A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Enhancing Electrode Location Assessment in Cochlear Implantation via Computed Tomography Image Fusion
In This Article
Summary
This protocol demonstrates a method for fusing pre-operative and post-operative computed tomography images for cochlear implant recipients. The approach may enhance the measurement accuracy of insertion depth and the center frequency of electrode array contacts. Additionally, this method has potential applications in anatomy-based fitting, an emerging area within the cochlear implant field.
Abstract
This study aimed to determine whether fusing pre- and post-operative computed tomography (CT) images could help in assessing electrode placement and center frequency (CF) in cochlear implant (CI) recipients. A secondary objective was to compare automatic fusion with manual methods for measuring cochlear parameters. The study included twenty ears with CIs that underwent both pre- and post-operative CT scans. Manual measurements of cochlear parameters were initially taken from the post-operative CT images, followed by automatic detection using fused pre- and post-operative CT images with otological software (OTOPLAN). Angular insertion depth (AID) and CF of each electrode contact were calculated using both methods, and error differences were assessed. The analysis showed significant differences between the two methods for cochlear width (B-value) and cochlear duct length (CDL); however, these differences were not clinically significant. Furthermore, there was no statistically significant difference in cochlear diameter (A-value). The mean differences were 0.04 mm for the A-value, 0.21 mm for the B-value, and 0.73 mm for the CDL. The comparison of AID and CF revealed non-significant differences between manual and automatic fusion methods across all electrode contacts, except for electrode number five. According to this study, fusing pre- and post-operative CT images can be used to determine electrode positions in CI recipients. Automatic fused images can potentially measure cochlear parameters, AID, and CF with reduced human interference. Therefore, this method may serve as another basis for creating anatomy-based fitting.
Introduction
Cochlear implant (CI) surgery is a transformative treatment for individuals with severe-to-profound sensorineural hearing loss, offering substantial improvements in auditory function and quality of life1. A critical factor for the success of CI surgery is the accurate placement of the electrode array within the correct cochlear compartment, specifically the scala tympani, as optimal electrode positioning, is consistently linked to superior hearing outcomes2. Thus, careful pre-operative planning and assessment of cochlear anatomy are essential to ensure the electrode array is positioned appropriately. Ensuring that the electrode array is fully inserted within the scala tympani is crucial for maximizing patient benefits and achieving optimal clinical outcomes3.
Pre-operative estimation of the insertion depth angle of CI electrode arrays is considered important for effective surgical planning. This estimation relies significantly on obtaining accurate measurements of key cochlear parameters, such as cochlear duct length (CDL), from pre-operative computed tomography (CT) scans4. These measurements enable essential predictions regarding cochlear coverage and angular insertion depth during CI surgery when the electrode length is known. A major predictor of CDL is the A-value, defined as the distance between the round window and the farthest point on the basal turn. Prior studies have highlighted the crucial role of pre-operative imaging and planning in guiding surgical decisions and optimizing outcomes for CI recipients5.
Pre-operative CT imaging is standard practice in many CI clinics for evaluating inner ear anatomy and cochlear parameters before surgery. Pre-operative CT provides clear, artifact-free images that support effective planning and optimize surgical procedures6. However, pre-operative CT analysis alone has limitations in accurately predicting the actual angular insertion depth (AID) and center frequency (CF) of electrode contacts along the CI electrode array. Consequently, post-operative imaging remains necessary to confirm electrode array positioning, assess any displacement or translocation, and determine the true AID of each contact7.
Post-operative imaging confirms the accuracy of electrode placement and aids in creating individualized fitting maps tailored to each patient's unique cochlear anatomy. These fitting maps are essential for optimizing auditory performance by ensuring precise stimulation of the auditory nerve fibers. Recent studies have shown that individualized fitting maps, or anatomy-based fitting (ABF), improve speech comprehension in both quiet and noisy environments compared to standard or clinical fitting maps8,9,10,11,12,13. Additionally, recipients tend to prefer ABF maps when their electrode array achieves adequate stimulation of the cochlea's apical region8. The frequency-to-place mismatch is the discrepancy between the CF of electrode contacts based on their physical location within the cochlea and the default settings4. Mertens et al. reported that the impact of frequency-to-place mismatch decreases with prolonged device use14. Other studies have demonstrated that reducing frequency-to-place mismatch with ABF in CI recipients enhances speech perception in noisy environments without affecting understanding in quiet settings11,13.
Various imaging modalities, including X-ray15,16, cone-beam CT17, and magnetic resonance imaging (MRI)18,19, have been used to evaluate CI recipients. However, CT imaging remains the preferred method due to its high spatial resolution and ability to capture detailed cochlear anatomy. CT imaging allows for precise assessment of inner ear structures, such as the scala tympani and scala vestibuli, facilitating accurate electrode placement.
Despite the many benefits of CT imaging, certain challenges persist, particularly with low-resolution post-operative scans. Metallic electrode contacts can produce image artifacts that obscure adjacent structures, complicating the accurate measurement of critical parameters like AID and CF. Recent studies have shown that fusing pre- and post-operative CT scans enhances image clarity over standard scans, enabling more accurate assessments and improving electrode location by providing complementary information from both pre- and post-operative images20.
Currently, post-operative CT image analysis often involves manual measurements using software tools, which require substantial training, are time-intensive, and may be prone to variability and error, limiting their efficiency and broader applicability. There is limited literature on the potential of automated measurements from fused images to address these limitations and provide reliable measurements of AID for electrode contacts. This research aims to evaluate whether the fusion of pre- and post-operative CT images can effectively assess cochlear characteristics and electrode placement in CI recipients. It further investigates how image fusion could advance anatomy-based fitting (ABF) and enhance measurement accuracy for both angular insertion depth (AID) and center frequency (CF).
Protocol
This study received approval from the principal Institutional Review Board (E-21-5737) and was conducted following the ethical principles outlined in the Declaration of Helsinki. Data were retrospectively collected from medical records at our tertiary CI center. Due to the anonymized nature of data analysis, informed consent was not required. The study included 20 ears from patients across various age groups.
Inclusion criteria were as follows: patients with normal inner ear anatomy who received CI between 2020 and 2023, underwent both pre- and post-operative CT scans, and were implanted with CI devices from the same manufacturer, compatible with the surgical planning software used. Temporal bone CT scans are routinely performed as part of the pre-operative assessment for cochlear implant patients at our center, while post-operative CT scans are only done when medically necessary to minimize radiation exposure. Exclusion criteria included CI patients who had undergone revision surgeries or had cochlear ossification, cochlear otosclerosis, or temporal bone fractures involving the otic capsule. Details of the equipment and software are provided in the Table of Materials.
1. Uploading and measuring cochlear parameters
NOTE: The measurement protocol employed in this study comprised a series of sequential steps designed to assess the effectiveness of the pre-and post-operative CT image fusion technique in evaluating electrode location in CI recipients (Figure 1).
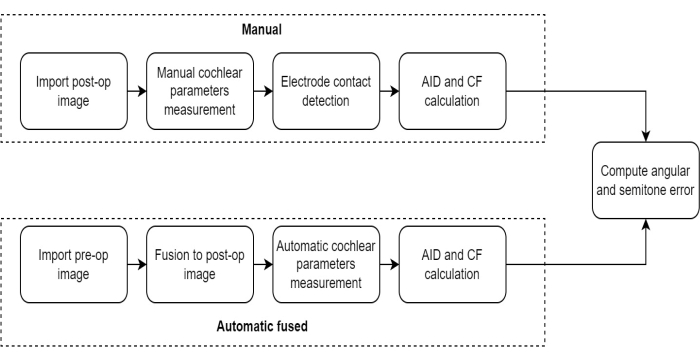
Figure 1: Measurement protocol comparison. This figure illustrates the protocol for measuring cochlear parameters using the Automatic Fusion method compared to the Manual method. Please click here to view a larger version of this figure.
- Press the Import button in the software's Data Management module to upload the post-operative and pre-operative CT images, and select the DICOM files.
- Manually define the center of modiolus, round window, and cochlear metrics, including the A-value (cochlear diameter), B-value (cochlear width), and H-value (cochlear height) in the 3D Ear-Cochlea module4 (Figure 2).
- Calculate the Cochlear Duct Length (CDL) using the A and B values, applying the Alexiades method to the Elliptic-Circular Approximation (ECA)2,4.
NOTE: The detailed steps of the post-operative analysis are described by Canfarotta et al.21. These measurements provide anatomical landmarks for CDL estimation.
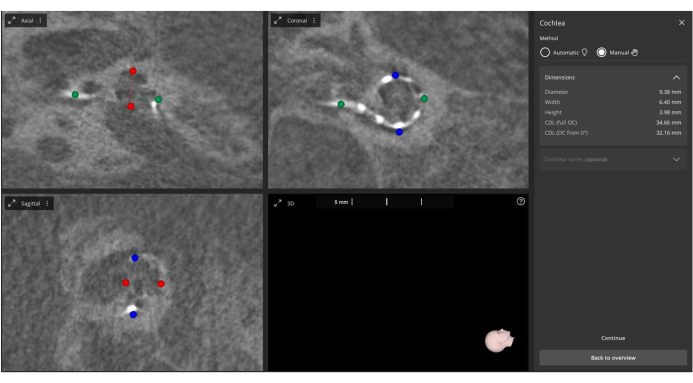
Figure 2: Manual measurement of post-operative A, B, and H values. Post-operative images are imported into the software, and the A-value (marked in green), B-value (marked in blue), and H-value (marked in red) are manually measured. Please click here to view a larger version of this figure.
2. Electrode contact identification and AID/CF calculation
- Open the Implant Assessment module. The software will automatically identify the twelve electrode contact points on the post-operative CT images.
- Manually adjust the detected contact points, if necessary, before confirming their positions.
- Allow the software to use these points, along with the pre-defined cochlear metrics, to automatically calculate the Angular Insertion Depth (based on Escude et al.22) and the Characteristic Frequency (based on Greenwood et al.23) for each contact (see Figure 3).
NOTE: This manual approach is considered the standard method for measuring AID and CF, and it is referred to as "Manual" in this study.
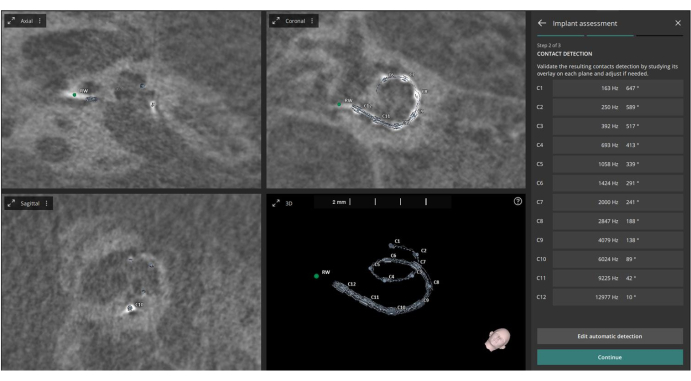
Figure 3: Identification of electrode contacts. This figure shows the identification of the twelve electrode contacts from the post-operative CT images. Please click here to view a larger version of this figure.
3. Image fusion
- Press the Add icon in the user interface to import the pre-operative CT images into the Image Fusion panel.
- Allow the software to automatically begin registering the pre-operative CT images with the pre-uploaded post-operative CT images using the automatic fusion feature, which applies a mutual information algorithm.
- Access the Manual Alignment feature in the pre-operative image menu if manual adjustments to the fusion are needed. This fusion process enhances visualization and allows a more comprehensive assessment of cochlear anatomy and electrode placement with fewer artifacts. The fusion aims to improve visualization for better evaluation (see Figure 4).
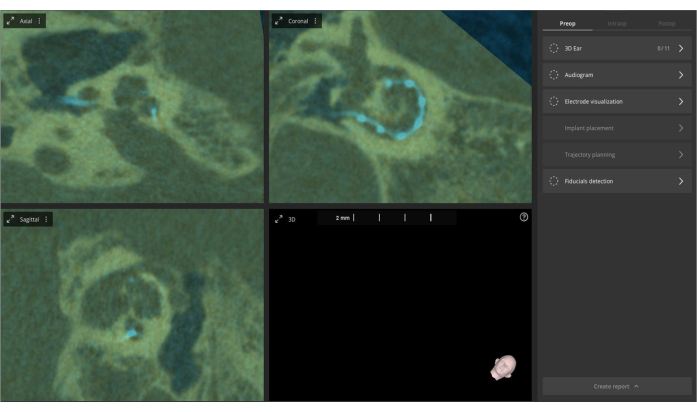
Figure 4: Fusion of pre- and post-operative CT images. Pre-operative CT images are imported into the software and fused with post-operative CT images. The overlay is displayed in olive color, with the electrodes highlighted in bright blue. Please click here to view a larger version of this figure.
4. Automatic cochlear parameter measurement on pre-operative image
- Re-measure the cochlear parameters on the newly imported pre-operative image within the 3D - Cochlea module.
- Select the Automatic option in the module menu to execute automatic cochlear parameter measurements. This feature is available only for pre-operative images without implant electrodes, as no metal artifacts exist.
- Use the automatic algorithm to reconstruct the inner ear anatomy in 3D. The 3D model will serve as the base for the automatic cochlear parameter measurements as described by Abari et al.24.
NOTE: The 3D reconstruction aids in precise measurement of cochlear parameters (see Figure 5).
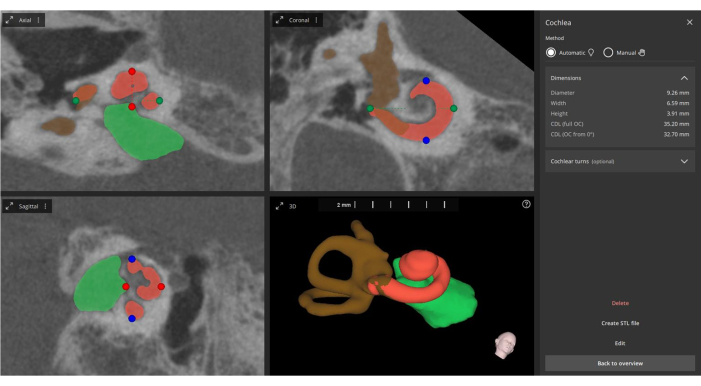
Figure 5: Automated measurement of cochlear parameters. From the fused images, the software automatically measures the main cochlear parameters (indicated by green, blue, and red points), performs 3D reconstruction (cochlea in orange, internal auditory canal in green, and semicircular canals in brown), and updates the information regarding the electrode contacts. Please click here to view a larger version of this figure.
5. Updating electrode contact points with new cochlear parameters
- Confirm the new Cochlear Duct Length (CDL) measurements obtained from the pre-operative images.
- Allow the previously identified electrode contact points from the post-operative images to automatically update with the new cochlear parameter information, defining the "Automatic Fusion" approach.
- Perform calculations based on the fused images (pre-operative and post-operative) for Angular Insertion Depth (AID) and Characteristic Frequency (CF), which are important for confirming electrode array placement, cochlear coverage, and building the Anatomy-Based Fitting (ABF) map (see Figure 5).
NOTE: AID and CF are critical for confirming electrode positioning and cochlear coverage in cochlear implant (CI) practice (see Figure 6).
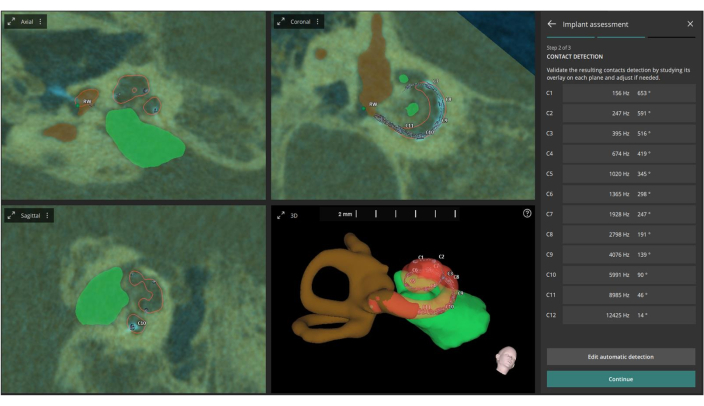
Figure 6: Automatic definition of AID and CF. This figure depicts the automatic definition of the AID (Acoustic Impedance Distribution) and CF (Center Frequency) for each individual electrode contact based on the fused images. Please click here to view a larger version of this figure.
6. Calculating the mean angular error and frequency error
- Calculate the mean angular error between the Manual and Automatic Fusion methods for each electrode contact.
- Calculate the frequency error in semitones between both methods (Manual and Automatic Fusion) for each electrode contact.
- Use these calculations to provide quantitative indicators of the difference and accuracy between the two methods. Note that semitones are the smallest musical intervals commonly used in Western music25.
7. Statistical analysis
- Feed the manually calculated cochlear parameters, Angular Insertion Depth (AID), and Characteristic Frequency (CF) from post-operative CT imaging, along with the automatically fused calculated data from the pre-and post-operative CT image fusion technique for each electrode contact, into statistical analysis software (R software).
- Use means and standard deviations for descriptive statistics of quantitative variables, while applying counts and percentages for categorical variables.
- Conduct a comparative analysis between the manually measured AID and frequencies and the automatically fused measurements using a pairwise Bonferroni-adjusted paired t-test or Wilcoxon signed rank test, depending on the normality assumption tested using the Shapiro-Wilk test.
NOTE: Assume that all comparisons have clinically relevant standardized effect sizes (Cohen's d)26. Consider a statistically significant P value to be less than 0.05.
Results
This study included 20 ears from CI recipients, with 11 implants on the left side and nine on the right side. The average age of the participants was 13.3 years, comprising 55% female and 45% male. Table 1 presents a comparative analysis of manual versus automatic fusion methods for measuring cochlear parameters, revealing statistically significant differences in certain measurements. Specifically, manual measurements showed a significantly higher B value and cochlear duct length (CDL) compared to automa...
Discussion
The findings of the present study revealed comparable measurements between Manual and Automatic Fusion methods for most cochlear parameters and frequencies. While Manual measurements exhibited marginally higher values for certain parameters, such as the B value and CDL, no clinically significant differences were observed overall. This discrepancy could be attributed to artifacts in the post-operative CT, which may affect measurement accuracy. Furthermore, this study builds upon prior research by specifically evaluating A...
Disclosures
Yassin Abdelsamad and Tahir Sharif work for MED-EL with scientific roles only. The authors have no financial interests in the products described in this manuscript and have nothing else to disclose.
Acknowledgements
The authors would like to thank Dr. Nikola Ivanovic for reviewing the protocol and Rahma Sweedy for doing the statistical analysis.
Materials
| Name | Company | Catalog Number | Comments |
| CI devices | MED-EL, Innsbruck, Austria | ||
| OTOPLAN software | CASCINATION, MED-EL, Innsbruck, Austria | version 4 (3.0.0) | Otology Planning Software |
| R software | R Foundation for Statistical Computing, Vienna, Austria | version 4.2.2 | Language and Environment for Statistical Computing |
References
- Dazert, S., Thomas, J. P., Loth, A., Zahnert, T., Stover, T. Cochlear Implantation. Dtsch Arztebl Int. 117 (41), 690-700 (2020).
- Jwair, S., Versnel, H., Stokroos, R. J., Thomeer, H. The effect of the surgical approach and cochlear implant electrode on the structural integrity of the cochlea in human temporal bones. Sci Rep. 12 (1), 17068 (2022).
- O'Connell, B. P., Hunter, J. B., Wanna, G. B. The importance of electrode location in cochlear implantation. Laryngoscope Investig Otolaryngol. 1 (6), 169-174 (2016).
- Aljazeeri, I., et al. Anatomy-based frequency allocation in cochlear implantation: The importance of cochlear coverage. Laryngoscope. 132 (11), 2224-2231 (2021).
- Oh, J., et al. Cochlear duct length and cochlear distance on pre-operative CT: imaging markers for estimating insertion depth angle of cochlear implant electrode. Eur Radiol. 31 (3), 1260-1267 (2021).
- Yoshimura, H., Watanabe, K., Nishio, S. Y., Takumi, Y., Usami, S. I. Determining optimal cochlear implant electrode array with OTOPLAN. Acta Otolaryngol. 143 (9), 748-752 (2023).
- Farnsworth, P. J., et al. Improved cochlear implant electrode localization using coregistration of pre-and post-operative CT. J Neuroimaging. 33 (3), 387-392 (2023).
- Kurz, A., Herrmann, D., Hagen, R., Rak, K. Using anatomy-based fitting to reduce frequency-to-place mismatch in experienced bilateral cochlear implant users: A promising concept. J Pers Med. 13 (7), 1109 (2023).
- Dillon, M. T., et al. Influence of the frequency-to-place function on recognition with place-based cochlear implant maps. The Laryngoscope. 133 (12), 3540-3547 (2023).
- Dillon, M. T., O'Connell, B. P., Canfarotta, M. W., Buss, E., Hopfinger, J. Effect of place-based versus default mapping procedures on masked speech recognition: Simulations of cochlear implant alone and electric-acoustic stimulation. Am J Audiology. 31 (2), 322-337 (2022).
- Creff, G., et al. Comparison of tonotopic and default frequency fitting for speech understanding in noise in new cochlear implantees: A prospective, randomized, double-blind, cross-over study. Ear Hearing. 45 (1), 35-52 (2024).
- Lassaletta, L., Calvino, M., Sanchez-Cuadrado, I., Gavilán, J. Does it make any sense to fit cochlear implants according to the anatomy-based fitting? Our experience with the first series of patients. Front Audiol Otol. 1, (2023).
- Kurz, A., Müller-Graff, F. -. T., Hagen, R., Rak, K. One click is not enough: Anatomy-based fitting in experienced cochlear implant users. Otol Neurotol. 43 (10), 1176-1180 (2022).
- Mertens, G., Van de Heyning, P., Vanderveken, O., Topsakal, V., Van Rompaey, V. The smaller the frequency-to-place mismatch the better the hearing outcomes in cochlear implant recipients. Eur Arch Otorhinolaryngol. 279 (4), 1875-1883 (2022).
- Liu, G. S., Cooperman, S. P., Neves, C. A., Blevins, N. H. Estimation of cochlear implant insertion depth using 2D-3D registration of post-operative X-ray and pre-operative CT images. Otol Neurotol. 45 (3), e156-e161 (2024).
- Alahmadi, A., et al. Advancing cochlear implant programming: X-ray guided anatomy-based fitting. Otol Neurotol. 45 (2), 107-113 (2024).
- Helal, R. A., et al. Cone-beam CT versus Multidetector CT in post-operative cochlear implant imaging: Evaluation of image quality and radiation dose. AJNR Am J Neuroradiol. 42 (2), 362-367 (2021).
- George-Jones, N. A., Tolisano, A. M., Kutz, J. W., Isaacson, B., Hunter, J. B. Comparing cochlear duct lengths between CT and MR images using an otological surgical planning software. Otol Neurotol. 41 (9), e1118-e1121 (2020).
- Swarup, A., et al. Comparing accuracy of cochlear measurements on magnetic resonance imaging and computed tomography: A step towards radiation-free cochlear implantation. J Otol. 18 (4), 208-213 (2023).
- Benson, J. C., Nassiri, A. M., Saoji, A. A., Carlson, M. L., Lane, J. I. Co-registration of pre- and post-operative images after cochlear implantation: A proposed technique to improve cochlear visualization and localization of cochlear electrodes. Neuroradiol J. 36 (2), 194-197 (2023).
- Canfarotta, M. W., Dillon, M. T., Buss, E., Pillsbury, H. C., Brown, K. D., O'Connell, B. P. Validating a new tablet-based tool in the determination of cochlear implant angular insertion depth. Otol Neurotol. 40 (8), 1006-1010 (2019).
- Escudé, B., et al. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurotol. 11 (SUPPL. 1), 27-33 (2006).
- Greenwood, D. D. A cochlear frequency-position function for several species-29 years later. J Acoust Soc Am. 87 (6), 2592-2605 (1990).
- Abari, J., Heuninck, E., Topsakal, V. Entirely robotic cochlear implant surgery. Am J Otolaryngol. 45 (5), 104360 (2024).
- Bell, A., Jedrzejczak, W. W. The 1.06 frequency ratio in the cochlea: Evidence and outlook for a natural musical semitone. Peer J. 5, e4192 (2017).
- Cohen, J. . Statistical Power Analysis for the Behavioral Sciences. , (2013).
- Mertens, G., Van Rompaey, V., Van de Heyning, P., Gorris, E., Topsakal, V. Prediction of the cochlear implant electrode insertion depth: Clinical applicability of two analytical cochlear models. Sci Rep. 10 (1), 3340 (2020).
- Paouris, D., Kunzo, S., Goljerova, I. Validation of automatic cochlear measurements using OTOPLAN(R) software. J Pers Med. 13 (5), 805 (2023).
- IbraheemAl-Dhamari Rania Helal, T. A. S. W., Paulus, D. Automatic cochlear multimodal 3D image segmentation and analysis using atlas-model-based method. Cochlear Implants Int. 25 (1), 46-58 (2024).
- Rivas, A., et al. Automatic cochlear duct length estimation for selection of cochlear implant electrode arrays. Otol Neurotol. 38 (3), 339-346 (2017).
- Breitsprecher, T., et al. CT imaging-based approaches to cochlear duct length estimation human temporal bone study. Eur Radiol. 32 (2), 1014-1023 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved