A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Behavioral and Network Pharmacology-Based Analyses for the Traditional Mongolian Medicine Zadi-5 in a Rat Model of Depression
In This Article
Summary
The present protocol describes a method for the behavioral test validation and bioinformatical prediction of the therapeutic efficacy of Zadi-5, a traditional Mongolian medicine, in depression.
Abstract
Zadi-5 is a traditional Mongolian medicine that is widely used for the treatment of depression and symptoms of irritation. Although the therapeutic effects of Zadi-5 against depression have been indicated in previously reported clinical studies, the identity and impact of the active pharmaceutical compounds present in the drug have not been fully elucidated. This study used network pharmacology to predict the drug composition and identify the therapeutically active compounds in Zadi-5 pills. Here, we established a rat model of chronic unpredicted mild stress (CUMS) and conducted an open field test (OFT), Morris water maze (MWM) analysis, and sucrose consumption test (SCT) to investigate the potential therapeutic efficacy of Zadi-5 in depression. This study aimed to demonstrate Zadi-5's therapeutic effects for depression and predict the critical pathway of the action of Zadi-5 against the disorder. The vertical and horizontal scores (OFT), SCT, and zone crossing numbers of the fluoxetine (positive control) and Zadi-5 groups were significantly higher (P < 0.05) than those of the CUMS group rats without treatment. According to the results of network pharmacology analysis, the PI3K-AKT pathway was found to be essential for the antidepressant effect of Zadi-5.
Introduction
Depression, also known as major depressive disorder (MDD), is a severe neuropsychiatric disease responsible for growing medical and economic burdens on society. Due to the associated complexity, morbidity, and mortality rates, a significant amount of research has been conducted to find remedies for the disorder1,2. According to a mental health survey by the World Health Organization, around 350 million people currently suffer from depression and its associated symptoms worldwide. It is predicted that depression will overtake cancer and cardiovascular diseases as the leading cause of disease burden globally by 2030. Thus, the prevention and treatment of depression will become a global priority in the near future3. The pathogenesis of MDD has not yet been elucidated. Still, it is commonly attributed to the following factors: genetic predisposition, dysfunction of the hypothalamus-pituitary-adrenal axis, reductions in neurotransmitter secretion, neuroimmune dysregulation-induced neuroinflammation, cell apoptosis, and reduced cell proliferation4,5.
Among these factors, neuroimmune dysregulation-induced neuroinflammation and altered secretion of neurotrophic factors have received particular attention for their roles in the development of depression and many other psychiatric diseases6. In the past decade, scholars have demonstrated that the hippocampus is the dominant site for regenerative nerve functions and is involved in regulating emotion and cognition. In this regard, the hippocampal neurons are recognized as novel therapeutic targets for antidepressant medicines under development7,8. Moreover, the hippocampus is also reported to be involved in short-term and long-term memory in learning and consolidating memories. Specifically, the shortage of pyramidal neurons in the CA1 region of the hippocampus causes retrograde and anterograde amnesia9. A typical antidepressant therapeutic strategy aims to enhance cell proliferation and neurogenesis in the dentate gyrus of the hippocampus. Natural product-derived compounds and small molecules synthesized based on medicinal chemistry techniques are considered the primary sources of innovative therapeutic agents for various neuropsychiatric conditions.
Traditional Mongolian medicines, which have a long history and a well-supported theoretical medical system, have descended from the nomads of the Mongolian plateau These medicines display multi-target and multi-pathway effects due to the various medicinal components that act in concert to generate synergistic functions. Zadi-5 is a well-established formulation among such drugs and was first recorded in "Clinical Experience of Dr. Gao Shi," written by an outstanding Mongolian clinician called Dr. Gao Shi (1804-1876). It has been clinical practice for a long time in Mongolia to use these pills to treat the symptoms of distress, palpitation, irritation, and cardiac stabbing pain10,11. Moreover, Zadi-5 has proven effects on alleviating post-stroke depression in affected patients12. The recent experimental research on CUMS has revealed that the Zadi-5 formulation alleviates depression by regulating the central neurotransmitters13; indeed, with Zadi-5, increased levels of brain-derived neurotrophic factor (BDNF) and tyrosine kinase receptor B (TrkB) have been detected and correlated with improved learning and memory in a rat model of depression14. However, the exact mechanism of action of Zadi-5 for such alleviation of depression has not been elucidated.
This study aimed to demonstrate the therapeutic effects of Zadi-5 against depression in rats using a behavioral test and identify the components of Zadi-5 using Traditional Chinese Medicine Systems Pharmacology (TCMSP) and Swiss Target Prediction to predict the potential mechanisms underlying the efficacy of Zadi-5, a traditional Mongolian medicine, in treating depression.
Access restricted. Please log in or start a trial to view this content.
Protocol
All the experimental protocols were approved by the Ethics of Animal Experiment Care Committee of Inner Mongolia Medical University and followed the guidelines of the National Institutes of Health on animal care and ethics. Male Sprague Dawley (SD) rats aged 8 weeks old (200 g ± 20 g) were housed in a room with a controlled temperature (22 °C ± 2 °C) and humidity (55% ± 15%) under a 12 h/12 h regulated light/dark cycle for 1 week. See Figure 1 for the workflow of the network pharmacology analysis.
1. Behavioral test in rats
- Establish a CUMS rat model
- Apply the following stimuli combined with isolation for 28 days to all the rats, except for the controls: inversion of the light/dark cycle for 24 h, food deprivation for 24 h, water deprivation for 24 h, high-speed level shaking for 15 min (one time/s), tail clamp for 2 min, swimming in cold water (4 °C) for 5 min, 45 °C heat stimulation, and wet padding for 24 h (Table 1). Raise the rats in individual cages.
NOTE: Avoid repeating the same type of stimuli for consecutive days. The above procedures to establish a CUMS rat model have been approved by the animal ethics committee and described previously15.
- Apply the following stimuli combined with isolation for 28 days to all the rats, except for the controls: inversion of the light/dark cycle for 24 h, food deprivation for 24 h, water deprivation for 24 h, high-speed level shaking for 15 min (one time/s), tail clamp for 2 min, swimming in cold water (4 °C) for 5 min, 45 °C heat stimulation, and wet padding for 24 h (Table 1). Raise the rats in individual cages.
- Drug preparation
- Pulverize the Zadi-5 pill in a grinder, and prepare a 1.16 g/mL solution in distilled water. Separately prepare a fluoxetine solution of 0.36 mg/mL in distilled water.
- Drug administration
- Divide the rats randomly into six groups (n = 10): control (CON), model (MOD), Zadi-5 group (Zadi-5, 1.6 g of Zadi-5/kg16), fluoxetine group (Fluoxetine, 3.6 mg of fluoxetine/kg). One time per day for 28 days, administer 1 mL/g per rat of the appropriate drug solution by gavage and treat the CON and MOD groups with an equal volume of distilled water.
NOTE: Gavage starts at the beginning of model establishment for all the groups.
- Divide the rats randomly into six groups (n = 10): control (CON), model (MOD), Zadi-5 group (Zadi-5, 1.6 g of Zadi-5/kg16), fluoxetine group (Fluoxetine, 3.6 mg of fluoxetine/kg). One time per day for 28 days, administer 1 mL/g per rat of the appropriate drug solution by gavage and treat the CON and MOD groups with an equal volume of distilled water.
- Open-field test (OFT)
- Divide a black box (50 cm x 50 cm x 30 cm) into nine square regions of equal area. Equip the box with a video tracking analysis system. One day after the last gavage, place the rat in the center square, and record its horizontal and vertical activities for 3 min.
- Score the number of squares crossed with all paws as a horizontal activity, and score standing and grooming as a vertical activity. After each test, clean the box with 75% alcohol to remove the smell of the rat for subsequent tests17.
- Sucrose consumption test (SCT)
- Weigh the respective bottles before and after consumption, and calculate the 60 min sucrose preference rates on day 0, day 7, day 14, day 21, and day 28 using equation (1):
Sucrose consumption =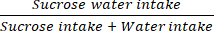 × 100% (1)
× 100% (1)
- Weigh the respective bottles before and after consumption, and calculate the 60 min sucrose preference rates on day 0, day 7, day 14, day 21, and day 28 using equation (1):
- Morris water maze (MWM)
- Divide the pool into four quadrants. Order the quadrants from one to four, and place the hidden platform in the third quadrant, 1 cm below the water surface.
- Place the rat subject into the maze in different quadrants to look for the platform for 120 s, and record the latency time using the MWM video trail analysis system.
- Place the rat subject in a fixed position in the pool. If the subject cannot find the hidden platform in 120 s, record the latency as 120 s.
- Next, dislodge the platform, place the rat in the water, and record the number of zone-crossings for 120 s.
- Add milk to the pool for some level of opacity. Maintain the water temperature at 23 °C ± 1 °C during the experiment.
2. Network pharmacological prediction
- Screen the active components in Zadi-5.
- Browse the Traditional Chinese Medicine Systems Pharmacology (TCMSP, https://old.tcmsp-e.com/tcmsp.php), and input "Myristicae Semen Seeds," "Aucklandiae Radix roots," and "Piperis Longi Fructus" in the "herb name" section to obtain the names of chemicals. Set the pharmacokinetic index of oral bioavailability (OB) to be >30% and the drug-like (DL) index to be >0.18 (Supplementary File 1).
- Search "Rou Dou Kou" (Myristica fragrans Houtt), "Tu Mu Xiang" (Inula helenium L.), "Mu Xiang" (Aucklandia lappa Decne.), "Guang Zao" (Choerospondias axillaris Roxb. Burtt Hill), and "Bi Ba" (Piper longum L.) in the Chinese Medicine Pharmacopeia (http://www.zhongyaocai360.com/zhongguoyaodian/) to identify the chemical names of each component.
- Search the identified chemical names in PubChem (https://pubchem.ncbi.nlm.nih.gov/) to find the isomeric SMILES or InChIkey
- Identify the target proteins of the active components in Zadi-5.
- Identify the target proteins of the active components using SEA (http://sea.bkslab.org/), BATMAN (http://bionet.ncpsb.org.cn/batman-tcm/), and Swiss Target Prediction (http://www.swisstargetprediction.ch/) with isomeric SMILES or InChIkey, and find the overlapping proteins.
- Use the protein database UniProt (http://www.uniprot.org/uploadlists/) to convert the identified targets to unified gene names.
- Search for the target proteins for depression.
- Search and identify the potential protein targets for depression by using the keywords "depression" and "depressive disorder" in Genecards (https://www.genecards.org/), Disgenet (https://www.disgenet.org/), and Drugbank (https://www.drugbank.com/).
- Find the target genes.
- Browse the Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/), upload the targets of the active components of Zadi-5 in List-1, upload the targets for depression in List-2, and submit. Obtain the Venn diagram, and filter out the overlapping target candidates.
- Construct the network.
- Construct a spreadsheet called "Type and Network" (Supplementary File 2). "Type" is the network's signature, and "Network" illustrates the relationship between the signs.
- Export the "Type and Network" to Cytoscape v3.9.0 to construct the network "Zadi-5 herbs-ingredients-disease targets."
- Analyze the protein-protein interaction (PPI) network of the target candidates.
- Set the common targets in the STRING database (https://cn.string- db.org/) to analyze their interactions. Set the protein type as "homo sapiens." Set the interaction threshold value to 0.9, and select only the experimentally verified types. Do not display the lonely island nodes.
- Conduct a Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses of the target-related pathways.
- Paste 86 potential antidepressant targets of Zadi-5 into the start analysis bracket in DAVID (https:// david.ncifcrf.gov/) to study the related signaling pathways by performing the Gene Ontology (GO) function-including biological process, cellular component, and molecular function-and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses.
NOTE: KEGG is visualized in the bubble chart using an online IMageGP (http://www.ehbio.com /ImageGP/index.php/Home/Index/). The bubble size represents the number of targets enriched in the indicated pathway, and the bubble color represents the enrichment’s P-value.
- Paste 86 potential antidepressant targets of Zadi-5 into the start analysis bracket in DAVID (https:// david.ncifcrf.gov/) to study the related signaling pathways by performing the Gene Ontology (GO) function-including biological process, cellular component, and molecular function-and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses.
- Construct the network to illustrate the active compounds in Zadi-5 that interact with the PI3K-AKT signaling pathway.
- Download the KEGG pathway document, select the genes of the PI3K-AKT pathway from the enrichment analysis, and paste them on the spreadsheet to construct a "Type and Network" document.
- Export the "Type and Network" document to the Cytoscape to generate the "PI3K-AKT visualized compounds-targets-pathways network" (Supplementary File 3).
NOTE: "Type" is the network's signature, and "Network" illustrates the relationship between the signs.
3. Statistical analysis
- Use a one-way analysis of variance (ANOVA), followed by Duncan's post hoc test, to determine the significant differences in biochemical and gene expression parameters. Calculate the mean ± standard deviation (SD), and visualize the data. Consider P < 0.05 as statistically significant.
Access restricted. Please log in or start a trial to view this content.
Results
Behavioral test in animals
Results of the behavioral tests in the CUMS-induced rat depression model
No significant differences between the tested groups were found for the OFT score, sucrose consumption, and MWM analysis before CUMS stimulation. After establishing the CUMS model, the MOD group's vertical and horizontal scores were lower than those of the CON group (P < 0.05). Compared with the MOD group, the vertical and horizontal scores of the POS and Zadi-5 groups ...
Access restricted. Please log in or start a trial to view this content.
Discussion
Depression is a mental disease characterized by low mood, anhedonia, and a lack of energy. This disorder is accompanied by distraction, cognitive dysfunction, social withdrawal, insomnia, sexual dysfunction, and gastrointestinal diseases18,19. In the study of depression, establishing an animal model is crucial for understanding the pathological mechanisms and effects of new drugs. In this study, a CUMS-induced rat depression model was established through the irri...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We are grateful for the instrumentation and laboratory provided by the Mongolian medical faculty of the Inner Mongolian Medical University, China. This study was supported by the National Natural Sciences Foundation of China (81760762) and the Science and Technology Plan Project of the Health Commission of Inner Mongolia, China (202201300).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Cytoscape software | version 3.7.0 | ||
| Fluoxetine | Lilly Suzhou Pharmaceutical Co., Ltd | J20160029 | |
| Morris water maze video trail analysing system | Tai Meng Tech Co., Ltd | WMT-200 | |
| Sprague Dawley rats | Beijing Biotechnology Co., Ltd, China | SCXK (JING) 2016-0002 | |
| video tracking system | Tai Meng Tech Co., Ltd | ZH-ZFT | |
| Zadi-5 pill | Pharmaceutical Preparation Center of International Mongolian Hospital, Inner Mongolia, China | M1301006 |
References
- Jiang, N., et al. The antidepressant-like effects of Shen Yuan in a chronic unpredictable mild stress rat model. Frontiers in Psychiatry. 12, 622204(2021).
- Yang, L. F., et al. The effects of psychological stress on depression. Current Neuropharmacology. 13 (4), 494-504 (2015).
- Liu, Q. Q., et al. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. Journal of Psychiatric Research. 126, 134-140 (2020).
- Pinto, B., Conde, T., Domingues, I., Domingues, M. R. Adaptation of lipid profiling in depression disease and treatment: A critical review. International Journal of Molecular Sciences. 23 (4), 2032(2022).
- Rahman, S., et al. Increased serum resistin but not G-CSF levels are associated in the pathophysiology of major depressive disorder: Findings from a case-control study. PLoS One. 17 (2), 0264404(2022).
- Liu, C., et al. Danzhi Xiaoyao powder promotes neuronal regeneration by downregulating Notch signaling pathway in the treatment of generalized anxiety disorder. Frontiers in Pharmacology. 12, 772576(2021).
- Tanti, A., Belzung, C. Hippocampal neurogenesis: a biomarker for depression or antidepressant effects? Methodological considerations and perspectives for future research. Cell and Tissue Research. 354 (1), 203-219 (2013).
- Zhu, C., et al. Silencing of RGS2 enhances hippocampal neuron regeneration and rescues depression-like behavioral impairments through activation of cAMP pathway. Brain Research. 1746, 147018(2020).
- Toda, T., Parylak, S. L., Linker, S. B., Gage, F. H. The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry. 24 (1), 67-87 (2019).
- Bold, S. History and Development of Traditional Mongolian Medicine, third edition. , Sodpress Kompanid Khevlv. Ulaanbaatar, Mongolia. (2013).
- Medical, E. committee of Mongolian Encyclopedia. Mongolian Studies' Encyclopedia: Mongolian Medicine, third edition. , Hohhot, Mongolia. (2012).
- Fan, L., Wang, W. Clinical observation of Mongolian medicine Zadi-5 combined with Western medicine to treat depression after stroke. China Practice Medicine. 14 (2), 115-116 (2019).
- Hu, R. L. B. G., et al. Experimental research on nutmeg wuwei pills against of depression model rats behavior and hippocampus monoamine neurotransmitters. Chinese Journal of ETMF. 21 (11), 146-149 (2015).
- Hu, R. L. B. G., et al. Effects of Rou kou Wuwei Pill on the learning and memory abilities and the expression of BDNF and TrkB in hippocampus of depression rats. CJTCMP. 32 (8), 3797-3800 (2017).
- Yang,, et al. Morinda officinalis oligosaccharides mitigate depression-like behaviors in hypertension rats by regulating Mfn2-mediated mitophagy. J Neuroinflammation. 20 (1), 31(2023).
- Hu, R. L. B. G., et al. Effect of Zadi Wuwei pills on behaviors and learning memory in the depression model rats. World Journal of ITWM. 10 (10), 1367-1370 (2015).
- Ghasemi, M., Raza, M., Dehpour, A. R. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. Journal of Psychopharmacology. 24 (4), 585-594 (2010).
- Zhang, Y., et al. tea attenuates chronic unpredictable mild stress-induced depressive-like behavior in rats via the gut-brain axis. Nutrients. 14 (1), 99(2021).
- Kandola, A., Ashdown-Franks, G., Hendrikse, J., Sabiston, C. M., Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neuroscience and Biobehavioral Reviews. 107, 525-539 (2019).
- Sun, J., et al. Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. Journal of Agricultural and Food Chemistry. 66 (31), 8415-8421 (2018).
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 52 (2), 90-110 (2005).
- Albrakati, A., et al. Neuroprotective efficiency of prodigiosins conjugated with selenium nanoparticles in rats exposed to chronic unpredictable mild stress is mediated through antioxidative, anti-inflammatory, anti-apoptotic, and neuromodulatory activities. International Journal of Nanomedicine. 16, 8447-8464 (2021).
- Chan, K., et al. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. Journal of Ethnopharmacology. 140 (3), 469-475 (2012).
- Su, H., et al. Exploration of the mechanism of Lianhua Qingwen in treating influenza virus pneumonia and new coronavirus pneumonia with the concept of "different diseases with the same treatment" based on network pharmacology. Evidence Based Complementary Alternative Medicine. 2022, 5536266(2022).
- Zhou, P., et al. Network pharmacology and molecular docking analysis on pharmacological mechanisms of Astragalus membranaceus in the treatment of gastric ulcer. Evidence Based Complementary Alternative Medicine. 2022, 9007396(2022).
- Hou, Y., et al. Salidroside intensifies mitochondrial function of CoCl2-damaged HT22 cells by stimulating PI3K-AKT-MAPK signaling pathway. Phytomedicine. 109, 154568(2023).
- Aoyagi, T., Matsui, T. Phosphoinositide-3 kinase signaling in cardiac hypertrophy and heart failure. Current Pharmaceutical Design. 17 (18), 1818-1824 (2011).
- Zhu, H., et al. The neuroprotection of liraglutide against ischaemia-induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Scientific Reports. 6, 26859(2016).
- Radak, Z., Zhao, Z., Koltai, E., Ohno, H., Atalayet, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxidants & Redox Signaling. 18 (10), 1208-1246 (2013).
- Wang, X., et al. Salidroside, a phenyl ethanol glycoside from Rhodiola crenulata, orchestrates hypoxic mitochondrial dynamics homeostasis by stimulating Sirt1/p53/Drp1 signaling. Journal of Ethnopharmacology. 2022, 115278(2022).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved