Для просмотра этого контента требуется подписка на Jove Войдите в систему или начните бесплатную пробную версию.
Method Article
Подготовка Poly(pentafluorophenyl acrylate) функционализированных SiO2 бусины очищение протеина
В этой статье
Erratum Notice
Резюме
Протокол для подготовки поли (пентафторфенилгидразин акрилатные) (poly(PFPA)) привитые кремнезема бусины представлены. Функционализированных поверхность poly(PFPA) затем прикол с антителами и успешно используется для разделения белков через иммунопреципитации.
Аннотация
Мы демонстрируем простой способ подготовить поли (пентафторфенилгидразин акрилатные) (poly(PFPA)) привитые кремнезема Бусины для иммобилизации антитела и последующих иммунопреципитации (IP) приложения. Poly(PFPA) привитые поверхность готова через простой двухэтапный процесс. На первом шаге как компоновщик молекулы на поверхности кремния осаждается 3-aminopropyltriethoxysilane (APTES). На втором шаге, poly(PFPA) гомополимер, синтезированных через реверсивный сложения и фрагментации цепной передачи (РАФТ) полимеризации, является привитыми к молекуле компоновщик через реакции обмена между пентафторфенилгидразин (ПРМ) единиц на полимерные и амины группы на APTES. Осаждения APTES и poly(PFPA) на кремний частицы являются подтверждается Рентгеновская фотоэлектронная спектроскопия (XPS), а также контролируется изменения размера частиц измеряется через Динамическое рассеяние света (DLS). Для улучшения поверхности гидрофильность бусы, частичная замена poly(PFPA) с Амин функционализированных poly(ethylene glycol) также производится (амино PEG). ПЭГ замещенных poly(PFPA) привитые кремнезема, который бусины затем прикол с антитела для IP приложения. Для демонстрации занятых антитело против протеинкиназы РНК активированный (PKR), и эффективность IP определяется Западный blotting. Результаты анализа показывают, что бусины иммобилизованные антитела действительно может использоваться для обогащения PKR, в то время как неспецифический белковых взаимодействий являются минимальными.
Введение
Реактивные полимерной щетки получили большой интерес в последние годы. Они могут быть использованы для иммобилизации функциональные молекулы органических или неорганических материалов для создания активированных поверхностей с приложениями в таких областях, как обнаружение и разделения1,2,3,4, 5. Среди полимеров сообщили содержащие пентафторфенилгидразин эфира единицы являются особенно полезными ввиду их высокой реактивности с аминами и стойкость к гидролизу6. Один из таких полимеров является poly(PFPA), и она может быть легко функционализированных после полимеризации с молекул, содержащих первичных или вторичных аминов7,8,9,10. Одним из примеров poly(PFPA) щетки были прореагировало с амино spiropyrans для создания поверхности свет отзывчивым7.
Подготовка poly(PFPA) и его приложений были описаны в ряде предыдущих публикаций6,,78,9,10,11,12 ,13,14,,1516,17. В частности Theato и коллеги сообщили синтез poly(PFPA) щетки через «прививки» и «прививки от «методы7,8,10,11,12 . В «прививки» подход, поли (methylsilsesquioxane)-поли (пентафторфенилгидразин акрилатные) (poly(MSSQ-PFPA)) гибридный полимер был синтезированных8,10,,1112. Компонент poly(MSSQ) был в состоянии формы сильная адгезия с рядом различных органических и неорганических поверхностей, таким образом позволяя poly(PFPA) компонент для формирования кистью слой на поверхности с покрытием материала. В «прививки от «подход, поверхность начато реверсивные сложения и фрагментации цепной передачи (SI-плот) полимеризации был нанят подготовить poly(PFPA) щетки7. В этом случае агент передачи поверхности иммобилизованных цепи (SI-CTA) был впервые ковалентно присоединяется к подложке через кремний силана реакции. Иммобилизованных SI-CTA затем участвовала в SI-плот полимеризации мономеров Стихийного, генерации плотно упакованных poly(PFPA) щетки с стабильной ковалентная связь к подложке.
Используя кисти poly(PFPA), синтезированных через SI-плот полимеризации, мы недавно продемонстрировали иммобилизации антител на poly(PFPA) привитые частиц кремнезема и их последующего применения в очистки белков18. Использование poly(PFPA) щетки для иммобилизации антитело было обнаружено решить ряд вопросов, связанных с текущей разделение белков через IP. Обычные IP основывается на использовании белка A/G как компоновщик для антитела иммобилизации19,,2021. Поскольку использование белка A/G позволяет антитела к быть присоединен с конкретной ориентации, высокие цели антигена восстановления эффективность достигается. Однако использование белка A/G страдает от неспецифических белков взаимодействия, а также потеря антител при восстановлении белка, оба из которых способствуют высокий уровень фонового шума. Для устранения этих недостатков, прямые сшивки антител к твердой поддержки был изучены22,,23-24. Эффективность таких методов обычно низка из-за случайной ориентации crosslinked антителами. Для poly(PFPA) привитые субстрата иммобилизация антител является постоянным, благодаря реакции обмена между подразделениями ПРМ и Амин функциональности на антитела. Хотя антитела ориентации по-прежнему случайных, система преимущества при использовании многих реактивной ПРМ сайты, управляемые по степени полимеризации. Кроме того мы показали, что путем частичной замены ПРМ единиц с амино PEG, гидрофильность поверхности могут быть настроены, дальнейшего повышения эффективности белка восстановления системы18. В целом частиц кремнезема poly(PFPA) привитые были продемонстрированы быть эффективной альтернативой традиционным IP с разумной эффективности, а также много чистых фона.
В этот вклад мы сообщаем альтернативный метод для подготовки poly(PFPA) привитые поверхность для иммобилизации антитела и IP приложения. В простой двухэтапный процесс, как показано на рисунке 1, молекула APTES компоновщика сначала осаждается на поверхности кремния, то poly(PFPA) полимер ковалентно прикрепляется к молекуле компоновщик через реакции между ПРМ единиц на полимерные и Амин функции на APTES. Этот метод подготовки позволяет постоянным сшивки poly(PFPA) к поверхности субстрата, но избегает многих осложнений, связанных с SI-CTA синтеза и SI-плот полимеризации poly(PFPA) кистей. Можно по-прежнему выполняться частичного замещения ПРМ единиц с амино PEG, позволяет тонкой настройки свойств поверхности кисти полимера. Мы покажем, poly(PFPA) привитые кремнезема бусы подготовленный таким образом может быть прикол с антителами и используется для обогащения белка через IP. Процедура подготовки подробных шарик, антитела иммобилизации и IP тестирования документируются в этой статье, для читателей, заинтересованных в поисках альтернативы обычного белка A/G на основе IP.
Access restricted. Please log in or start a trial to view this content.
протокол
1. Подготовка гомополимер Poly(PFPA)
- Рекристаллизация Динитрила
- Комбинат 5 g 2,2'-azobis(2-methylpropionitrile) (АО) с 25 мл метанола в стакан 250 мл. Погружать стакан в масляной ванне 60 ° C, а затем энергично перемешать смесь с баром перемешать до полного растворения Динитрила.
- Фильтр теплым раствором через фильтровальную бумагу (удержание частиц 5-8 мкм) и хранить фильтрата на 4 ° C, чтобы позволить кристаллов форме медленно.
- Соберите рекристаллизованном Динитрила фильтрации. Совместить собранного продукта с 25 мл свежего метанола и повторите процесс перекристаллизации.
- Сухие 2 x рекристаллизованном Динитрила в вакуумной печи при комнатной температуре (RT) на ночь. Хранить продукт в темноте в <-10 ° C.
- Синтез бензил dithiobenzoate25
- Подготовка 500 мл три шея раунд нижней колбе оснащены магнитной перемешать бар, refluxing конденсатора, воронку снижается и резиновые перегородки. Подключите колбы к линии газ азот через конденсатор refluxing и промыть внутри воздуха азотом. Вставьте термометр через носовой перегородки. Добавление 41 мл (0,041 моль) 1 М раствора бромида phenylmagnesium в тетрагидрофуран (THF) через шприц через же перегородки.
- Теплый phenylmagnesium бромида раствор до 40 ° C в масляной ванне. Затем добавьте 3.1 g (0,041 моль) сероуглерод через воронку снижается медленно, поддержание температуры раствора на 40 ° C.
- Добавьте 7.1 g (0,042 моль) бензиловый метила в полученную смесь через воронку снижается более чем 15 мин увеличение температуры реакции до 50 ° C. При этой температуре 45 мин по-прежнему перемешивания.
- Передача смеси реакции в separatory воронку и разбавляют 15 мл холодной воды, льда. Извлечь продукт, добавив 15 мл диэтиловым эфиром и удалите нижний слой воды. Повторите экстракции диэтиловым эфиром еще два раза.
- Вымойте комбинированных органические фазы с большим количеством воды, затем рассол (раствор 50% (w/v) NaCl в воде) и сухого продукта за безводный сульфат магния.
- Удаление растворителя в вакууме при 35 ° C, с помощью роторный испаритель.
- Очищайте продукт от колоночной хроматографии с использованием 400 мл силикагель (размер пор 60 Å, 63-200 сетка размер частиц) и петролейный эфир как элюента, уступая 5 g бензил dithiobenzoate (BDB) как Красного масла. 1H ЯМР (400 МГц, CDCl3) для подтверждения чистоты продукта: δ 8.02-7,99 (2 H, m), 7.55-7.50 (Ч. 1, м), 7.41-7,29 (7 H, m), 4.60 (2 H, s).
- Синтез poly(PFPA) через плот полимеризации9,26
- Коммерчески доступные Стихийного мономера содержит небольшое количество ингибиторов. До полимеризации удалите ингибиторы, передав мономер через одноразовый шприц, Упакованные с основными глинозема.
- Добавьте 0,4 мг (0.0024 ммоль) рекристаллизованном Динитрила, 4,3 мг (0,018 ммоль) BDB, 1012 мг (4.25 ммоль) ингибитор бесплатно Стихийного и 0,7 мл безводного Анизол Шленк флакон 20 мл.
- Соедините колбу Шленк линию и Дега в смеси с по крайней мере трех циклов замораживания насос оттаивания. Вкратце заморозить реакционной смеси в ванну жидкого азота. Применение вакуума удалить газ в headspace. Печать колбу, затем удалите от жидкого азота для оттаивания на RT. содержимого
- Фляга в масляной ванне 70 ° C и реагируют на 4 h под N2 очистка.
- Для завершения реакции, удалите фляга из масляной ванне и предоставления контента реакции воздуха.
- Осадок полимера в холодных метанола, а затем высушите восстановленные полимера в вакуумной печи при температуре 40 ° C на ночь.
- Чтобы измерить молекулярная масса полимера, используйте гель пропитывание хроматографии (ГКТ). Используйте ТГФ как подвижная фаза при 35 ° C с 1 мл/мин скорость потока и построения калибровочной кривой с помощью монодисперсных полистирола стандартов. Приобрести GPC измерения, Растворите полимера в ТГФ (1-2 мг/мл) и фильтра через 0,2 мкм фильтр одноразовые политетрафторэтилена (ПТФЭ). Inject 100 мкл пример в GPC инструмент. Преобразование времени удержания измеряемых образца в молекулярный вес с помощью пенополистирола калибровочной кривой.
2. Подготовка Poly(PFPA) функционализированных SiO2 бусины
- Лечение SiO2 бусины с APTES
- SiO2 частицы доступны в виде водной суспензии 5% (w/v). Объединить 0,8 мл SiO2 подвеска с 40 мг APTES и 8 мл метанола в 20 мл флаконе Сцинтилляционный с баром перемешать.
- Разрешить реакции перейти на RT для 5 h с энергичным перемешиванием.
- Решение передать Конические трубки. Чтобы изолировать APTES функционализированных SiO2 бусины, центрифуга решение на 10000 x g 5 минут, а затем удалить супернатант. Вымойте бисер, повторно рассеивая их в 3 мл свежего метанола. Shake трубки вручную для смешивания, но при необходимости улучшения дисперсии, sonication на водяной бане в течение нескольких секунд. Центрифуга бусы на 10000 x g для 5 минут удалить супернатант и повторите шаг мыть еще один раз.
- Объединить метанола, промывают SiO2 бусины с 3 мл диметилсульфоксида (ДМСО). Встряхните смесь вручную, или если необходимо sonicate на несколько секунд, до тех пор, пока бисер полностью рассеяны в ДМСО. Центрифуга бусы на 10000 x g 5 минут, а затем удалить супернатант. Повторите шаг для обеспечения полной растворителей обмена от метанола в ДМСО.
Примечание: Окончательный суспензии содержит APTES функционализированных SiO2 бусины рассеяны в 4 мл ДМСО. - Чтобы проверить распределение частиц по размерам, анализ DLS. Возьмите одну каплю подвеска, подготовленный в шаге 2.1.4 и место в одноразовых кювет УФ. Разбавить образца, заполнив кювета с свежим ДМСО, до тех пор, пока это 2/3 полной. Вставьте образец в ячейку держатель для начала сбора данных. Для измерения размера частиц, используйте следующие параметры: температура: 25 ° C; Уравновешивания время: 120 сек; Длительность измерения: автоматическая.
- Чтобы проверить состав поверхности, анализ XPS. Сухие небольшую выборку из подвеска, подготовленный на шаге 2.1.4 в вакуумной печи при температуре 40 ° C на ночь. Возьмите сушеные полимера и пакет равномерно на держатель образца 0,5 х 0,5 см. Загрузить образец в высокой вакуумной камеры (10-8 торр) и начать сбор данных. Для использования конкретного инструмента XPS, генерировать фотоэлектронов, с использованием монохроматического рентгеновского Аль Kα, работал на 15 кв и 6.7 мА и собирать с помощью гибридный режим увеличения с помощью анализатора в 50 eV передать энергию для спектры высокого разрешения, и 100 eV энергии для элементарного обследований.
- Прививки poly(PFPA) для APTES функционализированных SiO2 бусины
- Приготовляют раствор poly(PFPA), растворяя 20 мг poly(PFPA) в 2 мл ДМСО в сцинтилляционном флакон 20 мл.
Примечание: В данном исследовании используется сравнительно низкой молекулярной массой poly(PFPA) (20 кг/моль). Таким образом несмотря на высокие полимерных концентрации (10 мг/мл), наблюдается никаких доказательств сшивки полимеров. Если используется выше молекулярная масса полимера, затем концентрация раствора полимера может потребоваться быть скорректирована во избежание возможных сшивки. - Добавьте 1 мл APTES функционализированных SiO2 бусины приостановлено в ДМСО (от шага 2.1.4) к решению poly(PFPA). Реагировать на RT 1 h с энергичным перемешиванием.
- Изолируйте poly(PFPA) привитые SiO2 бусины центрифугированием на 10000 x g 5 мин, после удаления супернатант. Вымойте бисер, добавив 3 мл ДМСО и микс либо пожав руку или несколько секунд sonication. Центрифуга бусы на 10000 x g 5 минут, а затем удалить супернатант. Повторите Стиральная poly(PFPA) привитые SiO2 бусины с ДМСО дважды.
- Вымойте бусины в два раза больше с тройной дистиллированной воды (TDW). В этот шаг сочетать бисер с 3 мл TDW, а затем смешать, пожав руку или несколько секунд sonication. Центрифуга бусы на 10000 x g 5 минут, а затем удалить супернатант.
- Чтобы проверить распределение частиц по размерам, выполняют DLS следуя процедуре, описанной в шаге 2.1.5. Чтобы проверить химии поверхности, выполняют после процедуры, описанной в шаге 2.1.6 XPS.
- Приготовляют раствор poly(PFPA), растворяя 20 мг poly(PFPA) в 2 мл ДМСО в сцинтилляционном флакон 20 мл.
3. Подготовка SiO2 бусины привитый с ПЭГ замещенных Poly(PFPA)
- Подготовить раствор poly(PFPA), растворяют 20 мг poly(PFPA) в 2 мл ДМСО в сцинтилляционном флакон 20 мл.
- Для приготовления раствора ПЭГ, Растворите Амин функционализированных КОЛЫШЕК в 1 мл ДМСО. Точное количество PEG используется определяется требуемой степени замещения ПРМ, определяется уравнением, показано ниже:
Количество аминокислот ПЭГ (g/g-poly(PFPA)) = (N_poly(PFPA) х % PEG-Sub) x (MW_PEG / MW_poly(PFPA))
где N_poly(PFPA) = poly(PFPA) степень полимеризации
% PEG-Sub = процент PEG замещения
MW_PEG = молекулярная масса амино PEG
MW_ poly(PFPA) = молекулярная масса poly(PFPA) - PEG решение передать poly(PFPA) решения. Реагировать на RT 1 h с энергичным перемешиванием.
- Подготовить APTES функционализированных SiO2 бусины приостановлено в ДМСО, следуйте же шаги, указанные в шаге 2.1. Перевести 1 мл суспензии шарик в PEG-замещенных poly(PFPA) раствор, приготовленный в шаге 3.3. Разрешить наращивание между poly(PFPA) и APTES функционализированных SiO2 бусины перейти на RT 1 h с энергичным перемешиванием.
- Изолируйте бисер центрифугированием на 10000 x g 5 мин, после удаления супернатант. Вымойте бисер, добавив 3 мл ДМСО и микс либо пожав руку или несколько секунд sonication. Центрифуга бусы на 10000 x g 5 минут, а затем удалить супернатант. Повторите ДМСО мыть дважды.
- Вымойте бусины в два раза больше с TDW. В этот шаг сочетать бисер с 3 мл TDW, а затем смешать, пожав руку или несколько секунд sonication. Центрифуга бусы на 10000 x g 5 минут, а затем удалить супернатант.
- Сухие шарики на 40 ° C в вакуумной печи на ночь.
4. антитела иммобилизации на Poly(PFPA) привитые SiO2 бусины
Примечание: Такая же процедура используется независимо от процент PEG замещения на poly(PFPA). Подготовка-фосфатный буфер (PBS), растворяя PBS таблетка в TDW. Подготовьте 0,1% (v/v)-фосфатный буфер с Tween-20 (PBST), добавляя 1/1000 Tween-20 в PBS.
- Добавьте 5 мг poly(PFPA) привитые SiO2 Бусины для пробки microcentrifuge 1,5 мл.
- Вымойте бусы путем добавления 800 мкл PBS и перемешать хорошо vortexing. Центрифуга бусы на 10000 x g в РТ за 1 мин удалить супернатант и мыть Повторите три раза.
- 350 мкл свежие PBS, 50 мкл 0,1% (v/v) PBST и 6.67 мкг антитела. Инкубировать ~ 20 h на вращателе при 4 ° C.
- Вымойте бусины удалить несвязанные антитела. Центрифуга бусы на 400 x g и 4 ° C в течение 1 мин удалить супернатант и 400 мкл буфера lysis тщательно. Аккуратно вновь приостановите бисер, закупорить вверх и вниз в пять раз.
Примечание: Литического буфера используется для стирки бисер должен быть же используется во время лизис клеток и IP, за исключением того, что добавление ингибитора протеазы и Дитиотреитол являются необязательными, (см. шаг 5). - Повторите этот шаг мыть три раза. После окончательного вымыть удалите супернатант насколько это возможно.
5. ячейки Lysis и иммунопреципитации
- Подготовка буфера lysis и мыть буфера
- Подготовьте литического буфера (50 Tris-HCl (рН 8,0), 100 мм мм KCl, 0,5% (v/v) NP-40, 10% (v/v) глицерина, 1 мм Дитиотреитол (DTT) и протеазы ингибитор коктейль).
- Подготовьте мыть буфера (50 мм трис-HCl (рН 8,0), 100 мм KCl, 0,1% (v/v) NP-40 и 10% (v/v) глицерин).
- Хранить буферных растворов при 4 ° C.
- Подготовка клетки
- Семян (клетки HeLa) один или два дня до IP эксперимент и расти клеток при 37 ° C и 5% CO2.
- Соберите около 1.4 x 107 клетки с скребок клетки и передачи в коническую пробирку 15 мл. Центрифуга клетки на 380 x g на RT на 3 мин удалить супернатант и вновь приостановить с 1 мл холодного PBS и передачи в пробки microcentrifuge 1,5 мл.
- Центрифуга клетки на 10000 x g при 4 ° C за 30 с. удалить супернатант чисто. Клетки гранулы могут храниться при температуре-80 ° C после удаления супернатант.
- Подготовка lysates клетки
- Вновь приостановите клетки лепешка с 400 мкл буфера lysis. Sonicate клетки, с помощью ultrasonicator.
- После sonication, вихревой кратко и центрифуги lysate на 20000 x g при 4 ° C на 10 мин.
- Передать супернатант новых пластиковых пробирок 1.5 мл.
- Иммунопреципитация
- Перевести 300 мкл lysate клетки poly(PFPA) привитые ранее подготовленных Антитела инкубированы SiO2 бусины. Сохраните 30 мкл lysate как образца ввода в новой пробки microcentrifuge ячейки. Храните образца ввода при 4 ° C.
Примечание: Общее количество белка в lysate клетки должно быть примерно 4 мг. - Инкубировать lysate/бисер смесь для 3 h на вращателе при 4 ° C.
- Центрифуга смеси на 400 x g при 4 ° C в течение 1 мин удалить супернатант и 400 мкл буфера мытья тщательно. Аккуратно вновь приостановите бисер, закупорить вверх и вниз примерно в пять раз.
- Повторите этот шаг мыть три раза. После окончательного вымыть удалите супернатант насколько это возможно.
- Готовить 2 x лаурилсульфат натрия (SDS) Загрузка краситель (глицерин 25% (v/v), 0,1% (w/v) bromo фенола синий (BPB), 60 мм трис-HCl (рН 6,8), 2% (w/v) SDS и 2,75 мм 2-меркаптоэтанол). Хранить краски загрузки SDS 2 x при-20 ° C. 30 мкл 2 x SDS загрузки краситель бисер и образце хранимой ввода и тепло их за 10 мин при 95 ° C.
- После нагрева, анализа образца с помощью западных blotting27, или хранить образца при-20 ° C.
- Перевести 300 мкл lysate клетки poly(PFPA) привитые ранее подготовленных Антитела инкубированы SiO2 бусины. Сохраните 30 мкл lysate как образца ввода в новой пробки microcentrifuge ячейки. Храните образца ввода при 4 ° C.
Access restricted. Please log in or start a trial to view this content.
Результаты
Схема для подготовки poly(PFPA) привитые SiO2 бусины, с или без ПЭГ замены показан на рисунке 1. Для контроля за APTES и poly(PFPA), прививки процесса, голые SiO2 бусины, APTES функционализированных SiO2 бусины, и poly(PFPA) привитые SiO2 бусины характеризуют...
Access restricted. Please log in or start a trial to view this content.
Обсуждение
Синтез poly(PFPA) привитые SiO2 бусины иллюстрируется на рисунке 1. Используя APTES как компоновщик молекулы, poly(PFPA) щетки ковалентно привитыми к SiO2 субстрата может быть подготовлен через простой двухэтапный процесс. Хотя некоторые подразделения ПРМ приносятся в жер?...
Access restricted. Please log in or start a trial to view this content.
Раскрытие информации
Авторы не имеют ничего сообщать.
Благодарности
Эта работа была поддержана агентство развития (Грант № обороны UD170039ID).
Access restricted. Please log in or start a trial to view this content.
Материалы
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
Ссылки
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
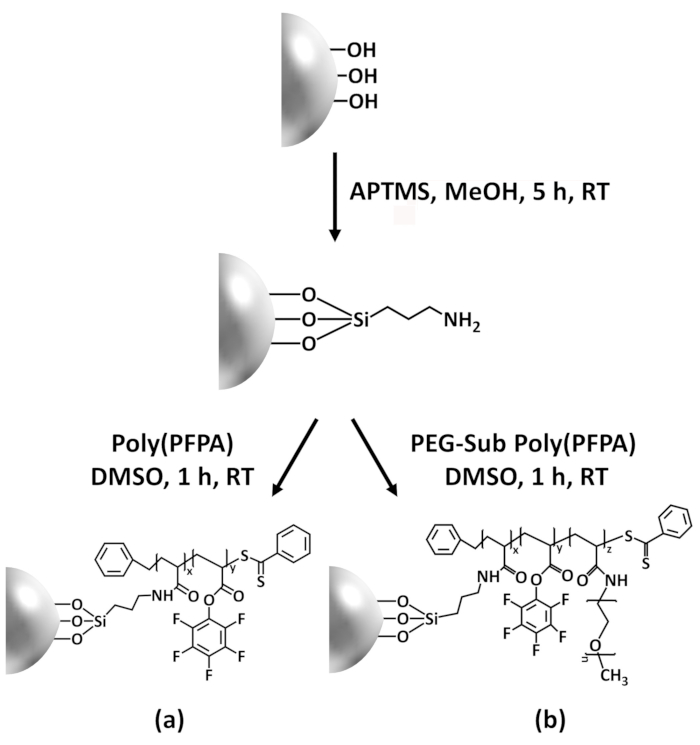
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
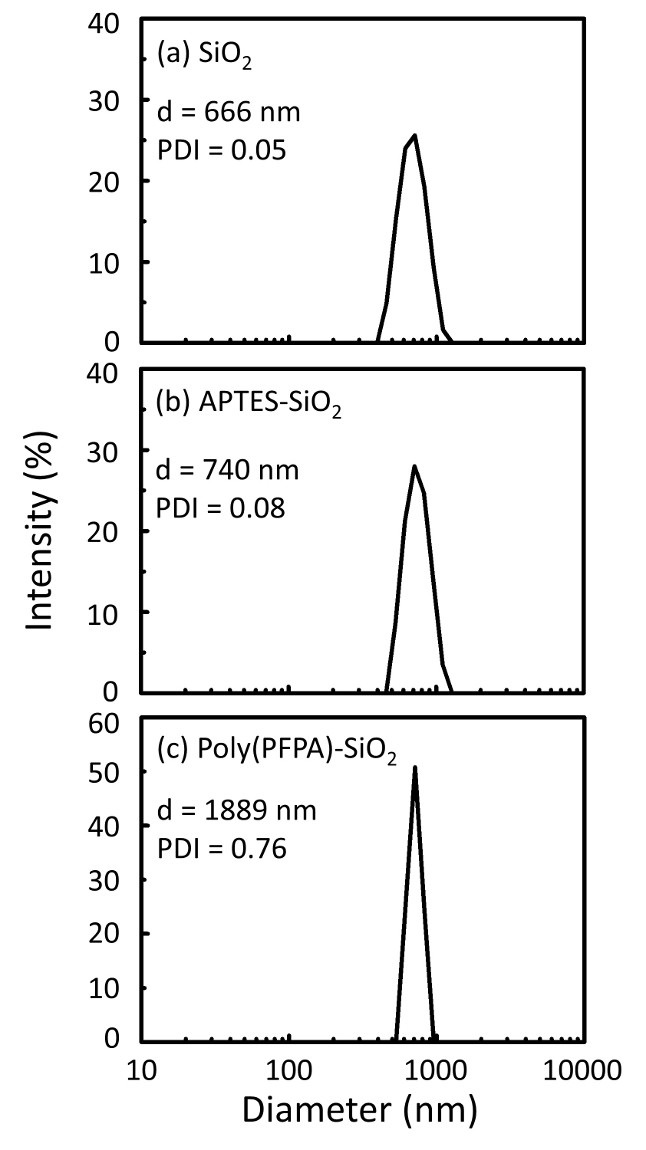
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
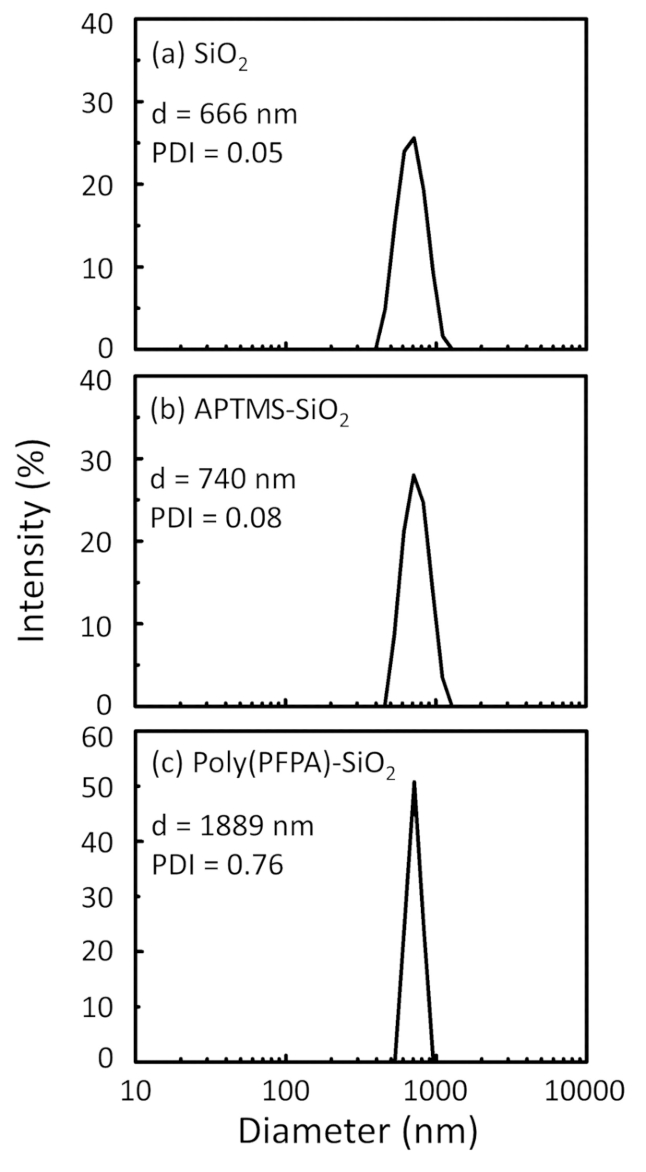
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
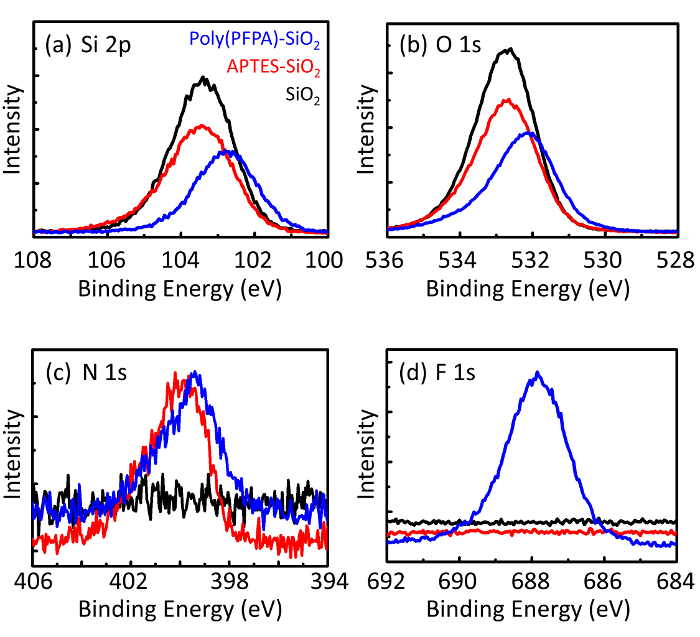
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
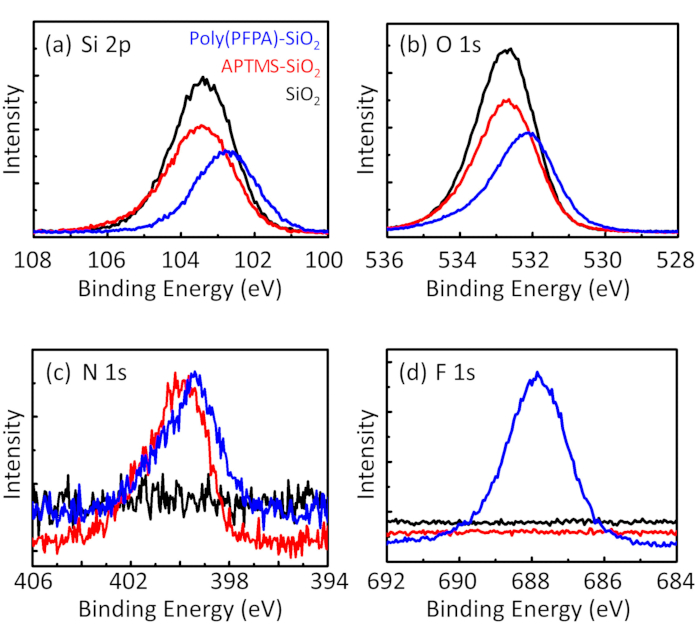
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеСмотреть дополнительные статьи
This article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены