A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
إعداد Poly(pentafluorophenyl acrylate) فونكتيوناليزيد SiO2 حبات "تنقية البروتين"
In This Article
Erratum Notice
Summary
بروتوكول لإعداد بولي (بينتافلوروفينيل أكريلاتي) (poly(PFPA)) المطعمة والسليكا الخرز يرد. سطح فونكتيوناليزيد poly(PFPA) ثم المعطل تداولها مع الأجسام المضادة واستخدمت بنجاح لفصل البروتين من خلال إيمونوبريسيبيتيشن.
Abstract
ندلل على طريقة بسيطة لتحضير بولي (بينتافلوروفينيل أكريلاتي) (poly(PFPA)) المطعمة الخرز السليكا لتجميد جسم وتطبيق إيمونوبريسيبيتيشن اللاحقة (IP). يتم إعداد السطح المطعمة poly(PFPA) عن طريق عملية من خطوتين بسيطة. في الخطوة الأولى، وتودع 3-أمينوبروبيلتريثوكسيسيلاني (أبتيس) كجزيء رابط على سطح السليكا. في الخطوة الثانية، هي المطعمة بولي poly(PFPA)، تصنيعه عن طريق إضافة عكسها، وتجزئة سلسلة نقل (الطوافة) البلمرة، للجزيء رابط من خلال رد فعل التبادل بين الوحدات بينتافلوروفينيل (الشراكة من أجل السلام) على بوليمر والمجموعات أمين على أبتيس. أبتيس و poly(PFPA) على السيليكا الجسيمات هي تؤكدها مطيافية الأشعة السينية النانومترية (XPS)، فضلا عن رصد بتغير حجم الجسيمات يقاس عن طريق نثر الضوء الحيوي (DLS). تحسين hydrophilicity السطحية من الخرز، الاستعاضة الجزئية من poly(PFPA) مع poly(ethylene glycol) فونكتيوناليزيد أمين (الأمينية شماعة) يتم أيضا. Poly(PFPA) محل شماعة المطعمة والسليكا ثم هي معطلة الخرز مع الأجسام المضادة لتطبيق الملكية الفكرية. للمظاهرة، يعمل جسم مضاد ضد كيناز البروتين المنشط الحمض النووي الريبي (PKR)، وتتحدد كفاءة الملكية الفكرية النشاف الغربية. وتظهر نتائج التحليل أن الخرز جسم معطلة يمكن استخدامها في الواقع لإثراء روبية باكستانية بينما تفاعلات البروتين غير محددة الحد الأدنى.
Introduction
فرش البوليمر رد الفعل قد تلقي الكثير من الاهتمام في السنوات الأخيرة. يمكن استخدامها لشل الجزيئات الوظيفية على المواد العضوية أو غير العضوية لإنشاء الأسطح المنشط مع تطبيقات في مجالات مثل الكشف والفصل1،2،،من34، 5. بين البوليمرات المتفاعلة التي ذكرت، تلك التي تحتوي على وحدات إستر بينتافلوروفينيل مفيدة بشكل خاص سبب بهم تفاعلية عالية مع الأمينات والمقاومة تجاه التحلل6. واحد هذه البوليمرات poly(PFPA)، ويمكن أن يكون فونكتيوناليزيد سهولة البلمرة اللاحقة مع الجزيئات المحتوية على الأمينات الأولية أو الثانوية7،،من89،10. وفي أحد الأمثلة، كانت رد فعل فرش poly(PFPA) الأمينية سبيروبيرانس لإنشاء الأسطح تستجيب للضوء7.
وقد وصف إعداد poly(PFPA) وتطبيقاتها في عدد من السابقة المنشورات6،،من78،،من910،11،12 ،13،،من1415،،من1617. على وجه الخصوص، أفادت تيتو وزملاء العمل تركيب فرش poly(PFPA) عبر كل "التطعيم إلى" و "التطعيم من" أساليب7،،من810،،من1112 . في "التطعيم إلى" النهج، بولي (ميثيلسيلسيسكويوكساني)-بولي (بينتافلوروفينيل أكريلاتي) (poly(MSSQ-PFPA)) المختلطة البوليمر كان المركب8،10،،من1112. المكون poly(MSSQ) كان قادراً على شكل التصاق قوية مع عدد من الأسطح المختلفة العضوية وغير العضوية، مما يسمح لعنصر poly(PFPA) تشكل طبقة فرشاة على سطح المواد المغلفة. في "التطعيم من" النهج، بدأ سطح إضافة عكسها وتجزئة سلسلة البلمرة نقل (SI-طوف) كان يعمل على إعداد poly(PFPA) فرش7. وفي هذه الحالة، عامل نقل سلسلة المعطل تداولها سطحية (SI-كبار المستشارين التقنيين) أولاً تعلق تساهمي على الركيزة عن طريق تفاعل السليكا-سيلاني. ثم شارك المعطل تداولها SI-كبار المستشارين التقنيين في البلمرة SI-طوف من مونومرات بفبا، توليد فرش poly(PFPA) كثافة مع الروابط التساهمية مستقرة للركيزة.
باستخدام الفرش poly(PFPA) توليفها عبر البلمرة SI-طوف، أظهرنا مؤخرا تجميد الأجسام المضادة جزيئات السليكا poly(PFPA) المطعمة وتطبيقها فيما بعد في تنقية البروتين18. تم العثور على استخدام فرش poly(PFPA) لتجميد جسم لحل عدد من المسائل المرتبطة بفصل البروتين الحالية من خلال الملكية الفكرية. الملكية الفكرية التقليدية التي تعتمد على استخدام البروتين A/G كرابط لجسم التثبيت19،،من2021. حيث يتيح استخدام البروتين A/G الأجسام المضادة التي يتم إرفاقها مع اتجاه محدد، هو تحقيق الكفاءة الانتعاش مستضد الهدف السامي. ومع ذلك، استخدام البروتين A/G يعاني من التفاعل البروتين غير محددة، فضلا عن فقدان الأجسام المضادة أثناء استرداد البروتين، التي تسهم في ارتفاع مستوى الضوضاء الخلفية. وقد crosslinking المباشرة للأجسام المضادة لدعم متين لحل أوجه القصور هذه، استكشفت22،،من2324. كفاءة هذه التقنيات منخفضة عادة بسبب التوجه العشوائي للأجسام المضادة كروسلينكيد. للركيزة poly(PFPA) المطعمة، تجميد للأجسام المضادة الدائمة، يتحقق من خلال رد فعل التبادل بين وحدات الشراكة من أجل السلام ووظائف أمين على أجسام. على الرغم من اتجاه جسم لا تزال عشوائية، يستفيد النظام من وجود كثير من رد الفعل الشراكة من أجل السلام من المواقع، يمكن التحكم بدرجة البلمرة. وعلاوة على ذلك، أظهرنا أن بالاستعاضة الجزئية لوحدات الشراكة من أجل السلام مع الأمينية شماعة، hydrophilicity السطحية يمكن ضبطها، مواصلة تحسين كفاءة نظام18استرداد البروتين. عموما، عرضت جزيئات السليكا المطعمة poly(PFPA) أن يكون بديل فعال للملكية الفكرية التقليدية بكفاءة معقولة، فضلا عن خلفية أنظف كثيرا.
في هذه المساهمة، يمكننا تقرير طريقة بديلة لإعداد سطح المطعمة poly(PFPA) لتجميد جسم وتطبيق الملكية الفكرية. في عملية من خطوتين بسيطة، كما هو مبين في الشكل 1، جزيء رابط أبتيس هو أولاً المودعة على سطح السليكا، ثم البوليمر poly(PFPA) تساهمي موصولة إلى جزيء رابط من خلال التفاعل بين وحدات الشراكة من أجل السلام على بوليمر ومهام أمين على أبتيس. هذا الأسلوب إعداد يسمح ل crosslinking الدائم من poly(PFPA) على سطح الركازة، لكن تجنب كثير من المضاعفات المرتبطة مع توليف SI-كبار المستشارين التقنيين والبلمره SI-طوف من فرش poly(PFPA). لا تزال تتم الاستعاضة الجزئية لوحدات الشراكة من أجل السلام مع الأمينية شماعة، السماح لصقل الخصائص السطحية فرشاة البوليمر. نعرض الخرز السليكا المطعمة poly(PFPA) وهكذا أعد يمكن المعطل تداولها مع الأجسام المضادة وتستخدم لتخصيب البروتين عن طريق الملكية الفكرية. الإجراء إعداد حبة مفصلة وتجميد جسم، واختبار الملكية الفكرية موثقة في هذه المقالة، للقراء المهتمين في البحث عن بديل للبروتين التقليدية A/G القائمة على الملكية الفكرية.
Access restricted. Please log in or start a trial to view this content.
Protocol
1-إعداد بولي Poly(PFPA)
- البلورة أيبن
- ضم 5 غ 2,2'-azobis(2-methylpropionitrile) (أيبن) مع 25 مل ميثانول في كوب 250 مل. تزج الكأس في حمام زيت 60 درجة مئوية، ثم يقلب الخليط مع شريط إثارة قوة حتى يذوب أيبن تماما.
- تصفية الحل الحارة من خلال ورق الترشيح (5-8 ميكرومتر احتباس الجسيمات) وتخزين فيلتراتي في 4 درجات مئوية للسماح البلورات للنموذج ببطء.
- جمع أيبن ريكريستاليزيد عن طريق الترشيح. الجمع بين المنتج التي تم جمعها مع 25 مل ميثانول الطازجة وكرر عملية البلورة.
- جاف 2 × يتطلب أيبن في فرن فراغ في درجة حرارة الغرفة (RT) بين عشية وضحاها. تخزين المنتج في الظلام في <-10 درجة مئوية.
- توليف لنزيل ديثيوبينزواتي25
- إعداد 500 مل ثلاثة-الرقبة أسفل جولة قارورة مزودة بشريط إثارة مغناطيسية ومكثف ريفلوكسينج وقمع إسقاط والغشاء المطاطي. الاتصال قارورة خط غاز النيتروجين عن طريق المكثف ريفلوكسينج وطرد الداخل الهواء مع النيتروجين. إدراج مقياس حرارة عن طريق الحاجز. إضافة مل 41 (0.041 mol) من الحل م 1 بروميد فينيلماجنيسيوم في رباعي هيدرو الفوران (THF) عن طريق حقنه عن طريق الشخص نفسه.
- الحل بروميد فينيلماجنيسيوم إلى 40 درجة مئوية في حمام زيت دافئ. ثم أضف ز 3.1 (0.041 mol) من ثنائي كبريتيد الكربون عن طريق القمع إسقاط ببطء، الحفاظ على درجة الحرارة الحل عند 40 درجة مئوية.
- إضافة ز 7.1 (0.042 mol) بروميد البنزيل الخليط الناتجة عن طريق القمع إسقاط ما يزيد على 15 دقيقة زيادة رد فعل درجة الحرارة إلى 50 درجة مئوية. تواصل إثارة في درجة الحرارة هذه لمدة 45 دقيقة.
- تحويل المزيج رد فعل إلى قمع سيباراتوري ويضعف مع 15 مل من الثلج والمياه الباردة. استخراج المنتج عن طريق إضافة 15 مل أثير ثنائي إثيل وإزالة طبقة المياه السفلي. كرر الاستخراج مع إثيل الاثير مرتين أخريين.
- غسل المراحل العضوية مجتمعة مع كمية وفيرة من المياه، ثم محلول ملحي (محلول 50% (w/v) كلوريد الصوديوم في الماء) وتجفيف المنتج عبر سلفات المغنيزيوم اللامائى.
- إزالة المذيب في فراغ في 35 درجة مئوية باستخدام مبخر دوراني.
- تنقية المنتج واسطة كروماتوغرافيا العمود باستخدام 400 مل هلام السليكا (حجم المسام 60 Å، حجم الجسيمات مش 63-200) والأثير البترول الوينت، مما أسفر عن 5 غ من البنزيل ديثيوبينزواتي (BDB) كزيت الأحمر. تأكيد نقاء المنتج بواسطة 1"ح الرنين المغناطيسي النووي" (400 ميجاهرتز، كدكل3): δ 8.02-7.99 (ح 2، m)، 7.50 7.55 (1 ح، م)، 7.29 7.41 (7 ح، م)، 4.60 (ح 2، ق).
- توليف poly(PFPA) عن طريق طوف البلمرة9،26
- متوفرة تجارياً بفبا مونومر يحتوي على كمية صغيرة من مثبطات. قبل البلمرة، إزالة مثبطات بتمرير مركب عن طريق حقنه المتاح معبأة مع شركة ألومينا الأساسية.
- إضافة 0.4 ملغ (0.0024 ملمول) يتطلب أيبن، مغ 4.3 (0.018 ملمول) BDB، مغ 1012 (4.25 ملمول) من بفبا خالية من مثبطات ومل 0.7 من انيسول اللامائى قارورة شلينك 20 مل.
- الاتصال قارورة خط شلينك وديغا المخلوط مع دورات تجميد أذاب مضخة ثلاثة على الأقل. بإيجاز، تجميد المخلوط رد فعل في حمام النتروجين سائل. تطبيق فراغ لإزالة الغاز في headspace. قم بإزالة الختم قارورة بعيداً عن النتروجين السائل للسماح للمحتوى لذوبان الجليد في الرايت
- وضع في قارورة في حمام زيت 70 درجة مئوية، والرد على ح 4 تحت التصريف2 ن.
- إنهاء رد فعل، إزالة قارورة من حمام الزيت وتعرض المحتوى رد فعل للهواء.
- يعجل البوليمر في الميثانول الباردة، ثم الجاف البوليمر المستردة في فرن فراغ عند 40 درجة مئوية بين عشية وضحاها.
- لقياس الوزن الجزيئي بوليمر, استخدام هلام تخلل اللوني (المؤتمر الشعبي العام). استخدام THF كالمرحلة المتنقلة في 35 درجة مئوية مع 1 مل/دقيقة تدفق معدل وبناء منحنى المعايرة باستخدام معايير البوليسترين مونوديسبيرسي. للحصول على قياس حزب المؤتمر الشعبي العام، حل البوليمر في THF (1-2 مغ/مل) وعامل التصفية من خلال 0.2 ميكرومتر المتاح تترافلوروايثيلين (PTFE) تصفية. حقن 100 ميكروليتر من العينة في الصك حزب المؤتمر الشعبي العام. تحويل وقت الاحتفاظ بالعينة المقاسة باستخدام منحنى المعايرة البوليستيرين الوزن الجزيئي.
2-إعداد Poly(PFPA) فونكتيوناليزيد SiO2 حبات
- العلاج من SiO2 حبات مع أبتيس
- SiO2 جزيئات متاحة في شكل تعليق مائي 5% (w/v). الجمع بين 0.8 مل SiO2 تعليق 40 مغ من أبتيس و 8 مل من الميثانول في قنينة التﻷلؤ 20 مل مزودة بشريط إثارة.
- السماح بأن رد الفعل على المضي قدما في الرايت ح 5 مع إثارة قوية.
- نقل الحل إلى أنبوب مخروطي. لعزل في أبتيس فونكتيوناليزيد SiO2 حبات، والطرد المركزي الحل في 10,000 س ز لمدة 5 دقائق، ثم إزالة المادة طافية. أغسل الخرز بإعادة تفريق لهم في 3 مل ميثانول الطازجة. هز الأنبوبة باليد للخلط، ولكن إذا لزم الأمر، تحسين التشتت قبل سونيكيشن في حمام مائي لبضع ثوان. الطرد المركزي الخرز في س 10,000 ز لأدنى 5 إزالة المادة طافية وكرر الخطوة يغسل مرة أخرى.
- الجمع بين الميثانول غسلها SiO2 حبات مع 3 مل من ثنائي ميثيل سلفوكسيد ([دمس]). اهتز هذا الخليط باليد، أو إذا لزم الأمر sonicate لبضع ثوان، حتى الخرز مشتتة تماما في [دمس]. الطرد المركزي الخرز في 10,000 س ز لمدة 5 دقائق، ثم إزالة المادة طافية. كرر هذه الخطوة لضمان صرف المذيبات كاملة من الميثانول إلى [دمس].
ملاحظة: تعليق نهائي يحتوي على أبتيس فونكتيوناليزيد SiO2 حبات موزعة في 4 مل من [دمس]. - للتحقق من توزيع حجم الجسيمات، إجراء تحليل لدائرة الأراضي والمساحة. تأخذ قطره واحدة من تعليق أعد الخطوة 2.1.4 ومكان في ومبومو الأشعة فوق البنفسجية المتاح. تخفف العينة بملء في ومبومو مع جديدة [دمس] حتى هو 2/3 كامل. إدراج العينة صاحب الخلية للبدء في الحصول على البيانات. لقياس حجم الجسيمات، استخدام معلمات الإعداد التالية: درجة الحرارة: 25 درجة مئوية؛ وقت الموازنة: 120 s; مدة القياس: التلقائية.
- للتحقق من تكوين السطحية، إجراء تحليل XPS. جاف عينة صغيرة من تعليق إعدادها في الخطوة 2.1.4 في فرن فراغ عند 40 درجة مئوية بين عشية وضحاها. تأخذ البوليمر المجففة وحزمة بالتساوي على حامل عينة 0.5 × 0.5 سم. تحميل العينة في الدائرة فراغ عالية (10-8 بالتور) والبدء في الحصول على البيانات. للصك XPS خاصة تستخدم، تولد فوتوليكترونس استخدام أحادي اللون Al Kα الأشعة السينية تعمل في 15 كيلوفولت وماجستير 6.7، وجمع استخدام التكبير الوضع المختلط مع المحلل في eV 50 تمرير الطاقة للاطياف عالية الدقة، و eV 100 تمرير الطاقة للدراسات الاستقصائية لعنصري.
- تطعيم poly(PFPA) إلى أبتيس فونكتيوناليزيد SiO2 حبات
- تحضير حل poly(PFPA) بإذابة 20 ملغ poly(PFPA) في 2 مل من [دمس] في قنينة التﻷلؤ 20 مل.
ملاحظة: يستخدم في هذه الدراسة، poly(PFPA) وزن الجزيئي منخفض نسبيا (20 كجم/مول). وهكذا، على الرغم من أن تركيز البوليمر عالية (10 مغ/مل)، يحتفل بأي دليل على وجود crosslinking البوليمر. إذا تم استخدام بوليمر وزن الجزيئي أعلى، ثم تركيز حل البوليمر قد تحتاج إلى تعديل لتجنب crosslinking ممكن. - أضف 1 مل أبتيس فونكتيوناليزيد SiO2 حبات علقت في [دمس] (من الخطوة 2.1.4) في حل poly(PFPA). الرد على RT ح 1 مع التحريك نشطة.
- عزل الخرز2 SiO poly(PFPA) المطعمة بالطرد المركزي في 10,000 س ز لمدة 5 دقائق، يليه إزالة المادة طافية. أغسل الخرز بإضافة 3 مل [دمس] ومزيج من أما المصافحة باليد أو بضع ثوان من sonication. الطرد المركزي الخرز في 10,000 س ز لمدة 5 دقائق، ثم إزالة المادة طافية. تكرار غسيل الخرز2 SiO poly(PFPA) المطعمة مع [دمس] مرتين.
- أغسل حبات مرتين أكثر مع الماء المقطر الثلاثي (إصلاح). في هذه الخطوة، ضم الخرز مع 3 مل إصلاح، ثم تخلط بالمصافحة باليد أو بضع ثوان من sonication. الطرد المركزي الخرز في 10,000 س ز لمدة 5 دقائق، ثم إزالة المادة طافية.
- للتحقق من توزيع حجم الجسيمات، نفذ DLS اتباع الإجراء الموضح في الخطوة 2.1.5. للتحقق من الكيمياء السطحية، أداء XPS اتباع الإجراء الموضح في الخطوة 2.1.6.
- تحضير حل poly(PFPA) بإذابة 20 ملغ poly(PFPA) في 2 مل من [دمس] في قنينة التﻷلؤ 20 مل.
3-إعداد SiO2 حبات المطعمة بالاستعاضة عن شماعة Poly(PFPA)
- إعداد الحل poly(PFPA)، حل 20 ملغ poly(PFPA) في 2 مل من [دمس] في قنينة التﻷلؤ 20 مل.
- لتحضير الوتد الحل، حل شماعة فونكتيوناليزيد أمين في 1 مل من [دمس]. استخدام المبلغ المحدد لشماعة تتحدد بالدرجة المطلوبة من الاستعاضة عن الشراكة من أجل السلام، تحددها المعادلة هو مبين أدناه:
مقدار الأمينية شماعة (g/g-poly(PFPA)) = (N_poly(PFPA) x % شماعة-Sub) x (MW_PEG/MW_poly(PFPA))
حيث N_poly(PFPA) = poly(PFPA) درجة بلمرة
% Sub الوتد = النسبة المئوية شماعة الاستبدال
MW_PEG = الوزن الجزيئي لامينيه شماعة
MW_ poly(PFPA) = الوزن الجزيئي ل poly(PFPA) - نقل الحل شماعة لحل poly(PFPA). الرد على RT ح 1 مع التحريك نشطة.
- لإعداد أبتيس فونكتيوناليزيد SiO2 حبات علقت في [دمس]، اتبع نفس الخطوات الموضحة في "الخطوة 2-1". نقل 1 مل تعليق حبة إلى حل محل شماعة poly(PFPA) إعدادها في "الخطوة 3، 3". السماح بتطعيم بين poly(PFPA) وأبتيس فونكتيوناليزيد SiO2 حبات المضي قدما في الرايت ح 1 مع التحريك نشطة.
- عزل الخرز بالطرد المركزي في 10,000 س ز لمدة 5 دقائق، يليه إزالة المادة طافية. أغسل الخرز بإضافة 3 مل [دمس] ومزيج من أما المصافحة باليد أو بضع ثوان من sonication. الطرد المركزي الخرز في 10,000 س ز لمدة 5 دقائق، ثم إزالة المادة طافية. تكرار الغسيل [دمس] مرتين.
- أغسل حبات مرتين أكثر مع إصلاح. في هذه الخطوة، ضم الخرز مع 3 مل إصلاح، ثم تخلط بالمصافحة باليد أو بضع ثوان من sonication. الطرد المركزي الخرز في 10,000 س ز لمدة 5 دقائق، ثم إزالة المادة طافية.
- جاف الخرز عند 40 درجة مئوية في فرن فراغ بين عشية وضحاها.
4-جسم التثبيت على Poly(PFPA) المطعمة SiO2 حبات
ملاحظة: يتم استخدام نفس الإجراء بغض النظر عن نسبة استبدال شماعة في poly(PFPA). تحضير فوسفات مخزنة المالحة (PBS) بإذابة قرص برنامج تلفزيوني في إصلاح. إعداد 0.1% (v/v) الفوسفات مخزنة المالحة مع توين-20 (ببست) بإضافة 1/1000 من توين-20 لبرنامج تلفزيوني.
- إضافة 5 ملغ poly(PFPA) المطعمة SiO2 حبات لأنبوب ميكروسينتريفوجي 1.5 مل.
- أغسل الخرز بإضافة 800 ميليلتر من برنامج تلفزيوني ومزيج جيد من قبل فورتيكسينج. الطرد المركزي الخرز في 10,000 س ز في RT 1 دقيقة إزالة المادة طافية وكرر الخطوة يغسل ثلاث مرات.
- إضافة 350 ميليلتر من برنامج تلفزيوني جديد، 50 ميليلتر 0.1% (v/v) ببست و 6.67 ميكروغرام من الجسم. احتضان ح ~ 20 في دوار عند 4 درجة مئوية.
- أغسل حبات إزالة الأجسام المضادة غير منضم. الطرد المركزي الخرز في 400 x ز و 4 درجات مئوية للحد الأدنى 1 إزالة المادة طافية وإضافة 400 ميليلتر من المخزن المؤقت لتحلل بعناية. بلطف إعادة تعليق الخرز من بيبيتينج صعودا وهبوطاً لخمس مرات.
ملاحظة: تحلل المخزن المؤقت المستخدم لغسل الخرز ينبغي أن تكون نفس واحد المستخدمة أثناء تحلل الخلية، والملكية الفكرية، إلا أن إضافة مثبطات ديثيوثريتول وحوزتي اختيارية، (راجع الخطوة 5). - كرر هذه الخطوة يغسل ثلاث مرات. بعد الغسيل النهائي، قم بإزالة المادة طافية بقدر الإمكان.
5-خلية تفسخ وإيمونوبريسيبيتيشن
- إعداد المخزن المؤقت لتحلل والمخزن المؤقت للمياه والصرف الصحي
- إعداد المخزن المؤقت تحلل (50 مم تريس-HCl (pH 8.0)، 100 ملم بوكل، 0.5% (v/v) NP-40، والغليسيرول 10% (v/v)، 1 مم ديثيوثريتول (DTT)، ومبطلات المانع كوكتيل).
- إعداد المخزن المؤقت الغسيل (50 مم تريس-HCl (pH 8.0)، 100 ملم بوكل، والغليسيرول NP-40، و 10% (v/v) 0.1% (v/v)).
- تخزين الحلول المخزن المؤقت عند 4 درجة مئوية.
- إعداد الخلايا
- بذور الخلايا (خلايا هيلا) يوم واحد أو يومين قبل التجربة الملكية الفكرية، ونمو الخلايا في 37 درجة مئوية و 5% CO2.
- جمع حوالي 1.4 × 107 الخلايا مع مكشطة الخلية ونقل إلى أنبوب مخروطي 15 مل. أجهزة الطرد المركزي الخلايا في 380 س ز على RT للحد الأدنى 3 إزالة المادة طافية وإعادة تعليق مع 1 مل من برنامج تلفزيوني ونقل إلى أنبوب ميكروسينتريفوجي 1.5 مل الباردة.
- الطرد المركزي الخلايا في 10,000 ز x عند 4 درجة مئوية لإزالة 30 س. المادة طافية نظيفة. ويمكن تخزين خلايا الكريات في-80 درجة مئوية بعد إزالة المادة طافية.
- إعداد خلية ليساتيس
- إعادة تعليق بيليه الخلية مع 400 ميليلتر من تحلل المخزن المؤقت. Sonicate الخلايا باستخدام أولتراسونيكاتور.
- بعد sonication ودوامه بإيجاز والطرد المركزي في ز 20,000 x عند 4 درجة مئوية لمدة 10 دقائق.
- نقل المادة طافية إلى أنبوب الطرد مركزي 1.5 مل جديدة.
- إيمونوبريسيبيتيشن
- نقل 300 ميليلتر من الخلية ليستي إلى جسم المعدة مسبقاً المحتضنة poly(PFPA) المطعمة SiO2 حبات. تحتفظ 30 ميليلتر من الخلية كنموذج الإدخال في أنبوب ميكروسينتريفوجي جديدة. تخزين العينة الإدخال عند 4 درجة مئوية.
ملاحظة: يجب أن يكون المبلغ الإجمالي للبروتين في الخلية ليستي حوالي 4 مجم. - احتضان هذا الخليط ليستي/الخرز ح 3 في دوار عند 4 درجة مئوية.
- الطرد المركزي المخلوط في 400 غرام x عند 4 درجة مئوية للحد الأدنى 1 إزالة المادة طافية وإضافة 400 ميليلتر من المخزن المؤقت للمياه والصرف الصحي بعناية. بلطف إعادة تعليق الخرز من بيبيتينج صعودا وهبوطاً حوالي خمس مرات.
- كرر هذه الخطوة يغسل ثلاث مرات. بعد الغسيل النهائي، قم بإزالة المادة طافية بقدر الإمكان.
- إعداد 2 × دوديسيل كبريتات الصوديوم (SDS) تحميل صبغ (والغليسيرول 25% (v/v)، 0.1% (w/v) برومو الفينول الأزرق (BPB)، 60 ملم تريس-HCl (6.8 درجة الحموضة)، 2% (w/v) الحزب الديمقراطي الصربي، ومم 2.75 2-ميركابتوثانول). تخزين مخزونات النشر الاستراتيجي x 2 تحميل صبغ عند-20 درجة مئوية. إضافة 30 ميليلتر من 2 × صبغ تحميل الحزب الديمقراطي الصربي إلى الخرز والعينة الإدخال المخزنة، والحرارة لهم لمدة 10 دقائق عند 95 درجة مئوية.
- بعد تدفئة، تحليل العينة باستخدام الغربية النشاف27، أو تخزين العينة في-20 درجة مئوية.
- نقل 300 ميليلتر من الخلية ليستي إلى جسم المعدة مسبقاً المحتضنة poly(PFPA) المطعمة SiO2 حبات. تحتفظ 30 ميليلتر من الخلية كنموذج الإدخال في أنبوب ميكروسينتريفوجي جديدة. تخزين العينة الإدخال عند 4 درجة مئوية.
Access restricted. Please log in or start a trial to view this content.
النتائج
تخطيطي لإعداد poly(PFPA) المطعمة SiO2 حبات، مع أو دون شماعة الاستبدال ويرد في الشكل 1. رصد أبتيس وتطعيم العملية، الخرز2 SiO العارية، poly(PFPA) فونكتيوناليزيد أبتيس SiO2 الخرز، والمطعمة poly(PFPA) SiO2 حبات تتميز بدائرة الأراضي والمساحة (الش...
Access restricted. Please log in or start a trial to view this content.
Discussion
توليف poly(PFPA) المطعمة SiO2 حبات يتضح في الشكل 1. باستخدام أبتيس كجزيء رابط، يمكن إعداد فرش poly(PFPA) تساهمي المطعمة ب SiO2 الركيزة عن طريق عملية من خطوتين بسيطة. على الرغم من أن بعض الوحدات الشراكة من أجل السلام هي التضحية للتفاعل مع أبتيس، عدد كبير من الوحدات الشراكة من أ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
الكتاب ليس لها علاقة بالكشف عن.
Acknowledgements
هذا العمل كان تدعمها الوكالة "تطوير الدفاع" (رقم المنحة UD170039ID).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
References
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
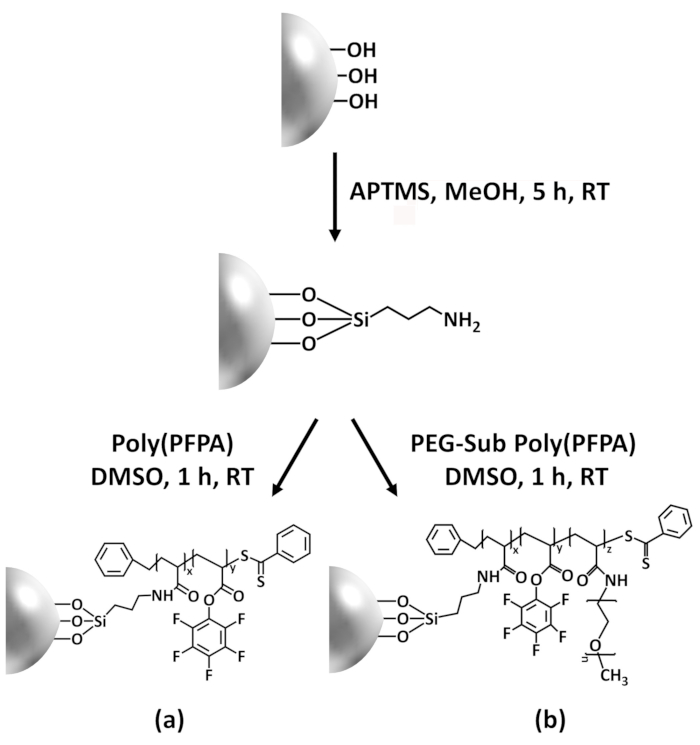
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
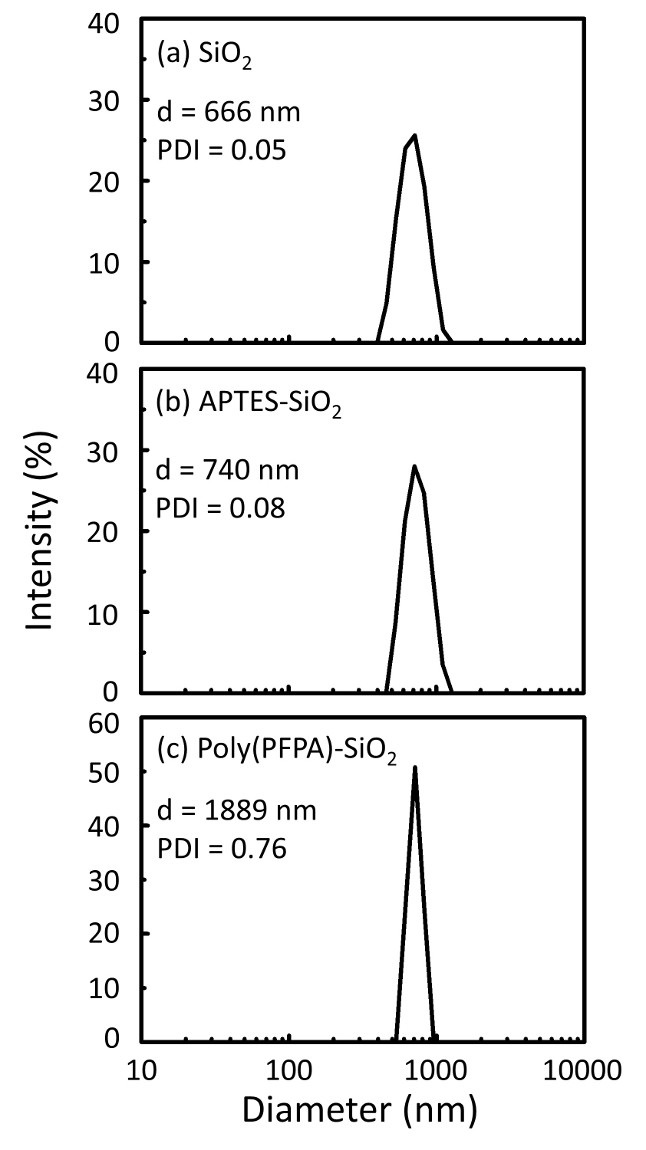
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
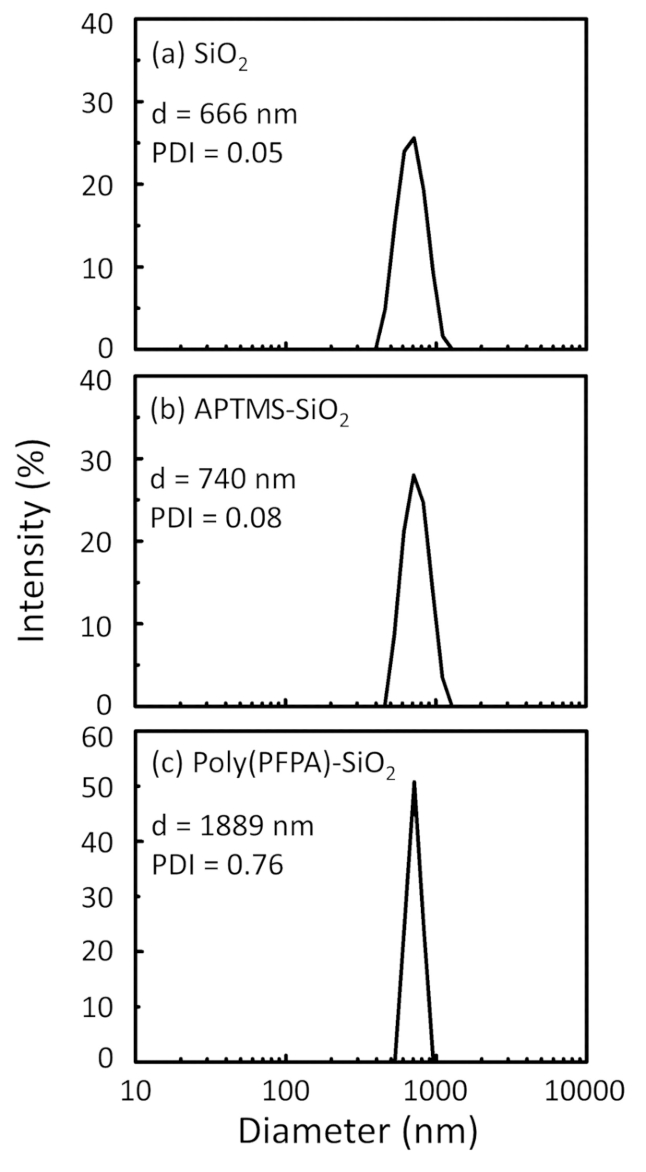
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
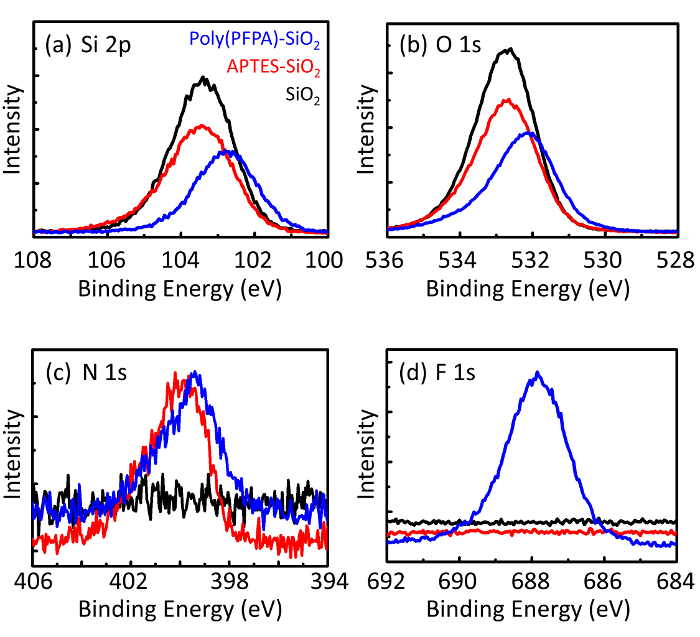
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
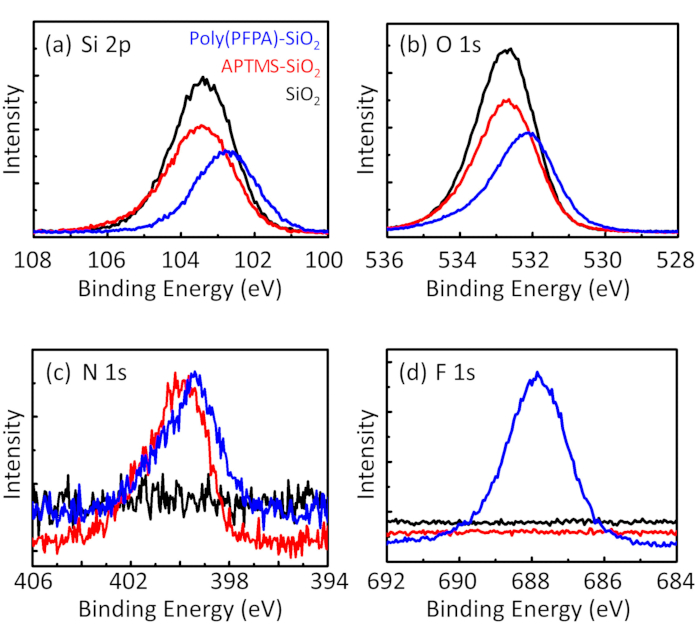
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved