A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Live Imaging of Arabidopsis Pollen Tube Reception and Double Fertilization Using the Semi-In Vitro Cum Septum Method
In This Article
Summary
Here, we describe an improvement of the semi-in vitro (SIV) method for observing pollen tube guidance and reception in Arabidopsis thaliana, which increases the receptivity of ovules. The high-throughput SIV cum septum method may be coupled with gametophyte marker lines and genetically encoded biosensors to monitor the dynamic process of fertilization.
Abstract
In flowering plants, the growth and guidance of the pollen tube (male gametophyte) within the pistil and the reception of the pollen tube by the female gametophyte are essential for double fertilization and subsequent seed development. The interactions between male and female gametophytes during pollen tube reception culminate in pollen tube rupture and the release of two sperm cells to effect double fertilization. As pollen tube growth and double fertilization are deeply hidden within the tissues of the flower, this process is difficult to observe in vivo.
A semi-in vitro (SIV) method for the live-cell imaging of fertilization in the model plant Arabidopsis thaliana has been developed and implemented in several investigations. These studies have helped to elucidate the fundamental features of how the fertilization process occurs in flowering plants and which cellular and molecular changes occur during the interaction of the male and female gametophytes. However, because these live cell imaging experiments involve the excision of individual ovules, they are limited to a low number of observations per imaging session, making this approach tedious and very time-consuming. Among other technical difficulties, a failure of the pollen tubes to fertilize the ovules in vitro is often reported, which severely confounds such analyses.
Here, a detailed video protocol for the imaging of pollen tube reception and fertilization in an automated and high-throughput manner is provided, allowing for up to 40 observations of pollen tube reception and rupture per imaging session. Coupled with the use of genetically encoded biosensors and marker lines, this method enables the generation of large sample sizes with a reduced time investment. Nuances and critical points of the technique, including flower staging, dissection, medium preparation, and imaging, are clearly detailed in video format to facilitate future research on the dynamics of pollen tube guidance, reception, and double fertilization.
Introduction
The generation of genetically unique offspring in sexually reproducing organisms is dependent on the successful fusion of male and female gametes. In flowering plants, the interaction of two male gametes (sperm cells) with two female gametes (egg cell and central cell) during double fertilization depends on sperm release from the pollen tube (the male gametophyte). This process, called pollen tube reception, is largely controlled by the synergid cells that reside within the embryo sac (the female gametophyte)1,2. As pollen tube reception takes place deep inside the flower, a method allowing for live-cell imaging of the process, called semi-in vitro (SIV) pollen tube reception, has been established3. With this method, excised Arabidopsis ovules are placed on semi-liquid pollen germination medium and targeted by pollen tubes that grow through the stigma and style of a pistil severed at the style-transmitting tract junction3,4. Since the development of this technique, detailed observations have led to several discoveries surrounding pollen tube guidance, reception, and fertilization. Among others, these discoveries include the acquisition of pollen tube targeting competence by growth through the stigma3, the onset of intracellular calcium oscillations in the synergids upon pollen tube arrival5,6,7,8,9, and the dynamics of sperm cell release and fertilization upon pollen tube burst10. Nevertheless, because this technique relies on the excision of ovules, the observations of fertilization are limited in number, and pollen tube reception is often aberrant, resulting in the failure of pollen tube rupture (Video 1 and Supplementary File 1). Therefore, there is a need for a more efficient approach allowing for high-throughput analyses of pollen tube reception and fertilization.
In developing this protocol, several new approaches to analyze pollen tube reception, spanning from the most "in vitro" to the most "in vivo" methods, were tested, and an efficient technique based on the excision of the entire septum was settled upon, which allows for up to 40 observations of fertilization per day. Here, the nuances and critical points of the technique are outlined, including flower staging, dissection, medium preparation, and imaging settings. By following this protocol, research focusing on pollen tube guidance, pollen tube reception, and double fertilization should be facilitated. The higher sample sizes the method allows for are expected to bolster the scientific soundness of the conclusions drawn from live imaging experiments. The potential applications of this technique include, but are not limited to, performing observations of the molecular and physiological changes in cytosolic calcium concentrations ([Ca2+]cyt), pH, or H2O2 during gametophyte interactions through the use of genetically encoded biosensors. Furthermore, cytological changes, such as degeneration of the receptive synergid, sperm cell migration, or karyogamy, can be more easily observed using this improved method. Finally, the timing of the different stages of fertilization can be monitored under widefield microscopy, and then more detailed analyses using confocal laser scanning microscopy (CLSM) or two-photon excitation microscopy (2PEM) can be conducted for higher resolution and 3D reconstruction.
Protocol
NOTE: See the Table of Materials for a list of the materials and equipment used in this protocol.
1. Considerations for designing the imaging experiment
- Predetermine the imaging duration and sampling frequency required to capture the desired phenomenon to be observed. For example, following Nyquist's sampling theorem, the sampling frequency must be greater than twice the highest frequency of the input sample. Therefore, to accurately capture synergid [Ca2+]cyt oscillations, perform imaging approximately every 10 s, but to measure the velocity of the sperm cells upon pollen tube discharge, ensure a temporal resolution of less than 1 s10.
- Select fluorescent markers for the cells of interest that are sufficiently bright beyond the background autofluorescence. For the use of biosensors, select the brightest lines that do not perturb the cell function, as they are generally more sensitive and require less exposure time.
- Consider which type of fluorescence microscopy and magnification are most suitable for capturing the desired phenomenon. Use widefield microscopy and low-magnification objectives to capture light from a greater depth of field, and maintain the focus over time, image larger objects such as whole ovules, or use ratiometric sensors that are sensitive to chromatic shift artifacts. Use CLSM or 2PEM with high-magnification objectives for better resolution of cellular and subcellular objects, but note the difficulty in maintaining focus over time.
- Estimate the number of samples to be imaged per experiment. For example, use a 10x objective with a minimum sampling time of ~30 s for imaging three septa and imaging up to 40 fertilization events per experiment because of the time required for the autofocus and multi-stage acquisition functions.
2. Pollen germination medium preparation
- Prepare liquid pollen germination medium (5mM CaCl2, 1mM MgSO4, 5mM KCl, 0.01% [w/v] H3BO3, and 10% [w/v] sucrose) using 1 M aliquot stocks of each micronutrient, which are stored at −20 °C. Adjust the pH to 7.5 with 0.1 M KOH. Store the medium at 4 °C for up to 2 weeks.
NOTE: The pH may drop over time. - Add 2.5 mL of liquid medium to a 10 mL beaker, and slowly add 32 mg of ultra-low melting point agarose (~1.3%) with a fast-rotating stir bar to avoid clumping.
- Melt agarose on a hotplate at 65 °C, and nutate the beaker to collect any condensation from the sides.
- Pipette 130 µL of medium into the 14 mm well of a 35 mm glass bottom dish, and rotate the dish until the medium covers the well.
- Aspirate a total of 40 µL with a pipette from the center of the dish, leaving behind a total volume of 90 µL.
NOTE: More medium can be aspirated out of the well to achieve a lower total volume; this may be necessary for high-magnification objectives with low working distances. However, the thinner the agar pad is, the quicker it will dry out. - Cool the dish on an aluminum block in a humidity chamber to ensure even cooling. Store the plates at 4 °C overnight for use the next morning.
3. Floral staging of septum donors, stigma donors, and pollen donors
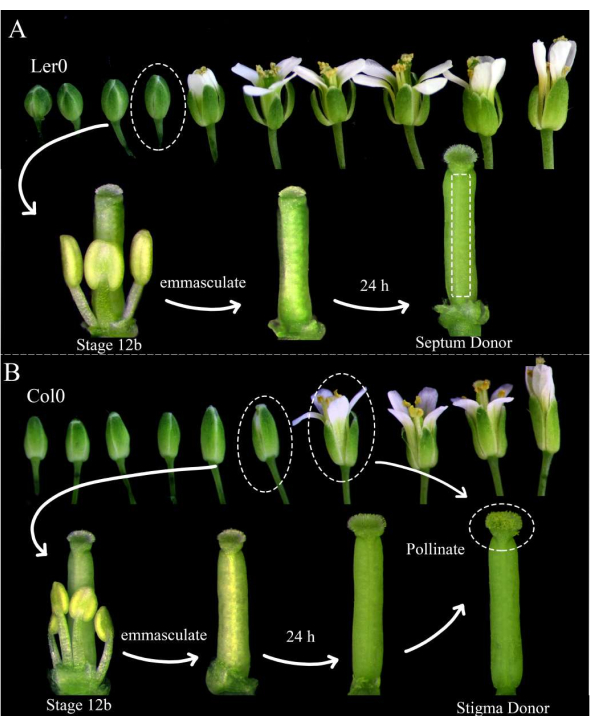
Figure 1: Floral staging of septum donors, stigma donors, and pollen donors. (A) Stages of Arabidopsis flowers (Ler-0) within an inflorescence. Buds at stage 12B, which have petals that are about to open and yellow indehiscent anthers, should be emasculated by removing all the sepals, petals, and stamens. Pistils (harboring the female gametophyte maker) are then usable as septum donors 24 h later. (B) Stages of Arabidopsis flowers (Col-0) within an inflorescence. Buds at stage 12B, which have yellow indehiscent anthers and stigmas that are just barely emerging from the petals, should be emasculated by removing all the sepals, petals, and stamens. The pistils are then usable as stigma donors 24 h later, when they should be pollinated by flowers (harboring the male gametophyte marker) that are open and shedding pollen. Pollinated stigmas should be dissected within 1 h of pollination. Please click here to view a larger version of this figure.
- The morning before imaging, choose Arabidopsis plants to be used as the septum donors and stigma donors; ensure that they are healthy and have recently begun to produce siliques.
NOTE: Landsberg erecta (Ler-0) is a good accession for use as a septum donor, since its septa are sturdy, and there are small internodes between the ovules. Columbia (Col-0) is a good accession to use as a stigma donor because they seem to be conducive to pollen tube growth. - Emasculate at least three stage 12B septum donor flowers and 12 stage 12B stigma donor flowers by removing their stamens, sepals, and petals (Figure 1A, B). Remove also older flowers on the same inflorescence, which could potentially pollinate the emasculated flower.
- In the morning, 24 h later, pollinate the stigma donors with the pollen donor flowers (e.g., harboring pollen tube or sperm cell markers) that are readily shedding pollen. Pollination is complete when the stigmas are almost completely covered with pollen (Figure 1B).
4. Septum dissection
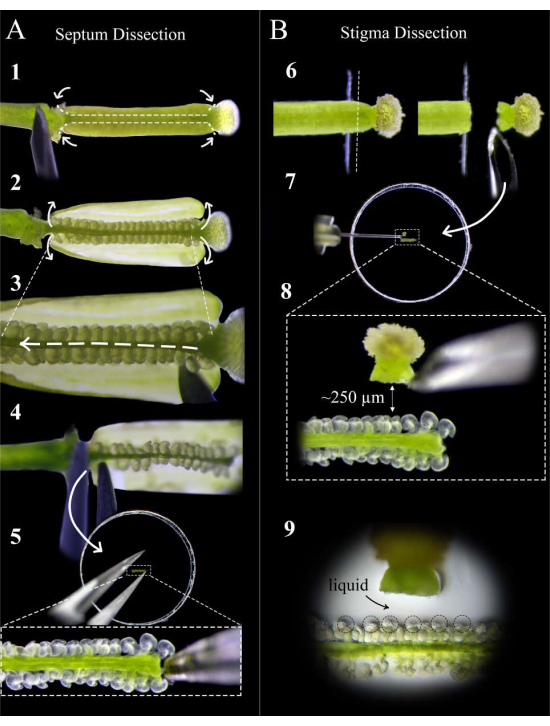
Figure 2: A quick guide to septum and stigma dissection. (A) Steps of septum dissection. Pin the pistil on double-sided tape with an insulin syringe needle, and make cuts at the style-ovary junction and the ovary-pedicle junction, followed by a shallow cut along the septum of either carpel (Step 1). Peel the carpel walls back onto the tape (Step 2). Cut the replum under the top septum (Step 3). Cut the septum at the style, and remove using forceps at the pedicel (Step 4). Place the septum on agar media, and embed gently with forceps. (B) Steps of stigma dissection. Pin the pistil (pollinated <1 h before) on double-sided tape, and cut at the style-ovary junction with a razor blade (Step 6). Place the stigma on agar medium with an insulin needle next to the septum, and adjust the distance to about 250 µm (Steps 7-8). Ensure that a semi-liquid pool forms around the micropyles and base of the styles (Step 9). Please click here to view a larger version of this figure.
- Thirty minutes after pollination, collect healthy and mature septum donor pistils at the pedicle, and place them onto fresh double-sided sticky tape mounted on a glass slide, with the stigma sticking just off the edge of the tape.
NOTE: Limit the dissection time for which the pistils are on the sticky tape to ensure it is as short as possible. The dissection should be conducted at high humidity, which can be achieved by placing a humidifier next to the slide. - Pin the pistil securely onto the tape by pressing gently on the pedicle and along the ovary using the back side of a new insulin syringe needle before removing the sepal, petal, and stamen debris left over from the emasculation.
- Under a stereoscope, use an insulin syringe needle to make two cuts per carpel at the style-ovary junction and the ovary-pedicle junction, followed by shallow cuts along the septum of each carpel (Figure 2, step 1).
NOTE: Cutting too deeply along the septum will sever ovules at the funiculus. - Peel back the ovary walls carefully onto the tape (Figure 2, step 2), and slide the needle gently between the two septa to cut the replum along the entire length of the pistil without disturbing the ovules (Figure 2, step 3).
NOTE: Cut the replum in the direction from the style to the pedicle to ensure that the ovules on the top septum are disturbed as little as possible. - Cut the top septum gently at both the junction with the style and with the pedicle, and remove the septum with forceps from the pedicle side (Figure 2, step 4).
NOTE: Keep this cut straight and gentle so that the septum stays flat and does not curve. - Place the septum as flat as possible on the medium such that the micropyles of the ovules are face up and in the same focal plane. Press the septum gently into the medium until the ovules are just slightly embedded in the medium (Figure 2, step 5).
NOTE: It is critical that the septum be placed such that the micropyles of the ovules are accessible to the pollen tubes (any inaccessible ovules can be gently rearranged with a needle). A small pool of liquid should form around the septum after embedding. - Repeat steps 4.1-4.6 for up to two more pistils, placing the septa end to end.
5. Stigma dissection
- Place the pollinated stigma donor pistils on fresh double-sided sticky tape mounted on a glass slide, such that the ovary-style junction is off the edge of the tape (Figure 2, step 6).
- Use a fresh razor blade to cut the style straight down and lift away to keep the stigma attached to the blade (Figure 2, step 6).
NOTE: Cut at the edge of the tape for the cleanest cut. - Remove the stigma from the blade with an insulin syringe needle, and place it flat approximately 250-300 µm from the ovules (Figure 2, steps 7-8).
NOTE: The style should be oriented horizontally on its side; otherwise, most of the pollen tubes will grow down under the septum. A small pool of liquid at the entry of the style should appear after it has been lying on the medium for 1 min; this is a good sign, as the pollen tubes will have a smooth exit from the style. - Repeat steps 5.1-5.3 for up to 12 stigmas, placing two on either side of each septum. Look for a small pool of liquid forming around the micropyle of the ovules; this aids the accessibility and targeting of the pollen tubes to the embryo sacs (Figure 2, step 9).
