核磁共振 (NMR)
Overview
资料来源: 实验室的博士 Henrik Sundén — — Chalmers 技术大学
核磁共振 (NMR) 是有机化学家重要的分析技术。核磁共振的帮助下,已极大地便利在有机实验室工作。它不仅可以提供有关分子的结构的信息也确定含量和纯度的一个样本。相比其他常见技术为有机化学家 — — 热分析和质谱 (MS) 等 — — 核磁共振是一种非破坏性的方法,是有价值的当样品的恢复非常重要。
有机化学的最常用核磁共振技术之一是质子 (1H) 核磁共振。目前在分子中质子将根据其周边的化学环境,使它能够阐明其结构的行为不同。此外,也可以通过比较 NMR 谱的起始原料到最终产品监测的一种反应完成。
充分体现了这个视频,如何在日常工作中有机化学家用于核磁共振波谱法。将显示以下: 我) 核磁共振样品的制备。ii) 使用1H NMR 监视反应。iii) 确定从1H NMR 反应获得的产品。将显示的反应是合成的E-查尔 (3) 从 (1) 醛和酮 (2) (方案 1)。1
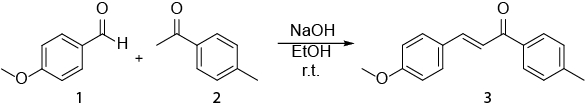
方案 1。合成 (2E)-3-(4-methoxyphenyl)-1-(4-methylphenyl)-2-propen-1-one.
Procedure
1.编制的核磁共振起始原料
- 添加开始到一个干净的核磁共振管材料的 ~ 10 毫克。
- 溶解在 ~0.7 毫升氘化溶剂 (扑热息痛3给出的例子) 的起始原料。一个好的光谱的溶剂适合身高 4.5-5 厘米。
- 仔细帽核磁共振管和在帽上写的示例名称。
- 摇动样品轻轻地确保所有材料都溶化了。小心避免溶剂和帽,这可能导致可能污染的样品之间的接触。
- 核磁共振管小心地插入微调框。微调按钮将旋转一次插入,确保整个样本经验产生匀强磁场的磁铁。以去除指纹和污垢的清洁核磁共振管和微调框 2-丙醇和实验室组织的外。
- 旋流器的采样深度的地方评估以确保如,可能破坏光谱仪,核磁共振管底不插入太远到核磁共振探头。不同探头有不同样品深度和用户应该知道的特定深度测量。
- 将标本放在核磁共振谱仪。瓦里安 400 MHz 光谱仪,配有自动进样器,在这里用。
- 完成后的核磁共振测量,过程的光谱和分配中的谱峰。
2.3 M 氢氧化钠的查尔酮合
Results
Application and Summary
References
- Ta, L., Axelsson, A., Bijl, J., Haukka, M., Sundén, H., Ionic Liquids as Precatalysts in the Highly Stereoselective Conjugate Addition of α,β-Unsaturated Aldehydes to Chalcones. Chem. Eur. J. 20 (43), 13889-13893 (2014).
- Table adapted from Graham Solomons, T. W. Fryhle, C. B., Organic Chemistry, 10th edition, Wiley, p. 387, 418 (2011).
- Clayden, J., Greeves, N., Warren, S., Wothers, P. Proton nuclear magnetic resonance. Organic Chemistry, Chapter 11, Oxford University Press, 269 (2001).
- Wu, X.-F., Neumann, H., Spannenberg, A., Schulz, T., Jiao, H., Beller, M.,Development of a General Palladium-Catalyzed Carbonylative Heck Reaction of Aryl Halides. J. Am. Chem. Soc. 132 (41), 14596-14602 (2010).
Tags
跳至...
此集合中的视频:

Now Playing
核磁共振 (NMR)
Organic Chemistry
246.8K Views

催化导论
Organic Chemistry
34.1K Views

程序集的激烈化学反应回流系统
Organic Chemistry
166.3K Views

进行室温下反应
Organic Chemistry
70.3K Views

溅镀转移的溶剂
Organic Chemistry
41.5K Views

随着冻融泵循环脱气液体
Organic Chemistry
55.9K Views

制备无水试剂和设备
Organic Chemistry
79.1K Views

重结晶法净化化合物
Organic Chemistry
705.6K Views

通过沉淀混合物的分离
Organic Chemistry
157.2K Views

固-液萃取
Organic Chemistry
237.2K Views

旋转蒸发来去除溶剂
Organic Chemistry
212.4K Views

分馏
Organic Chemistry
332.9K Views

X 射线衍射分析晶体生长
Organic Chemistry
32.3K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
288.5K Views

柱层析法
Organic Chemistry
358.6K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。