JoVE 비디오를 활용하시려면 도서관을 통한 기관 구독이 필요합니다. 전체 비디오를 보시려면 로그인하거나 무료 트라이얼을 시작하세요.
Method Article
단백질 정화에 대 한 Poly(pentafluorophenyl acrylate) 기능성 SiO2 구슬의 준비
Erratum Notice
요약
폴 리 (pentafluorophenyl 아크릴)의 준비에 대 한 프로토콜 (poly(PFPA)) 융합 실리 카 비즈 제공 됩니다. Poly(PFPA) 기능성된 표면 다음 항 체와 함께 움직일 하 고 immunoprecipitation 통해 단백질 분리에 대 한 성공적으로 사용.
초록
폴 리 (pentafluorophenyl 아크릴)을 준비 하는 간단한 방법을 보여 줍니다 (poly(PFPA)) 융합 항 체 동원 정지 및 후속 immunoprecipitation (IP) 응용 프로그램에 대 한 실리 카 구슬. 간단한 2 단계 프로세스를 통해 poly(PFPA) 이식할된 표면 준비 된다. 첫 번째 단계에서 3-aminopropyltriethoxysilane (항)으로 링커 분자 실리 카 표면에 입금 됩니다. 두 번째 단계에서 poly(PFPA) 단일 중합체, 가역 추가 및 조각화 체인 전송 (뗏목) 중 합을 통해 합성에 pentafluorophenyl (PFP) 단위 사이 교환 반응을 통해 링커 분자에 투입 되는 폴리머와 항에 아민 그룹입니다. 항 및 실리 카 입자는 엑스레이 광전자 분광학 (XPS)에 의해 확인으로 입자 크기 변화에 의해 감시에 poly(PFPA)의 증 착 동적 산란 (DL)을 통해 측정. 구슬, 아민 기능성된 poly(ethylene glycol)와 poly(PFPA)의 부분 대체의 표면 화란 개선 (아미노 PEG)도 수행 됩니다. 못 대체 poly(PFPA) 실리 카 구슬 다음 IP 응용 프로그램에 대 한 항 체와 함께 움직일 수 투입. 데모, 단백질 키 니 아 제 RNA 활성화 (PKR)에 대 한 항 체, 고용 및 IP 효율 서쪽 blotting에 의해 결정 됩니다. 분석 결과 항 체 움직일 구슬 실제로 일반적인 단백질 상호 작용은 최소 PKR을 풍부 하 게 사용 될 수 있다는 것을 보여준다.
서문
반응성 폴리머 브러쉬 최근 몇 년 동안에 많은 관심을 받았습니다. 활성화 된 표면 탐지 및 분리1,2,3,4,등의 분야에서 응용 프로그램과 함께 만드는 유기 또는 무기 재료에 기능성 분자를 고정을 사용할 수 있습니다. 5. 보고 반응 고분자 중 pentafluorophenyl 에스테 르 단위를 포함 하는 아민 및 가수분해6향해 저항 그들의 높은 반응성 때문에 특히 유용 합니다. 이러한 한 폴리머 poly(PFPA), 이며 1 차 또는 2 차 아민7,8,,910를 포함 하는 분자와 쉽게 기능성된 후 중 합 될 수 있습니다. 한 예로, poly(PFPA) 브러쉬 빛 반응 표면7만들려고 아미노 spiropyrans로 반응 했다.
Poly(PFPA)와 그 응용 프로그램의 준비 이전 간행물6,7,,89,10,11,12의 숫자에 설명 되었습니다. ,13,,1415,,1617. 특히, Theato와 동료 보고 "에 접목"와 "에서" 방법7,,810,,1112 접목을 통해 poly(PFPA) 브러쉬의 합성 . "접목 에" 접근 방식, 폴 리 (methylsilsesquioxane)에서-폴 리 (pentafluorophenyl 아크릴) (poly(MSSQ-PFPA)) 하이브리드 폴리머 합성된8,10,,1112했다. Poly(MSSQ) 구성 요소 형태로 강한 접착 코팅된 소재 표면에 브러시 레이어를 형성 하는 poly(PFPA) 구성 요소 되므로 다른 유기 및 무기 표면 수가 있었습니다. "접목 에서" 접근에서 표면 가역 추가 시작 하 고 조각화 체인 전송 (SI-뗏목) 중 합 poly(PFPA) 브러쉬7을 준비 하기 위해 고용 되었다. 이 경우에, 표면 고정된 체인 전송 에이전트 (SI-CTA) covalently silane 실리 카 반응을 통해 기판에 첨부를 먼저 되었다. 고정된 시 CTA 다음 기판에 안정적인 공유를 밀도가 포장된 poly(PFPA) 브러쉬 생성 PFPA 단위체의 뗏목 시 합에 참가 했다.
시-뗏목 중 합을 통해 합성 poly(PFPA) 브러쉬를 이용 하 여 우리는 최근 poly(PFPA) 융합 실리 카 입자와 단백질 정화18에서 그들의 후속 응용 프로그램에 항 체의 동원 정지를 시연. 항 체의 동원 정지에 대 한 poly(PFPA) 브러쉬를 사용 하 여 다양 한 IP 통해 현재 단백질 분리와 관련 된 문제를 해결 하기 위해 발견 되었다. 기존의 IP 항 체 immobilization19,,2021단백질 A/G는 링커로의 사용에 의존합니다. 이후 단백질 A/G를 사용 하 여 항 체를 특정 방향으로 장착할 수, 높은 대상 항 원 복구 효율성 달성 된다. 그러나, 단백질 A/G를 사용 하 여 단백질 복구, 둘 중 배경 잡음의 높은 수준에 기여 하는 동안 일반적인 단백질 상호 작용 뿐만 아니라 항 체의 손실에서 겪고 있다. 이러한 단점을 해결 하려면 고체 지원에 항 체의 직접 가교 탐험된22,,2324되었습니다. 이러한 기술의 효율은 일반적으로 낮은 가교 된 항 체의 임의의 방향으로. Poly(PFPA) 투입 기판에 대 한 항 체의 동원 정지는 영구적, PFP 단위 및 항 체에 아민 기능 사이 교환 반응을 통해 달성입니다. 비록 항 체 오리엔테이션은 여전히 무작위, 시스템 많은 반응 PFP 사이트, 중 합도 의해 제어 하는 데에서 혜택. 또한, 우리 아미노-말뚝와 PFP 단위의 부분 대체 하 여 보여주는 표면 화란 수 수 조정, 추가 시스템18의 단백질 복구 효율성을 향상. 전반적으로, poly(PFPA) 융합 실리 카 입자 합리적인 효율성 뿐만 아니라 많은 청소기 배경 전통적인 ip 효과적인 대안이 될를 표시 했다.
이 기여에 우리는 poly(PFPA) 이식할된 표면 항 체 동원 정지 및 IP 응용 프로그램에 대 한 준비 하는 다른 방법을 보고 합니다. 간단한 2 단계 프로세스에 그림 1에서 볼 수 있듯이 항 링커 분자는 먼저 입금 실리 카 표면에 그 후에 poly(PFPA) 폴리머 covalently에 PFP 단위 사이 반응을 통해 링커 분자에 연결 된 고분자 고 항에 아민 기능입니다. 이 준비 방법은 기판 표면에 poly(PFPA)의 영구 가교에 대 한 수 있지만 시 CTA 합성 및 poly(PFPA) 브러쉬의 중 합 시-뗏목과 관련 된 많은 합병증을 피 한다. 아미노 페그와 PFP 단위의 부분 대체 여전히 수행할 수 있습니다, 고분자 브러쉬 표면 특성의 미세 조정 허용. 우리는 따라서 준비 poly(PFPA) 융합 실리 카 구슬 항 체와 함께 움직일 수 및 IP 통해 단백질 농축 사용할 수 보여줍니다. 자세한 비드 준비 절차, 항 체 immobilization, 그리고 IP 테스트이 문서에 설명 되어 있습니다, 그리고 추구에 관심이 있는 독자에 대 한 기존의 단백질 A/G 대신 IP 기반.
Access restricted. Please log in or start a trial to view this content.
프로토콜
1입니다. Poly(PFPA) 단일 중합체의 준비
- AIBN의 recrystallization
- 2,2'-azobis(2-methylpropionitrile) (AIBN)의 5 g을 250ml 비 커에 메탄올 25 mL 결합. 60 ° C 기름 목욕에서 비 커를 담가 다음 적극적으로 AIBN 완전히 녹아 때까지 저 어 바 혼합물을 저 어.
- 필터 종이 (5-8 μ m 입자 보존)를 통해 따뜻한 해결책을 필터링 하 고 천천히 형태를 결정 수 있도록 4 ° C에서 여과 액을 저장.
- 여과 의해 박막된 AIBN을 수집 합니다. 신선한 메탄올 25 mL 수집된 제품 결합 고 recrystallization 프로세스를 반복 합니다.
- 하룻밤 실 온 (RT)에서 진공 오븐에서 박막된 AIBN x 2를 건조. <-10 ° c.에 어둠 속에서 제품을 저장
- 벤 dithiobenzoate25 의 합성
- 마그네틱 볶음 바, refluxing 콘덴서, 떨어지고 퍼 널 및 고무 심장 500 mL 3 목 라운드-하단 술병을 준비 합니다. Refluxing 콘덴서를 통해 질소 가스 라인에 플라스 크를 연결 하 고 내부 밖으로 플러시 질소와 공기. 심장 통해 온도계를 삽입 합니다. 같은 심장 통해 주사기를 통해 tetrahydrofuran (THF)에 41 mL (0.041 mol) phenylmagnesium 브 로마 이드의 1 분 솔루션을 추가 합니다.
- 따뜻한는 기름 목욕에서 40 ° C에 phenylmagnesium 브 로마 이드 솔루션. 다음 추가 떨어지고 퍼 널을 통해 이황화 탄소의 3.1 g (0.041 mol) 천천히, 40 ° c.에 솔루션 온도 유지
- 7.1 g (0.042 mol) 벤 질 브 로마 이드의 떨어지고 퍼 널 이상 15 분 증가 반응 온도 50 ° c.를 통해 결과 혼합물에 추가 45 분이 온도에서 교 반을 계속 합니다.
- Separatory 깔때기에 반응 혼합물을 전송 하 고 얼음 냉 수의 15 mL로 희석. Diethyl 에테르의 15 mL을 추가 하 여 제품을 추출 하 고 낮은 물 레이어를 제거 합니다. Diethyl 에테르로 추출 두 번 더 반복 합니다.
- 풍부한 양의 물, 다음 소금물 (50% (w/v) 물에서 NaCl의 솔루션)와 결합 된 유기 단계를 세척 하 고 무수 황산 마그네슘 이상 제품을 건조.
- 진공 회전 증발 기를 사용 하 여 35 ℃에서 용 매를 제거 합니다.
- 칼럼 크로마토그래피 eluent, 저조한 레드 오일으로 벤 dithiobenzoate (BDB)의 5 g으로 실리 카 젤 (기 공 크기 60 Å, 63-200 메쉬 입자 크기) 및 석유 에테르 400ml를 사용 하 여 제품을 정화. 1H NMR (400 MHz, CDCl3) 제품 순도 확인: δ 8.02-7.99 (2 H, m), 7.55-7.50 (1 H, m), 7.41-7.29 (7 H, m), 4.60 (2 H, s).
- 통해 뗏목 합9,26 poly(PFPA)의 합성
- 상용 PFPA 단위체 억제제의 소량을 포함 되어 있습니다. 중 합, 전에 억제제는 단위체 기본 알 루미나 포장 일회용 주사기를 통해 전달 하 여 제거 합니다.
- 20 mL Schlenk 플라스 크를 BDB, 억제제 무료 PFPA 1012 mg (4.25 mmol)과 무수 anisole의 0.7 mL의 박막된 AIBN, 4.3 mg (0.018 mmol)의 0.4 mg (0.0024 mmol)를 추가 합니다.
- Schlenk 선 플라스 크를 연결 하 고 적어도 3 개의 펌프 freeze-thaw 주기를 가진 혼합물을 드. 간단히, 동결 액체 질소 욕조에 반응 혼합물. Headspace에 가스를 제거 하는 진공을 적용 합니다. 플라스 크를 밀봉 다음 콘텐츠를 실시간에 녹여 수 있도록 액체 질소에서 제거
- 70 ° C 기름 목욕에서 플라스 크를 놓고 N2 제거에서 4 h 반응 합니다.
- 반응 종료, 기름 목욕에서 플라스 크를 제거 하 고 공기에 반응 한 내용을 표시 합니다.
- 차가운 메탄올에 폴리머를 침전 후 진공 오븐 40 ° C에 복구 된 폴리머 하룻밤 말리 면.
- 고분자 분자량 측정, 젤 투과 크로마토그래피 (GPC)를 사용 합니다. THF를 사용 하 여 1 mL/min 35 ° C에서 모바일 단계 유량 고 단 분산 폴리스 티 렌 표준을 사용 하 여 보정 곡선을 건설. GPC 측정을 취득, 0.2 μ m 일회용 소계 (PTFE) 필터를 통해 THF (1-2 mg/mL)와 필터에 폴리머를 분해. GPC에는 샘플의 100 μ를 주사. 폴리스 티 렌 교정 곡선을 사용 하 여 분자량을 측정된 샘플 보존 시간을 변환 합니다.
2. 준비 Poly(PFPA)의 공업화 SiO2 구슬
- 항으로 SiO2 구슬의 치료
- SiO2 입자는 5% (w/v) 수성 현 탁 액의 형태로 사용할 수 있습니다. 항 및 저 어 바 장비 20 mL 섬광 유리병에 메탄올의 8 mL 40 mg에 0.8 mL SiO2 서 스 펜 션의 결합.
- 활발 한 감동 5 h에 대 한 실시간 진행에 반응을 수 있습니다.
- 원뿔 튜브에 솔루션을 전송. 항을 공업화 SiO2 구슬, 원심에서 5 분, 10000 x g 솔루션 다음 제거는 상쾌한. 다시 3 mL 신선한 메탄올에에서 분산 하 여 구슬을 씻어. 혼합, 튜브를 손으로 흔들어 하지만 필요한 경우 향상 분산 몇 초 동안 물 욕조에 쥡니다. 원심 5 분 제거에 대 한 10000 x g에서 비즈는 상쾌한 고 한 번 더 세척 단계를 반복 합니다.
- 디 메 틸 sulfoxide (DMSO) 3 mL와 SiO2 구슬 씻어 메탄올을 결합 한다. 혼합물을 손으로 흔들어 또는 구슬 DMSO에 완벽 하 게 분산 때까지 몇 초 동안 sonicate 필요한 경우. 5 분 동안 10000 x g에서 구슬 원심 다음는 상쾌한을 제거 합니다. DMSO에 메탄올에서 완전 한 용 매 교환 되도록 단계를 반복 합니다.
참고: 마지막 정지 포함 기능성된 항 SiO2 구슬 DMSO의 4 mL에 분산. - 입자 크기 분포를 확인 하려면 DL 분석을 수행 합니다. 일회용 UV 멧으로 준비 단계 2.1.4와 장소에서 현 탁 액의 한 방울을 가져가 라. 그것은 2/3까지 신선한 DMSO와는 베트를 작성 하 여 샘플을 희석 전체. 샘플 데이터 수집을 시작 하려면 셀 홀더에 삽입 합니다. 입자 크기 측정을 위해 다음과 같은 설치 매개 변수를 사용 하 여: 온도: 25 ° C; 평형 시간: 120 s; 측정 기간: 자동.
- 표면 조성, 확인 하려면 XPS 분석을 수행 합니다. 준비 단계에서 40 ℃ 진공 오븐에서 2.1.4에서 하룻밤 정지에서 작은 샘플을 건조. 말린된 폴리머와 0.5 cm x 0.5 cm 샘플 홀더에 균일 하 게 팩을 가져가 라. 높은 진공 챔버 (10-8 torr)에 샘플을 로드 하 고 데이터 수집을 시작 합니다. 15에서 운영 단색 알 Kα X-ray를 사용 하 여 photoelectrons를 생성 하는 특정 XPS 악기 사용에 대 한 kV와 6.7 mA 및 수집 50 eV에서 분석기와 하이브리드 모드 확대를 사용 하 여 고해상도 스펙트럼에 대 한 에너지를 통과 하 고 100 eV 에너지 전달 대 한 원소 설문 조사.
- Poly(PFPA) 항에 접목 한 기능성 SiO2 구슬
- 20 mL 섬광 유리병에 DMSO의 2 mL에 poly(PFPA)의 20 밀리 그램을 용 해 하 여 poly(PFPA) 솔루션을 준비 합니다.
참고:이 연구는 상대적으로 낮은 분자량 poly(PFPA) (20 k g/mol) 사용 됩니다. 따라서, 고분자 농도 (10mg/mL)에 불구 하 고 고분자 가교의 증거가 관찰 됩니다. 높은 분자량의 폴리머를 사용 하는 폴리머 솔루션 농도 가능한 가교를 피하기 위해 조정 해야 할 수 있습니다. - 항의 1 mL 기능성 SiO2 구슬 poly(PFPA) 솔루션 (2.1.4 단계)에서 DMSO에 추가 합니다. 활발 한 교 반으로 1 시간에 대 한 RT에 반응.
- Poly(PFPA) 융합 SiO2 구슬 5 분 뒤에 상쾌한의 제거에 대 한 10000 x g에서 원심 분리 하 여 격리 합니다. 어느 손 또는 쥡니다의 몇 초 떨고 DMSO와 섞어 3 mL를 추가 하 여 구슬을 씻어. 5 분 동안 10000 x g에서 구슬 원심 다음는 상쾌한을 제거 합니다. DMSO와 poly(PFPA) 융합 SiO2 구슬의 세척 두 번 반복 합니다.
- 워시 트리플 증류수 (TDW)으로 두 배 이상 구슬. 이 단계에서 TDW의 3 mL 구슬 결합 다음 손이나 쥡니다의 몇 초를 흔들어 혼합. 5 분 동안 10000 x g에서 구슬 원심 다음는 상쾌한을 제거 합니다.
- 입자 크기 분포를 확인 하려면 DL 2.1.5 단계에 설명 된 절차를 다음을 수행 합니다. 표면 화학을 확인 하려면 XPS 2.1.6 단계에 설명 된 절차를 다음을 수행 합니다.
- 20 mL 섬광 유리병에 DMSO의 2 mL에 poly(PFPA)의 20 밀리 그램을 용 해 하 여 poly(PFPA) 솔루션을 준비 합니다.
3. SiO2 의 준비 구슬 못 대체 Poly(PFPA)와 융합
- Poly(PFPA) 솔루션을 준비, 20mg 20 mL 섬광 유리병에 DMSO의 2 mL에 poly(PFPA)의 분해.
- 못 솔루션을 준비, DMSO의 1 mL에 아민 기능성된 못 분해. 말뚝의 정확한 양을 사용 PFP 대체, 아래 방정식에 의해 결정의 원하는 정도에 따라 결정 됩니다:
양의 아미노 말뚝 (g/g-poly(PFPA)) = (N_poly(PFPA) x % 말뚝-서브) x (MW_PEG / MW_poly(PFPA))
여기서 N_poly(PFPA) = poly(PFPA)도 중 합
% 말뚝-서브 % 말뚝 대체 =
MW_PEG 아미노 PEG의 분자량 =
MW_ poly(PFPA) poly(PFPA)의 분자량 = - Poly(PFPA) 솔루션을 페그 솔루션을 전송 합니다. 활발 한 교 반으로 1 시간에 대 한 RT에 반응.
- 항을 준비 하려면 기능성 DMSO에 SiO2 구슬, 같은 단계 2.1에 표시 된 단계에 따라. 3.3 단계에서에서 준비 못 대체 poly(PFPA) 솔루션으로 비드 서 스 펜 션의 1 mL를 전송 합니다. Poly(PFPA) 고 항 간의 접목 기능성 SiO2 구슬 활발 한 교 반으로 1 시간에 대 한 실시간 진행을 허용 합니다.
- 5 분 뒤에 상쾌한의 제거에 대 한 10000 x g에서 원심 분리 하 여 구슬을 격리 합니다. 어느 손 또는 쥡니다의 몇 초 떨고 DMSO와 섞어 3 mL를 추가 하 여 구슬을 씻어. 5 분 동안 10000 x g에서 구슬 원심 다음는 상쾌한을 제거 합니다. DMSO 세척 두 번 반복 합니다.
- 워시 TDW와 두 번 더 구슬. 이 단계에서는 3 mL TDW, 구슬 결합 다음 손이나 쥡니다의 몇 초를 흔들어 혼합. 5 분 동안 10000 x g에서 구슬 원심 다음는 상쾌한을 제거 합니다.
- 하룻밤 건조 진공 오븐에서 40 ° C에서 구슬.
4. 항 체 Immobilization Poly(PFPA)에 투입 SiO2 구슬
참고: 동일한 절차는 poly(PFPA)에 % 말뚝 대체에 사용 됩니다. TDW에서 PBS 태블릿을 용 해 하 여 버퍼링 하는 인산 염 (PBS)를 준비 합니다. PBS에 트윈-20의 1/1000을 추가 하 여 0.1% (v/v) 버퍼링 인산 염 분 트윈-20 (PBST)와 함께 준비 합니다.
- Poly(PFPA)의 5 mg 투입 SiO2 구슬 1.5 mL microcentrifuge 튜브를 추가 합니다.
- Vortexing에 의해 잘 800 µ L의 PBS와 믹스를 추가 하 여 구슬을 씻어. 원심 1 분 제거에 대 한 실시간에 10000 x g에서 비즈는 상쾌한 고 세 번 세척 단계를 반복 합니다.
- 신선한 PBS, 50 µ L 0.1% (v/v) PBST, 및 항 체의 6.67 µ g 350 µ L를 추가 합니다. ~ 20 h 4 ° c.에 회전자에 품 어
- 워시 언바운드 항 체를 제거 하는 구슬. 400 x g에서 구슬 원심 1 분에 4 ° C는 상쾌한을 제거 하 고 세포의 용 해 버퍼의 400 µ L를 신중 하 게 추가. 부드럽게 다시 5 번 위아래로 pipetting으로 구슬 정지.
참고: 세포의 용 해 버퍼 구슬 세척 하는 데 사용 해야 같은 세포 세포의 용 해 및 IP, 사용 제외 하 고 dithiothreitol 및 프로 테아 제 억제제의 추가 선택 사항 (단계 5 참조). - 이 세척 단계를 세 번 반복 합니다. 최종 세척 후 상쾌한 가능한 한 많이 제거 합니다.
5. 세포 세포의 용 해 및 Immunoprecipitation
- 세포의 용 해 버퍼와 워시 버퍼의 준비
- 세포의 용 해 버퍼 (50 m m Tris HCl (pH 8.0), 100 m m KCl, 0.5% (v/v) NP-40, 10% (v/v) 글리세롤, 1 m m dithiothreitol (DTT), 그리고 프로 테아 제 억제 물 칵테일)를 준비 합니다.
- 워시 버퍼 (50 mM Tris-HCl (pH 8.0), 100 m m KCl, 0.1% (v/v) NP-40, 및 10% (v/v) 글리세롤)를 준비 합니다.
- 4 ° c.에 버퍼 솔루션 저장
- 셀의 준비
- 시드합니다 세포 (HeLa 세포) IP 실험 하기 전에 하나 또는 두 일 37 ° C, 5% CO2세포를 성장 하 고.
- 15 mL 원뿔 튜브에 약 1.4 x 10 셀 스 크레이 퍼와 전송7 셀을 수집 합니다. 원심 분리기 3 분에 대 한 RT에서 380 x g에서 세포는 상쾌한을 제거 하 고 다시 1 mL의 PBS 춥고 1.5 mL microcentrifuge 튜브로 전송 된 일시 중단
- 30 미 제거 상쾌한 청결을 위한 4 ° C에서 10000 x g에서 세포를 원심. 셀 펠 릿은 상쾌한 제거 후-80 ° C에 저장할 수 있습니다.
- 세포 lysates의 준비
- 다시 세포의 용 해 버퍼의 400 µ L로 셀 펠 릿을 일시 중단 합니다. ultrasonicator을 사용 하 여 셀 sonicate
- 쥡니다, 짧게 소용돌이 및 원심 분리기 lysate 20000 x g 4 ° c에서 10 분 후.
- 새로운 1.5 mL 원심 분리기 튜브는 상쾌한 전송.
- Immunoprecipitation
- 알을 품는 이전 준비 항 체 융합 poly(PFPA) SiO2 구슬에 세포 lysate의 300 µ L를 전송. 새로운 microcentrifuge 튜브에서 입력된 샘플으로 lysate 셀의 30 µ L을 유지 합니다. 4 ° c.에 입력된 샘플 저장
참고: 세포 lysate에서 단백질의 총 금액은 약 4 밀리 그램 해야 합니다. - 4 ° c.에 회전에 3 h lysate/구슬 혼합물을 품 어
- 원심 1 분 제거를 위한 4 ° C에 400 x g에서 혼합물은 상쾌한 고 워시 버퍼의 400 µ L를 신중 하 게 추가 합니다. 부드럽게 다시 아래로 약 5 배 pipetting으로 구슬 정지.
- 이 세척 단계를 세 번 반복 합니다. 최종 세척 후 상쾌한 가능한 한 많이 제거 합니다.
- 나트륨 라우릴 황산 염 (SDS) 염료 (25% (v/v) 글리세롤, 0.1% (w/v) 브로 모 페 놀 블루 (BPB), 60 mM Tris HCl (pH 6.8), 2% (w/v) SDS, 그리고 2.75 m m 2-mercaptoethanol) 로드 x 2를 준비 합니다. -20 ° c.에 2 x SDS 로드 염료를 저장 구슬과 저장된 입력된 샘플을 SDS 로드 염료 x 2의 30 µ L을 추가 하 고 95 ° c.에서 10 분 동안 그들을 열합니다
- 난방, 후27, 더럽혀 서를 사용 하 여 샘플 분석 또는-20 ° c.에 샘플 저장
- 알을 품는 이전 준비 항 체 융합 poly(PFPA) SiO2 구슬에 세포 lysate의 300 µ L를 전송. 새로운 microcentrifuge 튜브에서 입력된 샘플으로 lysate 셀의 30 µ L을 유지 합니다. 4 ° c.에 입력된 샘플 저장
Access restricted. Please log in or start a trial to view this content.
결과
Poly(PFPA)의 준비에 대 한 회로도와 SiO2 구슬, 투입 하거나 못 없이 대체 그림 1에 표시 됩니다. 항와 접목 과정, 맨 손으로 SiO2 구슬, poly(PFPA) 항 기능성 SiO2 구슬, 그리고 poly(PFPA) 융합 SiO2 구슬 DL (그림 2)와 XPS (그림 3)에 의해 특징입니다. 구슬의 IP 효율성 서쪽 blotting에 의해 ?...
Access restricted. Please log in or start a trial to view this content.
토론
Poly(PFPA)의 합성 SiO2 구슬은 그림 1에 나와 있는 투입. 채용 함으로써 항으로 링커 분자, poly(PFPA) 브러쉬 covalently SiO2 기판에 투입 간단한 2 단계 프로세스를 통해 준비 될 수 있습니다. PFP 단위 중 일부 항에 대 한 반응에 대 한 희생은, 비록 많은 PFP 단위 아미노 말뚝 또는 항 체 이상 반응에 대 한 사용할 수 있는 예상 된다. PFP 그룹 poly(PFPA) 브러쉬 할 물
Access restricted. Please log in or start a trial to view this content.
공개
저자는 공개 없다.
감사의 말
이 작품 국방 개발 (부여 번호에 대 한 기관에 의해 지원 되었다 UD170039ID)입니다.
Access restricted. Please log in or start a trial to view this content.
자료
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
참고문헌
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
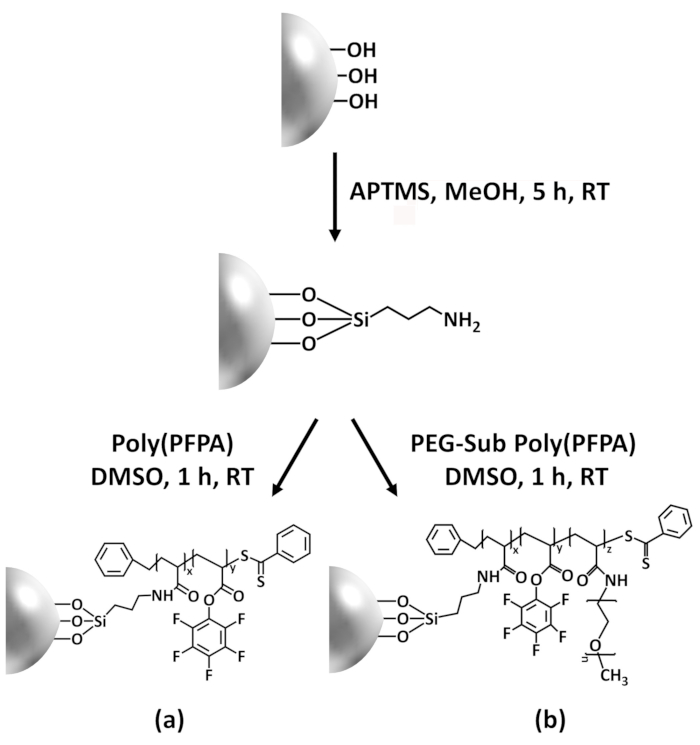
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
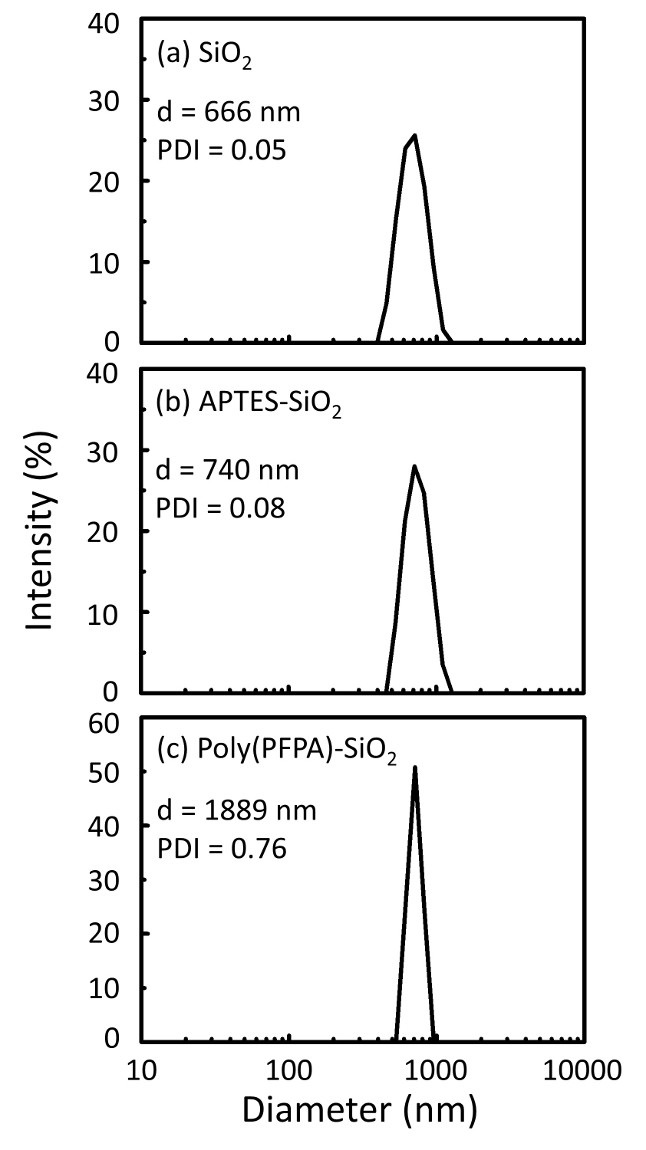
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
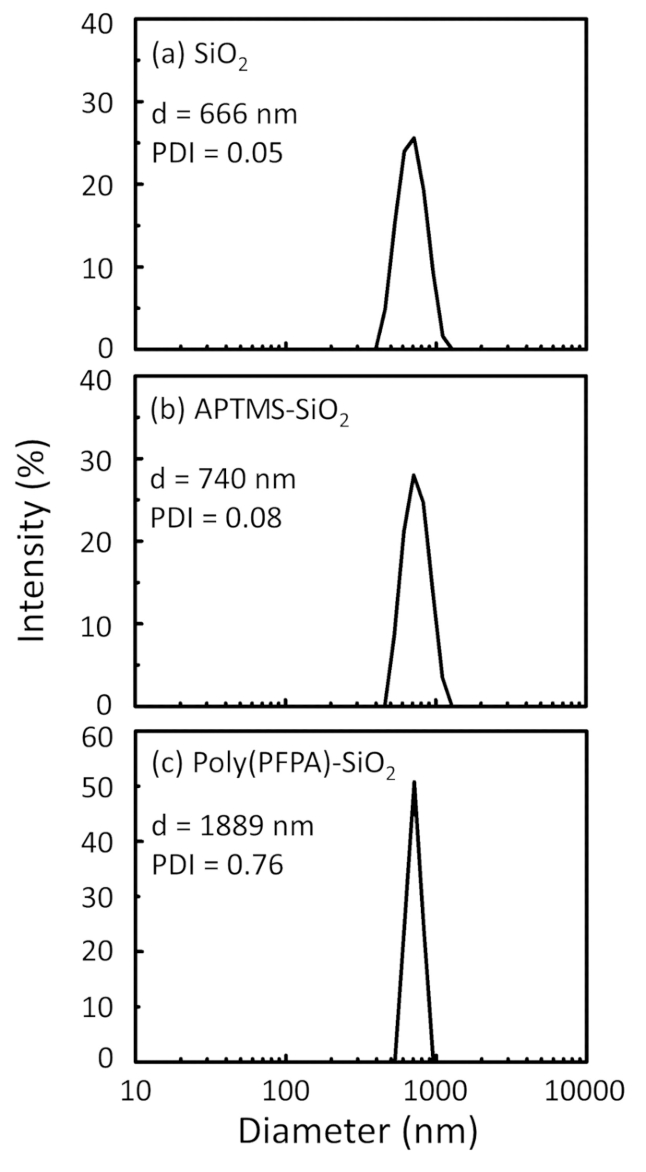
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
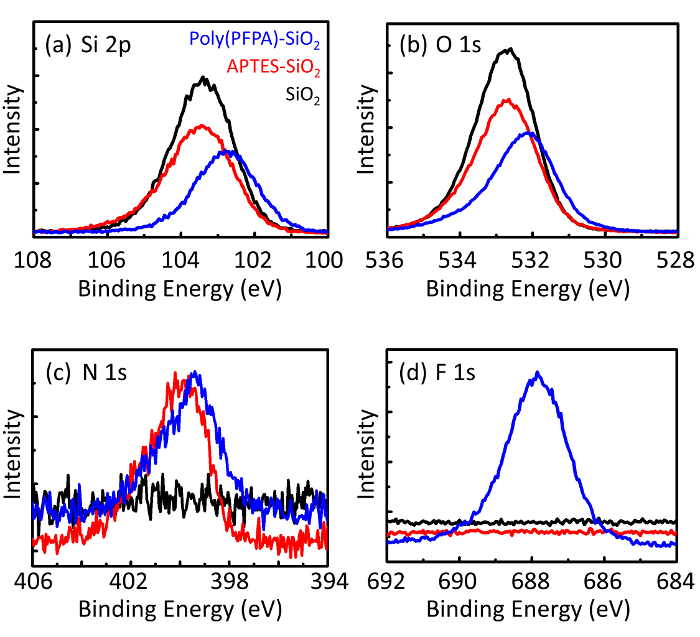
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
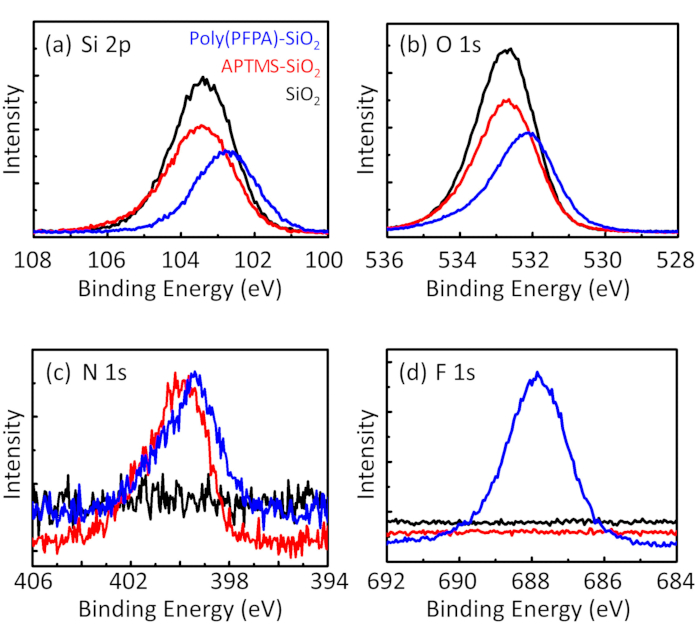
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유