Method Article
Fluid-cell Raman Spectroscopy for operando Studies of Reaction and Transport Phenomena during Silicate Glass Corrosion
In This Article
Summary
Fluid-cell Raman Spectroscopy (FCRS) enables in operando observations of reaction and transport phenomena during the aqueous corrosion of silicate glasses at a microscopic level, at elevated temperatures, and in real-time. Without interrupting ongoing processes, FCRS provides information about reaction mechanisms, kinetics, and transport processes.
Abstract
Fluid-cell Raman Spectroscopy (FCRS) enables the real-time and space-resolved (operando) study of reaction mechanisms, kinetics, and their mutual interactions with transport processes during silicate glass corrosion at the micrometer scale and at elevated temperatures. This manuscript provides a detailed protocol for setting up an FCRS experiment, exemplified by a corrosion experiment with a ternary Na borosilicate glass and a 0.5 M NaHCO3 solution at a temperature of 86 ± 1 °C. The protocol involves (i) sample preparation, (ii) assembly of the fluid cell, and (iii) setting of Raman measurement parameters for collecting Raman spectra across the sample/solution interface in regular time intervals. The results from the experiment show the formation of a water-rich zone between a silica-based surface alteration layer (SAL) and the pristine glass, which is an intrinsic feature of an interface-coupled dissolution-precipitation model for the formation of a SAL during silicate glass corrosion. The ability to track the reaction and transport processes during the corrosion of silicate glasses and potentially of other transparent materials, spatially resolved and in real-time, represents a unique strength of this technique, overcoming the disadvantages of conventional analysis of multi-step quenching experiments. The corrosion of the top side of the glass sample represents a current issue, reducing spatial resolution at depth due to precipitation within the laser pathway. This is caused by a solution-filled gap between the sapphire window of the fluid cell lid and the top side of the monolith, which is difficult to avoid during the experimental setup. This must be taken into account when choosing the depth at which the measurement should be made. In a few cases, the formation of air bubbles was observed, which disrupted or even led to the termination of the experiment. However, this can be avoided by carefully setting up the experiment, which requires little practice.
Introduction
Silicate glasses represent metastable materials that are susceptible to aqueous corrosion by atmospheric water vapor or liquid water in engineered environments and technological applications such as solar energy conversion systems1, pharmaceutical use2, and the immobilization of high-level nuclear waste from spent nuclear fuel3,4,5. Besides its role in the technological fields of application, natural volcanic glasses represent a major component of Earth's exposed surface and are thus involved in global biogeochemical cycles and the evolution of long-term climate6,7. The atmospheric alteration by water vapor is a key factor in both technological applications and natural environments.Distinguishing this effect from that of the alteration by liquid water is important as the surface-to-volume (S/V) ratio of glass to solution is significantly different8,9. The present work, however, focuses on the corrosion behavior of silicate glass in liquid water. When a glass comes in contact with an aqueous solution, a number of coupled reaction and transport processes occur at the glass/water interface, generally forming a structurally complex surface alteration layer (SAL). However, the mechanisms, kinetics, and rate-limiting factors of silicate glass corrosion are still a matter of intensive debate, reflected by the existence of various corrosion models5,10,11,12,13. Therefore, understanding the complex interplay between reaction and transport processes at the glass/water interface in time and space and at a microscopic level is fundamental for the development of analytical and numerical models predicting the long-term silicate glass corrosion14.
One widely accepted model presumes that the SAL forms by volume interdiffusion of hydronium from the solution into the glass and network modifiers from the glass into the solution (leaching), whereby silanol groups form by the exchange with the cations15,16. In the course of the reaction, it is assumed that the silanol groups recombine, releasing molecular water by forming new siloxane bonds and eventually leaving behind a porous residual silica-rich SAL (Figure 1A). However, experimental results obtained by atom probe tomography and transmission electron microscopy revealed an atomically sharp interface between the glass and the SAL14,17,18, which contradicts a diffusion-controlled process. In addition, new results of isotope tracer experiments are not consistent with a leaching model19,20. Instead, such observations can be explained by the interface-coupled dissolution-precipitation (ICDP) model that is based on a stoichiometric dissolution of the glass spatially and temporally coupled to the precipitation of amorphous silica once a solution boundary layer at the glass surface is supersaturated with respect to silica 5,21,22. Recently, the ICDP model has been expanded to include an ion exchange zone that may evolve ahead of an ICDP front if the dissolution-precipitation rate slows down dramatically23 (Figure 1B). For a more detailed review of further models considering the formation of SAL, refer to the PhD thesis of M. Fritzsche24.
Commonly performed corrosion experiments follow a multi-step workflow, including the alteration experiment itself, quenching (rapid cooling), drying, sawing, and polishing of the altered glass sample for postmortem analysis15,19. This, however, is critical as it may change the structural and chemical properties of the main corrosion product, i.e., the hydrous amorphous silica-based SAL, due to condensation and/or polymerization, loss of water (dehydration) and/or cracking and flacking15,19,25. In chemically complex systems (multicomponent glasses and solutions), quenching and drying of the sample may also induce the precipitation of secondary minerals that are not involved in the reaction itself. Besides this, a quenched sample represents only one point in time of the glass corrosion process, requiring a high effort to derive glass dissolution rates and information about the reaction mechanism(s) from multiple quench experiments26. Therefore, most glass corrosion kinetic data obtained during glass corrosion experiments from aliquot analyses of the bulk solution are less informative for studying the reaction mechanisms and accompanying transport processes during silicate glass corrosion.
To overcome the disadvantages of ex situ experiments, including sample preparation and post mortem analysis, in situ techniques have gained increasing interest over the past years27,28. For instance, atomic force microscopy (AFM) and vertical scanning interferometry (VSI) became vital tools to study mineral surfaces that are in direct contact with aqueous solution27,29. However, both approaches are limited to studying the very first steps of a corrosion process, i.e., until a secondary layer has formed that impedes the investigation of the glass/SAL interface28,30. Fluid-cell Raman spectroscopy (FCRS) overcomes the aforementioned shortcomings by providing real-time and space-resolved (operando) observations of reaction and transport processes at solid/solution interfaces at a microscopic scale and at elevated temperatures if the parent and product phase(s) are transparent to visible light (Figure 2). First FCRS studies were conducted by Geisler et al.5, who investigated the aqueous corrosion behavior of ternary borosilicate glass (TBG) in a 0.5 M NaHCO3 solution, i.e., under near-neutral pH conditions at 85 °C. The formation of a water-rich zone between the SAL and pristine glass was observed, an intrinsic feature of the ICDP model. By using a bicarbonate solution, pH gradients at the glass surface and within the SAL could also be detected by estimating the local pH from the intensity ratio of the carbonate and bicarbonate band (c.f.5). Moreover, without disturbing the ongoing corrosion process, an exchange with a deuterated solution showed that the transport of water through the SAL was not a rate-limiting step for the corrosion process. In summary, this work showcased the strength of FCRS to identify key reaction mechanisms and feedback on transport phenomena within a single experiment under well-controlled conditions.
Subsequent FCRS experiments have further demonstrated that this method is suitable for routine use, producing consistent and reproducible results24,31,32. For instance, FCRS was applied to study the effect of heavy ion irradiation on the forward dissolution rate of borosilicate glasses, showing a significant increase in the forward dissolution rate by a factor of 3.7 ± 0.531. Moreover, FCRS experiments conducted with a Ba-bearing, soda-lime boroaluminosilicate glass in a hyperalkaline solution documented operando the direct transformation of the glass into Mg-clay, zeolite, and carbonates. These authors found a decrease in the initial glass dissolution rate, the magnitude of which appears to be related to the composition and structure of the alteration layer33. Moreover, changes in the shape of the convoluted water band were used to monitor ionic strength operando, revealing rhythmic fluctuations32. Sulzbach and Geisler34 performed FCRS experiments to study the replacement of celestine (SrSO4) by strontianite (SrCO3), both transparent to visible light, in a carbonate solution, which provided new details about an ICDP mechanism and gave the first evidence for three kinetic regimes. Recently, as part of a doctoral thesis24, the experimental setup was extended to include an external heating station, facilitating long-term studies over several months with glasses of higher durability, such as the six-component International Simple Glass (ISG)14,24. For this, the fluid cell was stored on the heating station between consecutive Raman measurements to avoid blocking the Raman spectrometer for several months. In addition, flow-through experiments were conducted by connecting the fluid cell to a syringe pump. This approach effectively prevented the precipitation of silica, which allowed the measurement of forward dissolution rates under turbulent flow conditions (Fritzsche24).
In general, FCRS provides a novel approach for studying coupled mechanisms of reactions and mass transfer occurring at solid-water interfaces, overcoming the shortcomings of commonly applied ex situ experiments. It is readily expandable to accommodate a wide range of samples and conditions. The aim of this article is to share the technical and experimental details of Fluid-cell Raman spectroscopy, exemplified by a corrosion experiment with a ternary borosilicate glass (TBG) and a 0.5 M NaHCO3 solution at a nominal temperature of 90 °C. The protocol will cover the sample preparation, the assembly of the fluid cell, and the setting of the measurement conditions at the Raman spectrometer. For determining the glass retreat rate, potential pH gradient, and the local temperature the authors refer to the study of Geisler et al.5 and Sulzbach and Geisler34 plus supplementary material and the doctoral thesis of Dohmen35 and Fritzsche24. Critical steps in setting up the experiment, such as filling and closing the cell, will be covered with additional advice on how to avoid repeating the pitfalls of previous work. This article provides a comprehensive overview of the technique, facilitating its implementation by newcomers to the field and contributing to the advancement of research in solid-fluid interaction.
Protocol
1. Preparation of the sample
NOTE: FCRS experiments can be conducted with crystalline or amorphous materials as long as the parent and product phase(s) are transparent to visible light and the reaction takes place at solution temperature below about 100 °C within time scales of days to months5,24,30,31,33,35. The sample should be prepared as a polished monolith about 10 x 10 x 0.9 mm3. The sample size can vary by up to a few mm. The Polytetrafluoroethylene (PTFE) sample holder, which must be manufactured for each experiment, can be adjusted accordingly (Figure 3A). A technical drawing of the PTFE holder used is given in Supplementary Figure 1. In the following, a sample of ternary Na borosilicate glass (TBG) is prepared.
- Grind the glass sample coupon with 600-grit silicon carbide (SiC) paper on two opposite sides until it fits into the PTFE sample holder. Ensure that the sample is held tightly and as vertically as possible in the holder, and carefully remove the abraded PTFE material.
NOTE: By inserting the sample coupon carefully into the holder, abrasion of the PTFE is possible, but it also ensures a stable and tight position of the sample. - Mount the PTFE holder with the sample in a larger metallic sample holder in preparation for grinding the top side of the glass coupon to the same level as the PTFE holder.
- Once the sample, PTFE, and metallic sample holder are almost in one plane, grind the surface with a finer 1000-grit SiC paper.
- Clean the sample within the holder first with ethanol and then with water (manually with spray bottles) and dry it with the aid of a compressed air pistol to remove any grinding (SiC) debris.
NOTE: The presence of SiC particles potentially damages the polishing rag and can disturb subsequent Raman analysis. Additional use of lint-free tissues for cleaning with water is recommended to wipe off residues. - Polish the top side of the sample within the PTFE holder with a 3 µm and 1 µm diamond paste for at least 20 min. Ensure that the surface of the glass sample and the PTFE holder form a thoroughly polished plane in order to enable direct contact with the sapphire disk (Figure 3A,D).
NOTE: This is essential for minimizing the reactive surface area and the gap between the sapphire window and the sample as much as possible in order to reduce the corrosion processes on the top side of the sample. Moreover, a well-polished top surface of the sample reduces laser light refraction36,37,38. - Clean the sample and PTFE holder as described in step 1.4 and additionally use an ultrasonic bath for a duration of approxiamtely 30 s with a frequency of 50-60 Hz.
- Check the polished top side surface of the glass and holder under a stereoscopic microscope with a magnification of 10 times.
2. Preparation of desired solution
- Measure the pH of the 0.5 M NaHCO3 solution after solution equilibration with ambient air by a pH meter. To remove potential solid particles, filter the solution with a sterile syringe filter (0.22 µm pore size) right before the injection.
NOTE: The solution is commonly prepared one day before the start of the experiment. Prior to the onset of the experiment, the pH value and temperature of the solution are measured in order to ensure that the pH value is consistent with the theoretically calculated pH value for the equilibrated solution. - Make sure that the solution is visually free of air bubbles. Determine the solution volume based on the size of the fluid cell and ensure it is enough to recover sufficient solution for chemical analysis at the end of the experiment.
3. Setup of Raman measurement parameters
- Ensure that the Raman spectrometer is calibrated with a suitable frequency standard (e.g., the first-order optical phonon band of a silicon single-crystal with a maximum at 520.7 cm-1). For FCRS experiments, use a Raman spectrometer equipped with a 532 nm laser with a high output power (> 1 W), a microscope with an automated x-y-z stage with a step size of ≤ 1 µm, a 100x long working distance (LWD) objective with a high numerical aperture, a less dispersive grating (e.g., with 600 grooves per mm) to cover a broad spectral range, a neon lamp for spectrometer shift correction during long-term measurements and adjustable confocal and spectrometer entrance slits to independently optimize the spatial and spectral resolution.
CAUTION: Laser safety goggles must be worn when undertaking Raman spectroscopic measurements (check the laser class). With an output power of 1 W or higher, the light scattering effect is intense. However, the high laser output power is a prerequisite for performing high-quality FCRS experiments since a lot of primary light is absorbed along the beam path through the sapphire window, the solution, and the solids. - Setup the Raman measurement parameters in the software.
- Define the ranges of spectral windows to measure the Raman modes characteristic for the sample and solution under investigation. For the borosilicate glass sample, the spectral window ranges from 200 to 1735 cm-1. The second window ranges from 2800 to 4000 cm-1 to measure the respective Raman modes of molecular water and silanol groups.
- If possible, close the confocal hole to 600 µm and open the spectrometer entrance slit width to 200 µm to optimize the depth resolution at the expense of spectral resolution (not critical for relatively broad Raman bands typical for amorphous materials and solutions).
- Select the acquisition time that achieves the best possible signal-to-noise ratio for the desired time resolution. For a sufficient intensity signal of the glass and water, measure the spectral windows for 7 s and 2 s, respectively, and accumulate over 5 rounds.
NOTE: The acquisition time may need to be to accomodate changes in the experiment adjusting to the changes the geometry of the altering sample/evolving solid/water interface with increasing reaction time. - Place the neon lamp alongside the beam path of the scattered light.
NOTE: The neon lamp is optional but recommended as an internal standard to correct the data for potential spectrometer shifts due to unavoidably fluctuations of the room temperature, which, however, should be best controlled within ± 0.5 °C (causing spectrometer fluctuations of up to ~ 0.2 cm-1). In addition, the Ne lines can be used to empirically determine the spectral resolution24.
4. Assembly of the fluid cell
NOTE: Technical drawings of the fluid cell components made out of polyether ether ketone (PEEK) are given in Supplementary Figure 2 and Supplementary Figure 3.
- Place the silicone washer on the inverted fluid-cell lid, then place the sapphire window and PTFE sample holder with the top side of the sample facing the sapphire window (Figure 3B-D). Fix the position of the silicone washer, sapphire window, and sample with the screw cap (Figure 3E-F).
- Before injecting the reactive solution, clean the tubes and valves on each side with deionized water. Then, inject air to remove the water inside the tubes and empty the reactor vessel.
- Insert the O-ring into the groove provided.
- Inject the solution from both sides of the reactor until the outlet of the tubing inside the reactor is fully covered. Close the valves before removing the syringe in order to prevent the accumulation of air within the tubing or valves.
- Add the remaining solution from the top of the reactor vessel until the solution forms a convex surface (meniscus).
- Fill the free spaces of the sample holding lid by carefully dripping the solution along the right and left sides of the sample coupon. Check the filled lid for possible air pockets.
- Turn around the lid for placing it on top of the reactor vessel and close the cell as quickly as possible using the 6 screws. Tighten the screws crosswise.
NOTE: In case of air enclosure, remove the lid and refill the cell and lid. The closed fluid cell represents a static system and provides a purely diffusive transport regime in solution. For flow-through experiments, it is possible to connect the cell to a syringe pump24. - The solution is expected to leak over the edge of the cell. Clean the cell from the outside to remove the solution that leaked over the edges.
- Mount the fluid cell on the x-y-z stage and connect the cell to the heating stage. Turn on the heating stage and set the desired nominal temperature (here 90 °C). The heating takes, for instance, about 14 min to reach a nominal temperature of 90 °C.
NOTE: A detailed description of the heater electronics is given in Supplementary Figure 4. Note also that due to a temperature gradient in the fluid cell, the real temperature at the depth of the measurements is lower. When using a bicarbonate solution, the temperature at any depth of the measurement can be determined from the position of the bicarbonate band near 1016 cm-1 (c.f., Geisler et al.5). For other carbonate-free solution compositions, we estimate the temperature at the measuring point using the empirically determined temperature gradient within the fluid-cell24.
5. Determining the gap size between the sapphire window and the top side of the sample and the position of the sample/solution interface
- Once the nominal temperature is reached, focus the laser beam on the sapphire window through the optical microscope in video mode.
NOTE: A defocusing of the laser spot inevitably takes place during heating up to the desired nominal temperature due to the slight thermal expansion of the sample and the fluid cell materials. Once the nominal temperature is achieved, the focus stabilizes after a few minutes. This can be controlled by using video mode. - Move the laser focus on the top of the sapphire window central to the surface in the x- and y-direction to ensure it is above the sample. Set the z-position (depth) to zero as a reference.
- Move the laser focus in the z-direction until the first Raman signals of water or solution species, such as, e.g., HCO3- and CO32-, appear (Figure 4).
NOTE: If available, a continuously repeating point measurement function (e.g., the Real Time Display function in the LabSpec 6 software) can be a very helpful tool for quickly determining the size of the gap between the top sample surface and the sapphire window. - Move the laser focus further downwards until a pure spectrum of the glass sample is detected using the Real Time Display function. This position is referred to as the top side of the sample.
- Move the laser focus further in the z-direction into the sample (> 50 µm for our observed glass corrosion rates).
NOTE: Step 5.5 is important to avoid disturbing signals from the expected corrosion from the top side of the sample. The depth of the laser focus, nevertheless, may have to be increased with time due to the corrosion from the top of the sample. The actual depth is larger in the order of tens of µm because of multiple refraction effects along the beam path37. - Move the stage in the x direction to determine the sample/solution interface based on the decreasing and increasing intensity of the Raman signal from the sample and solution, respectively. Repeat this step by monitoring the intensity signal of the H2O Raman modes in the x direction.
NOTE: Experience has shown that when the sample surface is at a perfect right angle to the sapphire window, a change in the intensity signal is observed over ~ 10 µm in the x direction, which corresponds to the lateral resolution. - Set the position of the sample/solution interface to x = 0. Set the total size of the line scan to 100 µm, ranging from -70 to 30 µm in the x direction so that the scanning direction is set from the sample into the bulk solution. In consideration of the expected reaction progress, the step size is set to 1 or 2 µm and the
NOTE: The line scan presents single point measurements in the x direction to monitor the moving glass/solution interface over time at one constant position in depth (z) and y-direction. The time needed per line scan is the sum of (1) the counting time, (2) the time to move to a new spot, and (3) the time the spectrometer needs to move to different frequency windows if a broad frequency range has to be measured, e.g., low-frequency region (100 to 1735 cm-1) and high-frequency region (2800 to 4000 cm-1). The length of the line scan may have to be extended into the sample depending on the reaction progress. - Start the line scan measurements. For the glass/solution interface, set a line scan of 100 µm with a 2 µm step size, taking 51 point measurements in the x direction. At each point, the first and second spectral window is measured for 7 s and 2 s, respectively, and 5 times accumulated. The line scan is automatically repeated across the moving glass/solution interface for several days to weeks, monitoring the dissolution of the glass and the precipitation of the silica-based surface alteration layer (SAL).
Results
In the following, the main features of the methodology are illustrated by the results from a corrosion experiment with a ternary Na borosilicate glass (TBG) sample and a 0.5 M NaHCO3 solution at a nominal temperature of 90 °C, i.e., the same conditions as for the experiments of Geisler et al.4. The actual temperature at the depth of measurement was 86 ± 1 °C, as determined from the temperature-dependent frequency shift of the bicarbonate band near 1016 cm-1 (at room temperature)4. The TBG has already been used in previous FCRS experiments24,30,31,35. The experiment was conducted with a Horiba Scientific HR800 confocal Raman spectrometer at the Institute for Geoscience of the University of Bonn, Germany. The system is equipped with a frequency-doubled Nd: YVO4 (532.11 nm) laser with an output power of 2.2 W and an electron-multiplier charged-coupled device (Figure 3B). A 100x long working distance (LWD) objective with a numerical aperture of 0.8, a spectrometer grating with 600 grooves per mm, a confocal hole of 600 µm, and a spectrometer entrance slit width of 200 µm was used. The spectrometer was initially calibrated with a silicon single-crystal that has a first-order Raman band at 520.7 cm-1. The Raman signal was measured in the wavenumber ranges from 200 to 1735 and 2800 to 4000 cm-1. The first wavenumber range includes a Ne line at 1707.36 cm-1 that was recorded as an internal wavenumber standard to correct each spectrum for any spectrometer shift during long-term measurements of up to several days (resulting from ± 0.5 °C temperature variations in the laboratory). The Raman data presented here were only corrected, if not otherwise stated, for possible frequency fluctuations by fitting a Gaussian function to the recorded Ne line24,39. In addition, the Ne line was used to empirically determine the spectral resolution, given by the full width at half maximum (FWHM), which was 5.1 cm-1.
Figure 5A displays the temporal evolution of the spatial distribution of the glass, solution, and SAL as displayed by the software using the recorded raw spectra. This false-color time versus position image was generated from line scans, i.e., from point-by-point measurements in the x direction across the glass/solution interface. The image shows a continuously retreating glass/solution interface within the first 4.0 h, indicating the congruent dissolution of the glass. The first signals of amorphous silica were detected after 8.3 h, capturing the precipitation of the SAL. Figure 5B shows representative raw Raman spectra recorded from one single point (pixel) of the solution, the SAL, and the glass, along with the individual frequency windows used for intensity integration to visualize their spatial distribution, as shown in Figure 5A and Figure 6A. The key observation from this experiment to highlight here is the formation of a water-rich zone between the SAL and the underlying glass. This water-rich zone began to form after approximately 80 h and, from this moment, has subsequently grown to a clear interfacial water layer with a width of about 6 to 8 µm (Figure 6A). In Figure 6B, representative average Raman spectra in the frequency range of water vibrations from the bulk solution, the SAL, the interface solution, and the nominal water-free glass are shown. The spectra highlight the H2O intensity difference between the bulk solution and the interface solution, but also that the spectrum from the SAL involves a new signal near 3600 cm-1 that can be assigned to Si-OH vibrations35. This additional OH band is not visible in the spectrum from the interfacial water layer and the bulk solution, which is further evidence that a clear gap between the pristine glass and SAL has slowly evolved during the experiment that was filled with water. The formation of such an interfacial water layer (or water-rich interface) while the reaction is in progress is an intrinsic feature of the ICDP model and cannot be explained by the widely accepted leaching mechanism4,17. The fact that this observation could be reproduced is of great importance as it obviously needs to be considered in modeling approaches that aim to realistically simulate the corrosion of silicate glass in aqueous solutions.
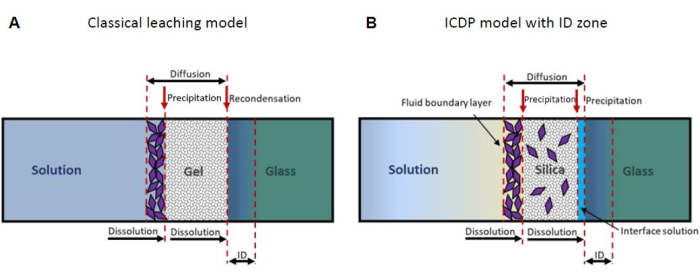
Figure 1: Schematic representation of the two most important mechanistic glass corrosion models. (A) The classical leaching model13,16 and (B) the interface-coupled dissolution-precipitation (ICDP) model19,20 with interdiffusion (ID) zone23. This figure has been modified from24. Please click here to view a larger version of this figure.
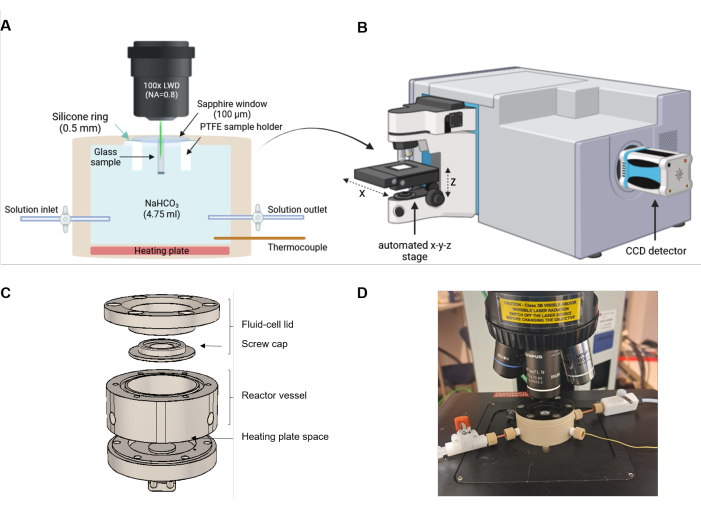
Figure 2: Experimental setup of fluid cell Raman spectroscopy. (A) The laser beam is aligned parallel to the direction of the reaction front. The reaction cell is equipped with a heating plate at the bottom and an inlet and outlet for optional solution exchange or flow-through experiments. (B) Sketch of a Raman spectrometer that is equipped with a microscope, an automated x-y-z stage, and a charge-coupled device (CCD) to detect the scattered light. (C) Schematic technical drawing of the PEEK components of the fluid cell. (D) Photo of a filled fluid cell mounted on the automated stage of the Raman microscope. The setup is not to scale. Please click here to view a larger version of this figure.
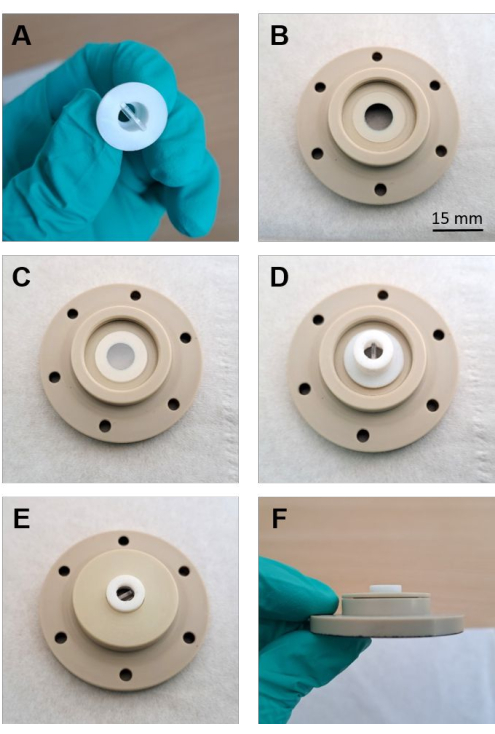
Figure 3: Assembling of the fluid-cell lid. (A) Polished sample and PTFE holder (B) Silicone washer placed on the lid of the fluid cell. (C) The sapphire window is precisely positioned on top of the washer. (D) PTFE holder with the sample facing the sapphire window placed on the window. (E) Screwed-on PEEK cap fixing the position of the sample. (F) Side-view of the cap screwed tightly onto the lid. Please click here to view a larger version of this figure.

Figure 4: Stack plot of raw Raman spectra at different depths. Stack plot from the presented experiment with a Na borosilicate glass and a 0.5 M NaHCO3 solution. The surface of the sapphire window is set to z = 0. Given the 100 µm thickness of the sapphire window, the distance between the bottom of the window and the top side of the glass monolith at -150 µm is approximately 50 µm. The blue spectra display mixed spectra of sapphire, the 0.5 M NaHCO3 solution, and the Na borosilicate glass. Please click here to view a larger version of this figure.
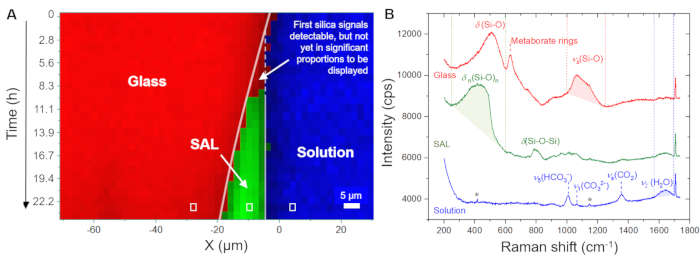
Figure 5: Distribution of the glass, solution, and silica-based SAL as a function of time and space at 86 °C. (A) Hyperspectral Raman line profiles across the glass/solution interface as a function of time for the first 24 h of the experiment with a Na borosilicate glass and a 0.5 M NaHCO3 solution. (B) Stack plot of raw Raman spectra rendered from single point measurement (white rectangles in (A)). The red, green, and blue dashed lines indicate the wavenumber ranges that were used to visualize the glass (1000 - 1250 cm-1), the bicarbonate solution (1560 - 1700 cm-1), and the silica-based SAL (250 - 600 cm-1), respectively. The Raman intensity within these boundaries was integrated after subtraction of a linear background that was defined by the intensity at the boundaries of the wavenumber window. The star symbol marks weak signals from the sapphire window. Please click here to view a larger version of this figure.
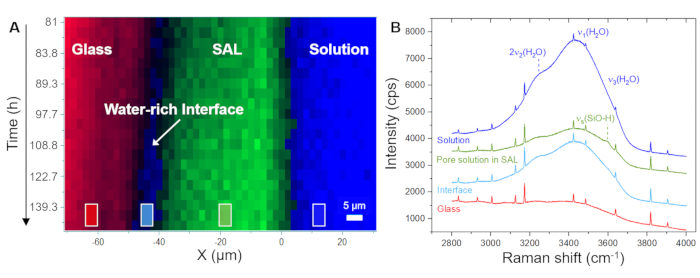
Figure 6: Distribution of the glass, a water-rich interface, and the SAL as a function of time-space along with representative Raman spectra. (A) Hyperspectral Raman line profiles across the glass/solution interface as a function of time for the period between 81 h and 140 h of the experiment with a Na borosilicate glass and a 0.5 M NaHCO3 solution, showing the formation of an interface water layer between the glass and the SAL. (B) Stacked raw Raman spectra averaged over the region marked by squares in (A), showing a decrease in the intensity signal of the respective Raman modes of water within the SAL and an increase between the SAL and the dissolving glass. Within the SAL, an additional signal intensity at around 3600 cm-1 is noted that can be assigned to the stretching mode of silanol groups (H-bonded to Si-O-)40. The visible weak water signal from the glass region results from the solution layer between the sapphire window and the glass sample surface. The sharp peaks are Ne lines. Please click here to view a larger version of this figure.
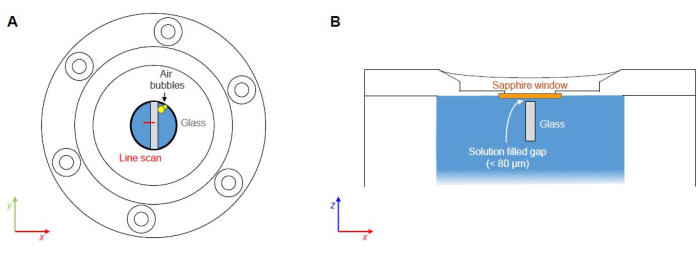
Figure 7: Critical points of FCRS experiments. (A) Potential entrapment and nucleation of air pockets along material interfaces (not to scale). (B) Solution-filled gap between the bottom of the sapphire window and the top side of the glass sample (not to scale). Please click here to view a larger version of this figure.
Supplementary Figure 1: Technical drawing of the sample holder made out of PTFE. Please click here to download this File.
Supplementary Figure 2: Technical drawing of the fluid-cell components made out of PEEK. Please click here to download this File.
Supplementary Figure 3: Technical exploded view drawing of the fluid-cell components made out of PEEK. Please click here to download this File.
Supplementary Figure 4: Detailed description of the heater of the reactor cell. The reactor cell heating element is a metal-ceramic heater disc (MCH), tolerating temperatures up to 400 °C. It has a diameter of 30 mm and a thickness of 1.5 mm. The heating element is connected to a temperature controller by means of a small electronic circuit. The connection between the heater and the controller is accomplished by flexible and heat-tolerant silicon wires. The temperature controller is housed together with the electronic circuit and a 5 V power supply in a small plastic box. Please click here to download this File.
Discussion
The present protocol focuses on setting up an FCRS experiment for the operando study of reaction and transport phenomena during the aqueous corrosion of borosilicate glass by presenting as-measured Raman data from an experiment with a simple ternary Na borosilicate glass in a 0.5 M NaHCO3 solution at 86 ± 1 °C. Critical steps represent (1) the possible entrapment of air pockets during the closing of the fluid cell and (2) top-side corrosion processes due to the gap between the bottom of the sapphire window and the top side of the glass sample (Figure 7). Top-side corrosion represents a problem, particularly during long-term experiments, as the corrosion products absorb and scatter the incident and scattered light, reducing the quality (signal-to-noise) ratio of the spectra and the spatial resolution with time. This issue is currently addressed by ex situ experiments with glasses that were thinly sputtered with a silicon nitride (SiN) layer in order to test the stability of the SiN layer against aqueous solutions. The Raman signals from the SiN surface layer are detectable to a depth of a few micrometers only and, thus, are not expected to have a disturbing effect during a FCRS experiment. Air trapping is always a potential problem that depends on how the solution is filled into the cell, but it can be avoided with a little practice and by removing air bubbles from the starting solution.
For a quantitative treatment, the Raman data must be properly corrected for background signals, temperature effects, and the wavelength dependence of the scattering process24,41. A detailed protocol for the data treatment is given in24. Moreover, it is important to note that the actual and apparent focal depth differ due to multiple refraction effects. However, the depth resolution and actual depth from which a Raman signal is recorded can be calculated with reasonable accuracy from geometrical and optical considerations of a multilayer system24. The spatial resolution can also empirically be determined4,27,28. In general, the depth resolution increases linearly with the apparent depth, depending on the numerical aperture of the objective and the refractive indices of the materials traversed by the laser beam, i.e., the sapphire window, solution, and sample/reaction products24,37.
FCRS provides real-time and in situ insights into coupled reaction and transport processes occurring at the solid-water interface at the microscopic scale. Without interrupting ongoing processes, FCRS can capture key reaction and transport processes, which is important, for instance, for the current debate on the mechanisms of glass corrosion used to describe the long-term performance of nuclear waste glasses. The exemplary data presented here show the formation of a water-rich zone (interfacial water layer/fluid film) between the SAL and the underlying glass, which is an intrinsic feature of the ICDP model. The results confirm previous FCRS studies5,24,30,33 and thus demonstrate the reproducibility of the experimental methodology. The presented method overcomes the disadvantages of multiple-step ex situ experiments involving quenching, drying, and mechanical sectioning of the reaction products and provides information on several quenching experiments by one single experiment while avoiding the risk of modifying alteration products for postmortem analysis.
Since the frequency of molecular vibration depends on the mass of the vibrating isotopes, the reaction and transport processes can even be traced operando by Raman spectroscopy using 2H and 18O that can be added to the system as stable isotope tracers4. The Raman measurement can also be extended to three dimensions, i.e., by sequential measurements in two dimensions, which can provide additional texture information27,28. However, it should be noted that the exposure time will increase dramatically. The rates of the processes to be imaged may thus be a limiting factor as they should be slower than the exposure time, which, of course, must also be considered for line scan measurements.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank G. Paulus (Schott AG) for synthesizing and characterizing the borosilicate glass. A special thanks goes to D. Lülsdorf and H. Blanchard (University of Bonn) as well as W. Bauer (Schott AG) for helping with the design and construction of the fluid-cell(s). We acknowledge Schott AG Mainz, Germany, the German Research Foundation (grant nos. GE1094/21-1 and GE1094/27-1 to T.G.), and the German Federal Ministry of Education and Research (BMBF) (grant no. 02NUK019F to T.G.) for financial support. T.G. and M.B.K.F. are also grateful for the financial support provided by Otto-Schott-Fond.
Materials
| Name | Company | Catalog Number | Comments |
| Cross screws (6 pieces) | --- | --- | |
| Blue silicone wire | Reichelt Elektronik | cross-section: 1.5 mm², length: 2 m | |
| Capacitor | Reichelt Elektronik | capacity: 100 nF | |
| Connectors according to user‘s choice | Reichelt Elektronik | ||
| Eurotherm Controller 3216 | Eurotherm/Emerson | Order Code 3216 CC VH LXXX X XXX G ENG XXXXX | |
| Flangeless Ferrules ETFE | Darwin Microfluidics | SKU: ID-P-200X | 1/4"-28 for 1/16" OD |
| Fluid Cell (container + lid) | home made | --- | technical drawing provided in the appendix (S1-S2) |
| Heating element, metal-ceramic heater (MCH) disc | Alibaba | D: 30 mm, H: 1.5 mm; https://german.alibaba.com/product-detail/DC-Power-Supply-30mm-MCH-Round-1600118228465.html?spm=a 2700.7724857.0.0.7cde5nqq5nqq2n | |
| Horiba HR800 Raman spectrometer | HORIBA | ||
| LabSpec 6.5.1.24 Spectroscopy Suite | |||
| Mains Cable with plug, load+neutral (L+N) | Reichelt Elektronik | length: 2 m | |
| Mains Fuse 1A | Reichelt Elektronik | 5x20 mm² | |
| Mains Fuse Holder | Reichelt Elektronik | 5x20 mm² | |
| Mains rocker switch | Reichelt Elektronik | 230VAC 2A | |
| Microfluidic Fittings Adapter Female Luer to 1/4-28 Male | Darwin Microfluidics | SKU: ID-P-618 | |
| Millex Syringe filters | Merck | SLGPR33RS | |
| NaHCO3 | Merck | CAS-144-55-8 | |
| NMOS Mosfet F1010N | Reichelt Elektronik | ||
| Plastic Enclosure | Reichelt Elektronik | TEKO KL22 | Enclosure 178x128x72 |
| PTFE sample holder | home made | --- | |
| PTFE Tubing | Darwin Microfluidics | BL-PTFE-1608-20 | 1/16" OD |
| Red silicone wire | Reichelt Elektronik | cross-section: 1.5 mm², length: 2 m | |
| Resistor | Reichelt Elektronik | 1/4W, 10kOhm | |
| Resistor | Reichelt Elektronik | 1/4W, 2.2kOhm | |
| Sapphire Window | Edmund Optics GmbH | #71-225 | D: 15 mm H: 100 µm |
| Shut-Off Valve 1/4-28 | Darwin Microfluidics | SKU: ID-P-782 | |
| Silicone washer | ART Elektromechanik | customized for institute | OD: 15mm, ID: 8 mm; H: 0.5 mm |
| Switching-mode power supply | Reichelt Elektronik | SNT RS 25 5 | 5V, 5A |
| Thermocouple Extension Cable, Plug/Socket, Type K | RS Components GmbH | 2 m | |
| Thermocouple Socket, Type K, Case Mount | RS Components GmbH | ||
| Thermocouple, Type K | TC Direct | D: 1 mm, L: 50-100mm | |
| Various cables for in-box wiring | Reichelt Elektronik |
References
- Belançon, M. P., Sandrini, M., Zanuto, V. S., Muniz, R. F. Glassy materials for Silicon-based solar panels: Present and future. J Non-Crystalline Solids. 619, 122548 (2023).
- Iacocca, R., et al. Factors affecting the chemical durability of glass used in the pharmaceutical industry. AAPS PharmSciTech. 11 (3), 1340-1349 (2010).
- Grambow, B. A general rate equation for nuclear waste glass corrosion. MRS Online Proc Lib. 44, 15-27 (1984).
- Gin, S., Taron, M., Arena, H., Delaye, J. M. Effect of structural disorder induced by external irradiation with heavy ions on the alteration of a four oxide borosilicate glass. NPJ Mater Degrad. 8, 64 (2024).
- Geisler, T., Dohmen, L., Lenting, C., Fritzsche, M. B. K. Real-time in situ observations of reaction and transport phenomena during silicate glass corrosion by fluid-cell Raman spectroscopy. Nat Mater. 18, 342-348 (2019).
- Stroncik, N. A., Schmincke, H. U. Evolution of palagonite: Crystallization, chemical changes, and element budget. Geochem Geophys Geosyst. 2 (7), 1017 (2001).
- Staudigel, H., Hart, S. R. Alteration of basaltic glass: Mechanisms and significance for the oceanic crust-seawater budget. Geochim Cosmochim Acta. 47, 337-350 (1983).
- Udi, U. J., Yussof, M. M., Ayagi, K. M., Bedon, C., Kamarudin, M. K. Environmental degradation of structural glass systems: A review of experimental research and main influencing parameters. Ain Shams Eng J. 14, 101970 (2023).
- Majérus, O., et al. Glass alteration in atmospheric conditions: crossing perspectives from cultural heritage, glass industry, and nuclear waste management. NPJMater Degrad. 4, 27 (2020).
- Grambow, B. Nuclear waste glasses - How durable. Elements. 2, 357-364 (2006).
- Hellmann, R., et al. Nanometre-scale evidence for interfacial dissolution-reprecipitation control of silicate glass corrosion. Nat Mater. 14, 307-311 (2015).
- Gin, S., et al. Origin and consequences of silicate glass passivation by surface layers. Nat Comm. 6, 6360-6360 (2015).
- Gin, S., et al. The controversial role of inter-diffusion in glass alteration. Chem Geol. 440, 115-123 (2016).
- Gin, S., et al. An international initiative on long-term behavior of high-level nuclear waste glass. Mater Today. 16, 243-248 (2013).
- Frugier, P., et al. SON68 Nuclear glass dissolution kinetics: Current state of knowledge and basis of the new GRAAL model. J Nuclear Mater. 380, 8-21 (2008).
- Bunker, B. C. Molecular mechanisms for corrosion of silica and silicate glasses. J Non-Crystalline Solids. 179, 300-308 (1994).
- Cailleteau, C., et al. Insight into silicate-glass corrosion mechanisms. Nat Mater. 7, 978-983 (2008).
- Gin, S., Ryan, J. V., Schreiber, D. K., Neeway, J., Cabié, M. Contribution of atom-probe tomography to a better understanding of glass alteration mechanisms: Application to a nuclear glass specimen altered 25years in a granitic environment. Chem Geol. 349 - 350, 99-109 (2013).
- Geisler, T., et al. The mechanism of borosilicate glass corrosion revisited. Geochim Cosmochim Acta. 158, 112-129 (2015).
- Geisler, T., et al. Aqueous corrosion of borosilicate glass under acidic conditions: A new corrosion mechanism. J Non-Crystalline Solids. 356, 1458-1465 (2010).
- Putnis, A. Why mineral interfaces matter. Science. 343, 1441-1442 (2014).
- Putnis, A. Mineral replacement reactions: from macroscopic observations to microscopic mechanisms. Mineralogical Magazine. 66, 689-708 (2002).
- Lenting, C., et al. Towards a unifying mechanistic model for silicate glass corrosion. NPJ Mater Degrad. 2, 28 (2018).
- Fritzsche, M. B. K. . The aqueous corrosion of borosilicate glasses studied in operando by in situ fluid-cell Raman spectroscopy. Dissertation. , (2023).
- Iler, K. R. . The chemistry of silica: Solubility, polymerization, colloid and surface properties and biochemistry of silica. , (1979).
- Gin, S., et al. The fate of silicon during glass corrosion under alkaline conditions: A mechanistic and kinetic study with the International Simple Glass. Geochim Cosmochim Acta. 151, 68-85 (2015).
- Putnis, C., Ruiz-Agudo, E. The mineral-water interface: Where minerals react with the environment. Elements. 9, 177-182 (2013).
- Icenhower, J. P., Steefel, C. I. Dissolution rate of borosilicate glass SON68: A method of quantification based upon interferometry and implications for experimental and natural weathering rates of glass. Geochim Cosmochim Acta. 157, 147-163 (2015).
- Icenhower, J. P., Steefel, C. I. Experimentally determined dissolution kinetics of SON68 glass at 90°C over a silica saturation interval: Evidence against a linear rate law. J Nuc Mater. 439, 137-147 (2013).
- Lenting, C., Geisler, T. Corrosion of ternary borosilicate glass in acidic solution studied in operando by fluid-cell Raman spectroscopy. NPJ Mater Degrad. 5, 37 (2021).
- Lönartz, M. I., et al. The effect of heavy ion irradiation on the forward dissolution rate of borosilicate glasses studied in situ and real time by fluid-cell raman spectroscopy. Materials. 12 (9), 1480 (2019).
- Müller, G., Fritzsche, M. B. K., Dohmen, L., Geisler, T. Feedbacks and non-linearity of silicate glass alteration in hyperalkaline solution studied by in operando fluid-cell Raman spectroscopy. Geochim Cosmochim Acta. 329, 1-21 (2022).
- Müller, G. . Raman spectroscopic ex situ and in situ. investigations of the aqueous corrosion of soda-lime silicate glasses. , (2019).
- Sulzbach, M., Geisler, T. The replacement of celestine (SrSO4) by strontianite (SrCO3) in aqueous solution studied in situ and in real time using fluid-cell Raman spectroscopy. Minerals. 14 (2), 164 (2024).
- Dohmen, L. . In situ .observations of reaction and transport phenomena during silicate glass corrosion by fluid-cell Raman spectroscopy: Method development and applications. Unpubl. PhD thesis. , (2019).
- Everall, N. Confocal Raman microscopy: Why the depth resolution and spatial accuracy can be much worse than you think. Appl Spectrosc. 54, 1515-1520 (2000).
- Everall, N. Depth profiling with confocal raman microscopy, part II. Spectroscopy. 19, 22-27 (2004).
- Everall, N. The influence of out-of-focus sample regions on the surface specificity of confocal Raman microscopy. Appl Spectrosc. 62, 591-598 (2008).
- Hauke, K., Kehren, J., Böhme, N., Zimmer, S., Geisler-Wierwille, T. In situ hyperspectral Raman Imaging: A new method to investigate sintering processes of ceramic material at high-temperature. Appl Sci. 9, 1310 (2019).
- Davis, K. M., Tomozawa, M. An infrared spectroscopic study of water-related species in silica glasses. J Non-Crystalline Solids. 201, 177-198 (1996).
- Long, D. A. . Raman spectroscopy. , (1977).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved