engineering functional vascularized beige adipose tissue from microvascular fragments of models of healthy and type II diabetes conditions. Journal of Tissue Engineering. 13, 20417314221109337 (2022).
- Gonzalez Porras, M. A., Stojkova, K., Acosta, F. M., Rathbone, C. R., Brey, E. M. Engineering human beige adipose tissue. Frontiers in Bioengineering and Biotechnology. 10, 906395 (2022).
- Herold, J., Kalucka, J. Angiogenesis in adipose tissue: The interplay between adipose and endothelial cells. Frontiers in Physiology. 11, 1861 (2021).
- McCarthy, M., et al. Fat-On-A-Chip models for research and discovery in obesity and its metabolic comorbidities. Tissue Engineering Part B: Reviews. 26 (6), 586-595 (2020).
- Klingelhutz, A. J., et al. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Scientific Reports. 8 (1), 523 (2018).
- Yang, J. P., et al. Metabolically active three-dimensional brown adipose tissue engineered from white adipose-derived stem cells. Tissue Engineering. Part A. 23 (7-8), 253-262 (2017).
- Vaicik, M. K., et al. Hydrogel-based engineering of beige adipose tissue. Journal of Materials Chemistry B. 3 (40), 7903-7911 (2015).
- Tharp, K. M., Stahl, A. Bioengineering beige adipose tissue therapeutics. Frontiers in Endocrinology. 6, 164 (2015).
- Tharp, K. M., et al. Matrix-assisted transplantation of functional beige adipose tissue. Diabetes. 64 (11), 3713-3724 (2015).
- Harms, M. J., et al. Mature human white adipocytes cultured under membranes maintain identity, function, and can transdifferentiate into brown-like adipocytes. Cell Reports. 27 (1), 213-225 (2019).
- Frueh, F. S., Später, T., Scheuer, C., Menger, M. D., Laschke, M. W. Isolation of murine adipose tissue-derived microvascular fragments as vascularization units for tissue engineering. Journal of Visualized Experiments. (122), e55721 (2017).
- Cannon, B., Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiological Reviews. 84 (1), 277-359 (2004).
- Unser, A. M., Tian, Y., Xie, Y. Opportunities and challenges in three-dimensional brown adipogenesis of stem cells. Biotechnology Advances. 33, 962-979 (2015).
- Dani, V., Yao, X., Dani, C. Transplantation of fat tissues and iPSC-derived energy expenditure adipocytes to counteract obesity-driven metabolic disorders: Current strategies and future perspectives. Reviews in Endocrine & Metabolic Disorders. 23 (1), 103-110 (2022).
- Xu, X., et al. Adipose tissue-derived microvascular fragments as vascularization units for dental pulp regeneration. Journal of Endodontics. 47 (7), 1092-1100 (2021).
- McDaniel, J. S., Pilia, M., Ward, C. L., Pollot, B. E., Rathbone, C. R. Characterization and multilineage potential of cells derived from isolated microvascular fragments. Journal of Surgical Research. 192 (1), 214-222 (2014).
- Gealekman, O., et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 123 (2), 186-194 (2011).
- Altalhi, W., Hatkar, R., Hoying, J. B., Aghazadeh, Y., Nunes, S. S. Type I diabetes delays perfusion and engraftment of 3D constructs by impinging on angiogenesis; which can be rescued by hepatocyte growth factor supplementation. Cellular and Molecular Bioengineering. 12 (5), 443-454 (2019).
- Altalhi, W., Sun, X., Sivak, J. M., Husain, M., Nunes, S. S. Diabetes impairs arterio-venous specification in engineered vascular tissues in a perivascular cell recruitment-dependent manner. Biomaterials. 119, 23-32 (2017).
- Laschke, M. W., et al. Adipose tissue-derived microvascular fragments from aged donors exhibit an impaired vascularisation capacity. European Cells & Materials. 28, 287-298 (2014).
- Später, T., et al. Vascularization of microvascular fragment isolates from visceral and subcutaneous adipose tissue of mice. Tissue Engineering and Regenerative Medicine. 19 (1), 161-175 (2021).
- Später, T., et al. Adipose tissue-derived microvascular fragments from male and female fat donors exhibit a comparable vascularization capacity. Frontiers in Bioengineering and Biotechnology. 9, 777687 (2021).
- Laschke, M. W., Menger, M. D. The simpler, the better: tissue vascularization using the body's own resources. Trends in Biotechnology. 40 (3), 281-290 (2022).
- Yang, F., Cohen, R. N., Brey, E. M. Optimization of co-culture conditions for a human vascularized adipose tissue model. Bioengineering. 7 (3), 114 (2020).
- Pilkington, A. -. C., Paz, H. A., Wankhade, U. D. Beige adipose tissue identification and marker specificity-Overview. Frontiers in Endocrinology. 12, 599134 (2021).
- Chiou, G., et al. Scaffold architecture and matrix strain modulate mesenchymal cell and microvascular growth and development in a time dependent manner. Cellular and Molecular Bioengineering. 13 (5), 507-526 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved