1. Information needed when pairing animals includes strain/stock of the animal utilizing proper nomenclature, dates of birth for the breeder male and female, and the setup date. Accurate recordkeeping is imperative with breeding colonies.
2. Sex determination of mice and rats is done by comparing the anogenital distances. In females, the distance between the anus and the external genitalia is shorter than it is for males. The presence of a scrotal sac in male animals is another sex indicator.
3. Selecting and setting up the mating scheme
NOTE: There are two mating schemes that can be used.
- Proestrus/estrus timed mating: This method relies on paring females with males at the point of maximum receptivity and fertility.
- The estrous cycle must be monitored in the females either by visual examination of the external genitalia for changes that are indicative of proestrus and estrous, or by cytology of vaginal secretions (see below).
- When a female is determined to be in proestrus or estrus, she is paired with a male at the end of the day, as the animals generally mate at night.
- The following morning, the female is examined for a copulatory plug (see below). If no plug is present, the female can remain with the male during the day and checked for a copulatory plug at the end of the day. Alternatively, if it is determined that she is no longer in proestrus or estrus, she is removed from the breeding cage.
- Random timed mating: This method is based on the fact the estrous cycle of rodents is very short, 4-5 days long.
- For this method, matings can be set up anytime, and the females are then checked for copulatory plugs every morning and evening until a plug is observed.
- A female is paired with a male in the evening.
- She is checked for a copulatory plug at the beginning and end of each day until a plug is observed. It commonly takes 3 or more days for a plug to be seen when using this method.
4. Predicting pregnancy
Since palpation of pups is difficult until later during pregnancy, around day 10-12, commercial ultrasound systems for rodents have been developed; however, few animal research facilities have this technology. Therefore, visualization of copulatory plugs, observation of vaginal changes, or vaginal cytology are commonly used to assist with the prediction of when a female has conceived a litter (see below). However, none of these methods are able to confirm pregnancy. Once a copulatory plug is observed, the female should be monitored for signs of pregnancy, such as weight gain.
5. Determining the estrous cycle stage
- Visual Inspection
NOTE: For timed mating of mice and rats, visual observation of the vagina for changes that are indicative of proestrus and estrus is the quickest method to determine the estrous cycle stage, and it requires no special equipment.
- When evaluating the estrous cycle using the visual method, it is important to perform the visual inspection in the same area with respect to room lighting, as the light source can change the perceived color of vaginal tissues and make evaluation difficult. For example, the purple hue cast by LED lights makes visual detection more difficult.
- To evaluate the stage of the estrous cycle by visual observation, each mouse must be manually restrained by the tail, with the forepaws resting on a cage lid.
- The vaginal opening of each female is evaluated based on the condition of the tissue surrounding the vaginal area and the size of the vaginal opening.2
- During proestrus, the vaginal opening is wide and is characterized by swelling of the surrounding tissue. The tissue is pink in color and very moist. Often there are wrinkles or striations along the dorsal and ventral edges of the opening.
- During estrus, the swelling of the tissues surrounding the vaginal opening is reduced, and the tissues are not as moist and pink.
- During metestrus the vaginal opening is minimal and there is negligible swelling.
- During diestrus, there is no swelling of the tissues around the vaginal area, and the vaginal opening is small and closed.
- Vaginal cytology
As both mice and rats are polyestrous, the estrus cycle length is very short, ranging from 4-5 days. It is sometimes necessary to identify all four stages of estrous: proestrus, estrus, metestrus, and diestrus. Vaginal cytology is a very accurate method to determine these stages. There are also two methods of sample collection: the noninvasive method of vaginal lavage, and the invasive method of vaginal canal swabbing.
- Vaginal lavage
- Materials needed are sterile 200 µl pipettes tips, latex bulbs, sterile double distilled water (ddH20), and clean glass slides.
- Place a latex bulb on the end of a sterile 200 µl tip. Draw up approximately 100 µl of sterile ddH2O into the pipette.
- Lift the mouse out of the cage, and place her on the wire bar cage top with her tail towards you.
- Firmly grasp the tail and elevate the hindquarters of the mouse. The mouse will now have only the front paws grasping the lid.
- If the mouse urinates, wait until urination stops. Should there be urine left at the entrance to the vaginal canal, the opening can be rinsed with a small squirt of ddH2O. Change the tip that was used for rinsing.
- Place the end of the ddH2O-filled tip at the opening of the vaginal canal without penetrating the orifice.
- Gently depress the bulb to expel a quarter to half of the volume of water (~25-50 µl) at the opening of vaginal canal. The liquid will spontaneously aspirate into the canal without tip insertion. Slowly release the pressure exerted on the bulb. The fluid will withdraw back into the tip.
- Avoid releasing pressure too quickly to prevent aspiration of fluid into the bulb. A filtered tip may be useful for this purpose.
- Repeat the previous step 4-5 times using the same tip, bulb, and fluid to obtain a sufficient number of cells in a single sample.
- Place the fluid on glass slide, and allow the smear to completely dry at room temperature.
- Use a new pipette for each mouse.
- Once dry, these estrous smears can be stained immediately or stored and stained at a later date. Wright-Giemsa staining is most commonly used to stain the slides. This stain is available commercially as a one-step stain that does not require fixation of the slide to prevent cells from washing off during the staining process. The slide is placed in the stain for 45-60 seconds, as per the manufacturer's instructions.
- The slides are then examined under a microscope, and the cells seen correspond to the stage of the cycle: 1) if the female is in proestrus, the cells are seen as clusters of round, well-formed nucleated epithelial cells with a nucleus that stains darker than the cytoplasm; 2) if the female is in estrus, the majority of the cells are cornified squamous epithelial cells that lack a nucleus, are angular in appearance, and occur in densely packed clusters; 3) if the female is in metestrus, the cells are typically white blood cells (specifically neutrophils with some cornified squamous epithelial cells present) with darkly-stained nuclei that are shaped like two sausages linked together; 4) during diestrus, the cells present are normally white blood cells with the occurrence of a few nucleated epithelial cells..
- Vaginal swabbing
- Materials needed are sterile cotton-tipped applicators with a 2 mm diameter tip, clean glass microscope slides, and sterile physiological saline.
- Wet a cotton-tipped applicator with saline.
- Insert the tip of the applicator into the vagina of the restrained mouse.
- Gently turn and roll the tip against the vaginal wall. Carefully remove the swab.
- Cells are transferred to a dry glass slide by rolling the swab across the slide.
- Once the slide is dry, it can be stained with Wright-Giemsa stain.
Swabbing is considered a stressful procedure, and-when stressed-mice can have disrupted estrous cycles. Vaginal and cervical stimulation caused by swabbing can induce pseudopregnancy. Repeated swabs of the vaginal mucosa can cause damage if not performed gently, with proper restraint, and with the correct-sized cotton swabs.3,4
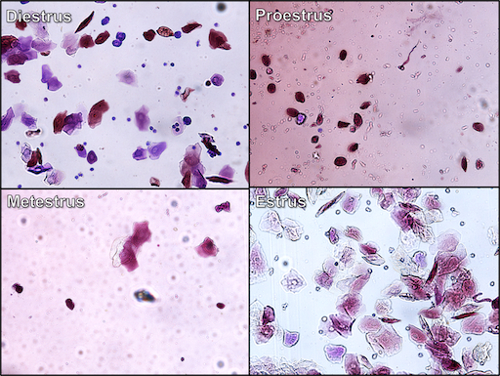
Figure 2. Vaginal cytology -- different stages of rodent estrus cycle
6. Visualizing a copulatory plug
This plug consists of vaginal fluid and semen, and persists in the vagina for 12-24 h postcopulation. The presence of the plug confirms mating, but does not guarantee that the female is pregnant. If the plugged female is pregnant, the first day of gestation is considered to be the day after the plug is found.
- Lift the mouse out of the cage and place her on the wire bar cage top with her tail towards you.
- Position the mouse by applying pressure just above the tail to arch the back to allow better presentation of the vaginal opening.
- Observe her vaginal opening for a whitish mass. The copulatory plug may not be visually obvious but can be confirmed with the use of a blunt probe.
- Using the tip of the probe, gently insert it into the vaginal opening. The presence of a copulatory plug will impede the advancement of the probe within 0.5 cm of the vaginal opening.
- The probe must be disinfected with alcohol and dried completely before each use.
As the female has litters, the date of birth, the litter size, the number born, the number weaned, the ratio of male:female pups, and the ratio of the genotypes should all be recorded. If the genotypes within a litter do not correspond to the genotypes of the parents, retesting must be done to verify the true genotype.
7. Weaning
Gestation for mice and rats is approximately 21 days. The young are weaned at 21-28 days of age. Both mice and rats can breed as early as 8 weeks of age, thus it is imperative that the pups are separated by gender at an early age. Intensive breeding requires that the pups of each litter be weaned at day 20 to prevent the older pups from being present when the next litter is born. For nonintensive breeding, the pups can be left with the mother past 20 days of age, often up to 28 days of age. This can be very beneficial for many genetically modified strains, as the pups may not be as vigorous as nonengineered or wildtype animals.
Male and female pups are separated at weaning. Whenever possible, newly weaned pups should not be housed singly. If a litter contains only one pup of a given sex, attempts should be made to house this pup with others of the same gender. Possible housing options are: 1) a single female pup may remain with the mother if not in an intensive breeding cage; 2) a single female or male pup may be placed with other same-gender pups from a different litter of the same age; 3) if the parents are a monogamous pair, the female can be removed from the cage to allow a single male pup to be housed with the father; and 4) a single male pup may be housed with female siblings up to 5 weeks of age. The gender of pups should be verified one week postweaning to prevent unwanted litters from improperly segregated pups.
Weanling mice and rats should be checked daily to assure that they are thriving. Although the Guide for the Care and Use of Laboratory Animals5 states that food must be presented to the animals in such a way to prevent it from being soiled by feces and urine, newly weaned mice should be provided a small amount of food (one pellet per mouse) placed in a glass dish (petri dish) on the cage floor. This will encourage the animals to transition to having rodent chow as their sole food source. Even for animals that are housed on racks that provide water to the cages through an automatic watering system, a water bottle can be added to the cage if the mice appear to be dehydrated.
| Name |
Colony Type |
Description |
| ICR |
Outbred |
Albino |
| Swiss-Webster |
Outbred |
Albino |
| Balb/c |
Inbred |
Albino |
| FVB |
Inbred |
Albino |
| C57BL/6 |
Inbred |
Black coat color |
| C3H |
Inbred |
Brown coat color |
| DBA/2 |
Inbred |
Brown/grey coat color |
| Athymic nudes (nu/nu) |
Inbred |
Hairless |
| SCID |
Inbred |
Severe combined immune-deficient mice-various coat colors |
Table 1. Commonly used mouse stains and stocks.
| Name |
Colony Type |
Description |
| Sprague-Dawley |
Outbred |
Albino |
| Wistar |
Outbred |
Albino |
| Fisher 344 |
Inbred |
Albino |
| Lewis |
Inbred |
Albino |
| Long Evans |
Inbred |
Hooded, black and white |
Table 2. Commonly used rat strains and stocks.