NOTE: Limit the amount of time the dish is open to prevent the medium and ovules from drying out.
6. Imaging
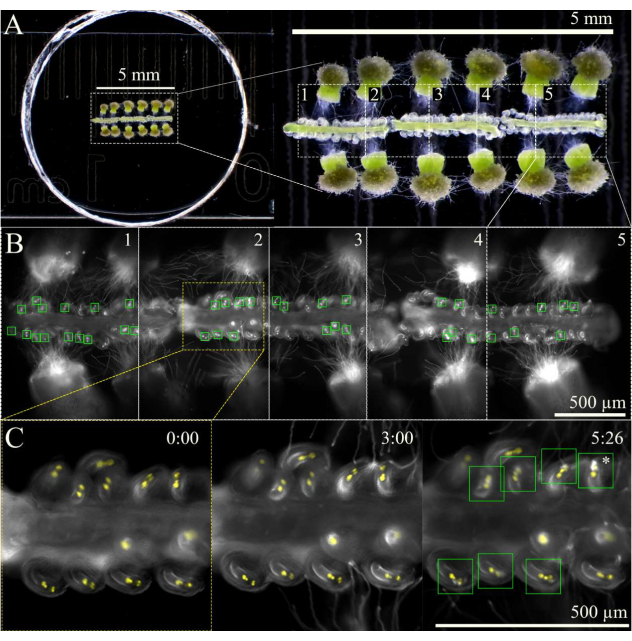
Figure 3: SIV cum septum imaging scheme. (A) Full SIV cum septum setup with 12 pollinated stigmas and 3 septa, as seen through a stereoscope. (B) Merged images of the full SIV cum septum setup seen in A 5 h after incubation using a 10x objective, with the five overview areas (~1 mm each) marked for multistage acquisition. The green boxes show the ovules that received pollen tubes undergoing an explosive burst in the synergids. (C) Closer view of overview area 2 at different time points. Approximately 3 h after incubating in the humidity chamber on the microscope, the pollen tubes should arrive near the micropyles, and by 6 h of imaging, most ovules should have properly received pollen tubes (green squares); these ovules then undergo explosive pollen tube burst (asterisk). Scale bars = (A) 5 mm, (B,C) 500 µm. Please click here to view a larger version of this figure.
- Once the dissection is done and the dish is ready to be imaged, let it incubate in a humidity chamber on the microscope maintained at 92% relative humidity and 21 °C.
NOTE: If no humidity chamber is available for the microscope, lining the outer edges of the lid and dish with a small volume of water will help to maintain a high level of humidity in the dish. - Bring the samples into focus under brightfield at a low light intensity, and then switch to fluorescent light, marking the areas of the sample on the stage overview to be imaged.
NOTE: At 10x magnification, about five areas can be marked for a total of three septa (Figure 3). Limit the amount of time used for marking the overview areas to minimize phototoxicity or the warming of the sample by the fluorescent light. - Begin imaging using a multi-stage acquisition scheme with an autofocus function at the beginning of each overview area. As pollen tubes will emerge from the style ~1 h after incubating the sample, delay the acquisition by 2 h or until the pollen tubes have reached the micropyle. Ensure the autofocus is set with a 100 µm range (7 µm large steps and 1 µm fine steps) to keep the ovules in focus as the septa slightly move and sink over time.
- Stop the image acquisition ~9 h after starting the sample incubation, as by this time, most of the ovules should have attracted and received a pollen tube.
NOTE: If a low number of ovules attract pollen tubes, careful observation of the dish under a stereoscope is useful to determine how the ovule or style orientation could be improved next time.
Results
To assess the timing of nuclear degeneration in the receptive synergid with respect to pollen tube rupture in Arabidopsis, as well as to observe whether the left or right synergid is predestined to become the receptive synergid, the SIV cum septum method described here was employed using a female gametophyte nuclear marker stacked with a synergid cytosolic marker (pFG:roGFP2-ORP1-NLS, pMYB98:roGFP2-ORP1) as the septum donor and a male gametophyte marker (pLAT52:R-GECO) as the poll...
Discussion
This manuscript introduces an efficient protocol for the imaging of pollen tube reception and double fertilization in Arabidopsis. The improved method, SIV cum septum, greatly increases the percentage and total number of successful pollen tube reception events that are observable per imaging session. The representative results shown here demonstrate an imaging session with 41 successful pollen tube reception events and 10 ovules showing reception defects (~80% efficiency). This is over double the n...
Disclosures
The authors report no conflicts of interest.
Acknowledgements
We thank Sara Simonini and Stefano Bencivenga for donating the pFG:roGFP2-ORP1-NLS construct and Christof Eichenberger, Johann Almendinger, Vincent Sutter, and Celia Baroux for their advice on microscopy. We kindly acknowledge advice from Ravi Palanivelu, Philipp Denninger, Sharon Kessler, Mark Johnson, Tomokazu Kawashima, and everyone else at the International Conference on Sexual Plant Reproduction 2022 who showed interest in a protocol on SIV cum septum. This work was supported by the University of Zurich and grants from the Swiss National Science Foundation to U.G.
Materials
| Name | Company | Catalog Number | Comments |
| 1 mm glass slide | Epredia | 16211551 | |
| 35 mm glass bottom dish (14 mm well) | Mattek | P35G-1.5-14-C | |
| Calcium Chloride | Roth | CN93.1 | |
| Columbia (Col-0) | Nottingham Arabidopsis Stock Centre (NASC) | stigma donor | |
| Dissecting Scope | Olympus | SZX2-ILLT | |
| Insulin needle (0.3 G) | BD | 304000 | |
| Landsberg erecta (Ler-0) | Nottingham Arabidopsis Stock Centre (NASC) | septum donor | |
| Magnesium Sulfate | Merck | 5886 | |
| Potassium Chloride | Roth | 6781.1 | |
| Razor blade | Beldura | 7026797 | |
| Scotch double sided tape | Scotch | 768720 | Less thick and good for stigma dissection |
| Sodium Chloride | Roth | 3957.1 | |
| Sucrose | ITW reagents | A2211,1000 | |
| Tesa double sided tape | Tesa | 05681-00018 | Very sticky and good for septum dissection |
| Ultra low gelling temperature agarose | FMC SeaPrep | 50302 |
References
- Huck, N., Moore, J. M., Federer, M., Grossniklaus, U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 130 (10), 2149-2159 (2003).
- Rotman, N., et al. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Current Biology. 13 (5), 432-436 (2003).
- Palanivelu, R., Preuss, D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biology. 6, 7 (2006).
- Susaki, D., Maruyama, D., Yelagandula, R., Berger, F., Kawashima, T. Live-cell imaging of F-actin dynamics during fertilization in Arabidopsis thaliana. Methods in Molecular Biology. 1669, 47-54 (2017).
- Iwano, M., et al. Cytoplasmic Ca2+ changes dynamically during the interaction of the pollen tube with synergid cells. Development. 139 (22), 4202-4209 (2012).
- Ngo, Q., Vogler, H., Lituiev, D., Nestorova, A., Grossniklaus, U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Developmental Cell. 29 (4), 491-500 (2014).
- Denninger, P., et al. Male-female communication triggers calcium signatures during fertilization in Arabidopsis. Nature Communications. 5, 4645 (2014).
- Hamamura, Y., et al. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nature Communications. 5, 4722 (2014).
- Ponvert, N., Johnson, M. Synergid calcium ion oscillations define a new feature of pollen tube reception critical for blocking interspecific hybridization. bioRxiv. , (2020).
- Hamamura, Y., et al. Live-cell imaging reveals the dynamics of two sperm cells during double fertilization in Arabidopsis thaliana. Current Biology. 21 (6), 497-502 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved