Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
Method Article
Préparation de Poly(pentafluorophenyl acrylate) fonctionnalisés SiO2 perles pour la Purification de protéines
Dans cet article
Erratum Notice
Résumé
Un protocole pour la préparation de poly (acrylate de pentafluorophényl) (perles de silice greffée de poly(PFPA)) est présentée. La surface fonctionnalisés poly(PFPA) est ensuite immobilisée avec des anticorps et utilisée avec succès pour la séparation de la protéine par immunoprécipitation.
Résumé
Nous démontrons une méthode simple pour préparer les poly (acrylate de pentafluorophényl) (poly(PFPA)) greffés perles de silice pour immobilisation des anticorps et l’application ultérieure immunoprécipitation (IP). La surface greffées poly(PFPA) est préparée par un processus en deux étapes simples. Dans un premier temps, 3-aminopropyltriethoxysilane (APTES) se dépose comme une molécule de l’éditeur de liens sur la surface de la silice. Dans la deuxième étape, poly(PFPA) homopolymère, synthétisé par l’addition réversible et polymérisation de transfert (RAFT) chaîne fragmentation, est greffée à la molécule de l’éditeur de liens par l’intermédiaire de la réaction d’échange entre les unités de pentafluorométhyl (PPP) sur le polymère et les groupes amine sur APTES. Les dépôts de APTES et poly(PFPA) sur la silice particules sont confirmées par spectrométrie de photoélectrons (XPS), ainsi que surveillés par le changement de taille de particules mesurées via la diffusion de la lumière dynamique (DLS). Pour améliorer la surface hydrophilicité des perles, substitution partielle de poly(PFPA) avec poly(ethylene glycol) amine fonctionnalisés (amino-PEG) est également réalisée. Le poly(PFPA) PEG-substitués greffés silice perles sont alors immobilisés avec des anticorps pour l’application de la propriété intellectuelle. Pour démonstration, un anticorps dirigé contre la protéine kinase activée par RNA (PKR) est employé, et efficacité de la propriété intellectuelle est déterminée par Western Blot. Les résultats d’analyse montrent que les perles d’anticorps immobilisé en effet peuvent servir à enrichir PKR, tandis que les interactions entre protéines non spécifiques sont minimes.
Introduction
Brosses de polymère réactif ont reçu beaucoup d’intérêt ces dernières années. Ils peuvent être utilisés pour immobiliser des molécules fonctionnelles sur les matières organiques ou inorganiques pour créer des surfaces activées avec des applications dans des domaines tels que la détection et la séparation1,2,3,4, 5. Parmi les polymères réactives signalés, ceux contenant des unités pentafluorophényl ester sont particulièrement utiles en raison de leur forte réactivité avec des amines et résistance envers l’hydrolyse6. Une tel polymère est poly(PFPA), et il peut être facilement fonctionnalisée après polymérisation avec des molécules contenant des amines primaires ou secondaires7,8,9,10. À titre d’exemple, poly(PFPA) brosses ont réagi avec amino-étude pour créer des surfaces sensibles à la lumière de7.
La préparation de poly(PFPA) et ses applications ont été décrites dans un certain nombre de précédents publications6,7,8,9,10,11,12 ,13,14,15,16,17. En particulier, Theato et ses collaborateurs a signalé la synthèse de poly(PFPA) brosses « greffage à » et « greffage de » méthodes7,8,10,11,12 par . Dans le « greffage de » approche, un poly (methylsilsesquioxane)-poly (acrylate de pentafluorophényl) (polymère hybride poly(MSSQ-PFPA)) a été synthétisé8,10,11,12. Le composant poly(MSSQ) réussit à adhérence forte forme avec un certain nombre de différentes surfaces organiques et inorganiques, permettant ainsi le composant poly(PFPA) former une couche de pinceau sur la surface du matériau enduite. Dans le « greffage de » approche, surface amorcée addition réversible et polymérisation par transfert (SI-RAFT) chaîne fragmentation a été utilisée pour préparer poly(PFPA) brosses7. Dans ce cas, un agent de transfert de chaîne immobilisée surface (SI-LTC) a été tout d’abord de façon covalente au substrat par réaction de la silice-silane. Le SI-Dec immobilisé participe ensuite à la polymérisation de SI-radeau de monomères PFPA, générant des brosses poly(PFPA) dense avec stable liaison covalente au substrat.
En utilisant les brosses poly(PFPA) synthétisés par polymérisation de SI-radeau, nous avons récemment démontré l’immobilisation des anticorps sur les particules de silice greffée de poly(PFPA) et leur application ultérieure dans la purification de protéines18. L’utilisation de brosses poly(PFPA) pour l’immobilisation de l’anticorps a été trouvée pour résoudre un certain nombre de questions liées à l’actuelle séparation de protéines par IP. IP classique repose sur l’utilisation de la protéine A/G comme un linker pour anticorps immobilisation19,20,21. Étant donné que l’utilisation de la protéine A/G permet les anticorps être attaché avec une orientation spécifique, efficacité de récupération de l’antigène cible élevé est atteint. Cependant, l’utilisation de la protéine A/G souffre d’interaction des protéines non spécifiques comme la perte d’anticorps lors de la récupération de protéines, qui contribuent à un niveau élevé de bruit de fond. Pour combler ces lacunes, réticulation directe des anticorps sur un support solide a été exploré22,23,24. L’efficacité de ces techniques est généralement faible en raison de l’orientation aléatoire des anticorps réticulé. Pour le substrat poly(PFPA) greffés, l’immobilisation des anticorps est permanente, obtenue par la réaction d’échange entre les unités de la PFP et fonctionnalités amine sur les anticorps. Bien que l’orientation de l’anticorps est toujours aléatoire, le système bénéficie d’avoir plusieurs sites réactifs de PPP, contrôlables par le degré de polymérisation. En outre, nous avons montré qu’en substitution partielle des unités ppp avec amino-PEG, hydrophilicité de surface peut être ajustée, améliorer davantage l’efficacité de récupération de protéines du système18. Dans l’ensemble, les particules de silice greffée de poly(PFPA) se sont avérés être une alternative efficace à la propriété intellectuelle traditionnelle avec une efficacité raisonnable, mais aussi beaucoup plus propre arrière-plan.
Dans cette contribution, nous présentons une méthode alternative pour préparer des poly(PFPA) surface greffées pour immobilisation des anticorps et l’application de la propriété intellectuelle. Dans un processus simple en deux étapes, comme illustré dans la Figure 1, une molécule de linker selon est tout d’abord déposée sur la surface de la silice, puis le polymère poly(PFPA) est covalente de la molécule de l’éditeur de liens par l’intermédiaire de la réaction entre les unités de PPP sur la polymère et les fonctions amines sur APTES. Cette méthode de préparation permet la réticulation permanente des poly(PFPA) sur une surface de substrat, mais évite les nombreuses complications associées de synthèse SI-LTC et polymérisation TR-radeau de brosses poly(PFPA). Une substitution partielle des unités ppp avec amino-PEG peut encore être effectuée, permettant un réglage fin des propriétés surface polymère brosse. Nous montrons les billes de silice poly(PFPA) greffés ainsi préparés peuvent être immobilisés avec des anticorps et utilisés pour l’enrichissement en protéines via IP. La procédure de préparation détaillées perle, immobilisation des anticorps et le test IP sont documentés dans cet article, pour les lecteurs intéressés par la recherche d’une alternative aux classique protéine A/G basés IP.
Protocole
1. préparation des Poly(PFPA) homopolymère
- Recristallisation de AIBN
- Mélanger 5 g de 2,2'-azobis(2-methylpropionitrile) (AIBN) avec 25 mL de méthanol dans un bécher de 250 mL. Plonger le bécher dans un bain d’huile de 60 ° C, puis remuer vigoureusement le mélange avec une barre de remuer jusqu'à AIBN est entièrement dissous.
- Filtrer la solution chaude sur papier filtre (5-8 μm rétention de particules) et conserver le filtrat à 4 ° C pour permettre les cristaux à forme lentement.
- Recueillir l’AIBN recristallisé par filtration. Combiner le produit recueilli avec 25 mL de méthanol frais et répéter le processus de recristallisation.
- Sécher toute la nuit le 2 x AIBN recristallisée dans une étuve à vide à température ambiante (RT). Stocker le produit dans l’obscurité à <-10 ° C.
- Synthèse de benzyle dithiobenzoate25
- Préparer un ballon à fond rond trois-cou 500 mL équipé d’une barre magnétique remuer, du condenseur, un entonnoir et un septum en caoutchouc. Raccorder le ballon à la ligne de gaz azote traversant le condenseur du et débusquer l’intérieur air avec de l’azote. Insérer un thermomètre à travers le septum. Ajouter 41 mL (0,041 mol) de la solution de bromure de phénylmagnésium 1 M dans le tétrahydrofurane (THF) via une seringue à travers le septum même.
- Chauffer la solution de bromure de phénylmagnésium à 40 ° C dans un bain d’huile. Ajouter ensuite 3,1 g (0,041 mol) de disulfure de carbone par le biais de l’entonnoir lentement, maintient la température de la solution à 40 ° C.
- Ajouter 7,1 g (0.042 mol) de bromure de benzyle au mélange qui en résulte à travers l’entonnoir pendant 15 min, augmentation de la température de réaction à 50 ° C. Continuer à remuer à cette température pendant 45 min.
- Transférer le mélange réactionnel dans une ampoule à décanter et diluer avec 15 mL d’eau froide glacée. Extraire le produit en ajoutant 15 mL d’éther diéthylique et enlever la couche inférieure de l’eau. Répéter l’extraction à l’éther deux fois plus.
- Laver les phases organiques combinées avec une quantité abondante d’eau, puis la saumure (solution de 50 % (p/v) de NaCl dans l’eau) et sécher le produit sur le sulfate de magnésium anhydre.
- Éliminer le solvant sous vide à 35 ° C à l’aide d’un évaporateur rotatif.
- Purifier le produit par chromatographie sur colonne à l’aide de 400 mL de gel de silice (taille des pores 60 Å, 63-200 mesh granulométrie) et éther de pétrole comme l’éluant, ce qui donne 5 g de dithiobenzoate de benzyle (BDB) comme l’huile rouge. 1H RMN (400 MHz, CDCl3) pour confirmer la pureté du produit : δ 8.02-7,99 (2 H, m), 7.55-7,50 (1 H, m), 7.41-7,29 (7 H, m), 4,60 (2 H, s).
- Synthèse de poly(PFPA) par l’intermédiaire de radeau polymérisation9,26
- Disponible dans le commerce PFPA monomère contient peu d’inhibiteurs. Avant la polymérisation, enlever les inhibiteurs en passant le monomère dans une seringue jetable emballée avec l’alumine basique.
- Ajouter 0,4 mg (0,0024 mmol) de AIBN recristallisé, 4,3 mg (0,018 mmol) de BDB, 1012 mg (4,25 mmol) de PFPA exempte d’inhibiteur et 0,7 mL d’anisole anhydre dans un ballon de Schlenk 20 mL.
- Raccorder le ballon à la ligne de Schlenk et dégazez le mélange au moins trois cycles de gel-dégel-pompe. En bref, congeler le mélange réactionnel dans un bain d’azote liquide. Appliquer le vide pour enlever le gaz dans l’espace de tête. Sceller la fiole puis retirer loin de l’azote liquide pour permettre le contenu décongeler à température ambiante.
- Placer le ballon dans un bain d’huile de 70 ° C et réagir pendant 4 h sous purge2 N.
- Pour terminer la réaction, enlever le ballon du bain d’huile et exposer le contenu de la réaction dans l’air.
- Précipiter le polymère dans le méthanol froid, puis séchez le polymère récupéré dans une étuve à vide à 40 ° C durant la nuit.
- Pour mesurer la masse moléculaire de polymères, utiliser la chromatographie par perméation de gel (GPC). Utilisez THF comme la phase mobile à 35 ° C avec un 1 mL/min de débit et de construire la courbe d’étalonnage monodispersés polystyrènes étalons. Pour obtenir la mesure de GPC, dissoudre le polymère en THF (1-2 mg/mL) et filtre par filtre de jetables en polytétrafluoroéthylène (PTFE) 0,2 μm. Injecter 100 μl de l’échantillon dans l’instrument GPC. Convertir le temps de rétention échantillon mesuré en poids moléculaire à l’aide de la courbe d’étalonnage en polystyrène.
2. Elaboration de Poly(PFPA) fonctionnalisés SiO2 perles
- Traitement de SiO2 perles avec APTES
- SiO2 particules sont disponibles sous la forme d’une suspension aqueuse de 5 % (p/v). Combiner les 0,8 mL de suspension2 SiO avec 40 mg de APTES et 8 mL de méthanol dans un flacon de 20 mL à scintillation équipé d’un bar de remuer.
- Permettre la réaction se déroulent en RT pendant 5 h avec un brassage vigoureux.
- Transvaser la solution dans un tube à fond conique. Pour isoler le APTES fonctionnalisés SiO2 perles, centrifuger la solution à 10 000 g pendant 5 min, puis retirez le surnageant. Laver les billes en les dispersant nouveau dans 3 mL de méthanol frais. Agiter le tube à la main pour le mélange, mais si nécessaire, améliorer la dispersion par sonication dans un bain d’eau pendant quelques secondes. Centrifuger les perles à 10 000 x g pendant 5 min. Retirez le surnageant et répétez l’étape de lavage une fois de plus.
- Combiner le méthanol lavé SiO2 perles avec 3 mL de diméthylsulfoxyde (DMSO). Agiter le mélange à la main, ou, si nécessaire, laisser agir pendant quelques secondes, jusqu'à ce que les perles sont complètement dispersées dans le DMSO. Centrifuger les perles à 10 000 g pendant 5 min, puis retirez le surnageant. Recommencez l’étape afin d’assurer l’échange complet de solvant méthanol de DMSO.
Remarque : La suspension finale contient les APTES fonctionnalisés SiO2 perles dispersées dans 4 mL de DMSO. - Pour vérifier la répartition granulométrique, effectuer des analyses DLS. Prendre une goutte de la suspension préparée à l’étape 2.1.4 et place dans une cupule UV jetable. Diluer l’échantillon en remplissant la cuve avec le DMSO fraîche jusqu'à ce qu’il soit 2/3 plein. Introduire l’échantillon dans le porte-cellule pour commencer d’acquisition de données. Pour mesure la taille des particules, utilisez les paramètres de configuration suivants : température : 25 ° C ; Temps d’équilibrage : 120 s ; Durée de la mesure : automatique.
- Pour vérifier la composition de la surface, effectuer une analyse XPS. Sécher un petit échantillon de la suspension préparée à l’étape 2.1.4 en étuve à vide à 40 ° C durant la nuit. Prendre le pack uniformément sur un porte-échantillon de 0,5 cm x 0,5 cm et polymère séchée. Charger l’échantillon dans la chambre à vide élevée (10-8 torr) et commencer l’acquisition de données. Pour l’instrument XPS particulier utilisé, générer des photoélectrons aide une radiographie panoramique Kα Al monochromatique exploité à 15 kV et 6,7 mA et grossissement de mode hybride avec l’analyseur à un 50 eV à frais virés passent l’énergie pour les spectres de haute résolution, puis une eV 100 énergie pour les enquêtes en élémentaires.
- Poly(PFPA) à selon la greffe fonctionnalisés SiO2 perles
- Préparer la solution de poly(PFPA) en dissolvant 20 mg de poly(PFPA) dans 2 mL de DMSO dans un flacon de 20 mL à scintillation.
Remarque : Dans cette étude, un poly(PFPA) de poids moléculaire relativement faible (20 kg/mol) est utilisé. Ainsi, malgré la concentration de polymère haute (10 mg/mL), est observé aucun signe de réticulation du polymère. Si l'on utilise un polymère de poids moléculaire plus élevé, puis concentration de solution de polymère peut devoir être ajustée pour éviter la réticulation possible. - Ajouter 1 mL de APTES fonctionnalisés SiO2 perles suspendues dans le DMSO (de l’étape 2.1.4) à la solution poly(PFPA). Réagir à ta pendant 1 h avec un brassage vigoureux.
- Isoler les poly(PFPA) greffés SiO2 perles par centrifugation à 10 000 x g pendant 5 min, suivie de l’élimination du surnageant. Laver les billes en ajoutant 3 mL de DMSO et mélange en l’agitant avec la main ou quelques secondes de la sonication. Centrifuger les perles à 10 000 g pendant 5 min, puis retirez le surnageant. Répéter le lavage des poly(PFPA) greffés SiO2 perles avec le DMSO deux fois.
- Laver les perles deux fois plus avec l’eau distillée triple (TDW). Dans cette étape, mélanger les billes avec 3 mL de TDW, puis mélanger en agitant avec la main ou quelques secondes de la sonication. Centrifuger les perles à 10 000 g pendant 5 min, puis retirez le surnageant.
- Pour vérifier la répartition granulométrique, effectuez DLS suivant la procédure décrite à l’étape 2.1.5. Pour vérifier la chimie de surface, effectuez XPS suivant la procédure décrite à l’étape 2.1.6.
- Préparer la solution de poly(PFPA) en dissolvant 20 mg de poly(PFPA) dans 2 mL de DMSO dans un flacon de 20 mL à scintillation.
3. préparation de SiO2 perles greffée avec Poly(PFPA) PEG-substitués
- Pour préparer la solution de poly(PFPA), dissoudre 20 mg de poly(PFPA) dans 2 mL de DMSO dans un flacon de 20 mL à scintillation.
- Pour préparer la solution de PEG, dissoudre fonctionnalisés amine PEG dans 1 mL de DMSO. Le montant exact du PEG utilisée est déterminée par le degré souhaité de la substitution de PPP, déterminée par l’équation ci-dessous :
Quantité d’amino-PEG (g/g-poly(PFPA)) = (N_poly(PFPA) x % PEG-Sub) x (MW_PEG / MW_poly(PFPA))
où N_poly(PFPA) = poly(PFPA) degré de polymérisation
% PEG-Sub = pourcentage PEG substitution
MW_PEG = masse moléculaire des amino-PEG
Poly(PFPA) MW_ = poids moléculaire de poly(PFPA) - Transférer la solution de PEG dans la solution poly(PFPA). Réagir à ta pendant 1 h avec un brassage vigoureux.
- Pour préparer selon fonctionnalisés SiO2 perles suspendues dans le DMSO, suivez les mêmes étapes indiquées au point 2.1. Transférer 1 mL de la suspension de perle dans la solution de poly(PFPA) PEG-substitués préparée à l’étape 3.3. Permettre la greffe entre poly(PFPA) et APTES fonctionnalisés SiO2 perles pour procéder à la droite pendant 1 h avec un brassage vigoureux.
- Isoler les perles par centrifugation à 10 000 x g pendant 5 min, suivie de l’élimination du surnageant. Laver les billes en ajoutant 3 mL de DMSO et mélange en l’agitant avec la main ou quelques secondes de la sonication. Centrifuger les perles à 10 000 g pendant 5 min, puis retirez le surnageant. Répéter deux fois le lavage de DMSO.
- Laver les perles deux fois plus avec TDW. Dans cette étape, mélanger les billes avec 3 mL TDW, puis mélanger en agitant avec la main ou quelques secondes de la sonication. Centrifuger les perles à 10 000 g pendant 5 min, puis retirez le surnageant.
- Sécher les perles à 40 ° C dans une étuve à vide toute la nuit.
4. anticorps immobilisation sur Poly(PFPA) greffés SiO2 perles
Remarque : La même procédure est utilisée indépendamment de pourcentage PEG substitution sur poly(PFPA). Préparer en solution saline tamponnée au phosphate (PBS) en dissolvant la tablette de PBS dans TDW. Préparer une solution saline tamponnée au phosphate de 0,1 % (v/v) avec Tween-20 (PBST) en ajoutant 1/1000 de Tween-20 à PBS.
- Ajouter 5 mg de poly(PFPA) greffés SiO2 perles dans un tube de microtubes de 1,5 mL.
- Laver les billes en ajoutant 800 µL de PBS et mélanger bien au vortex. Centrifuger les perles à 10 000 g RT pendant 1 min. Retirer le surnageant et répétez l’étape de laver trois fois.
- Ajouter 350 µL de PBS frais et 50 µL 0,1 % (v/v) PBST 6,67 µg de l’anticorps. Incuber pendant environ 20 heures sur un rotateur à 4 ° C.
- Laver les perles pour éliminer les anticorps non fixés. Centrifuger les perles à 400 x g et 4 ° C pendant 1 min. Retirer le surnageant et ajouter 400 µL de tampon de lyse soigneusement. Doucement remettre en suspension les perles de pipetage de haut en bas pour cinq fois.
NOTE : Tampon de lyse utilisé pour laver les perles soit identique à celui utilisé lors de la lyse cellulaire et de la propriété intellectuelle, sauf que l’ajout de l’inhibiteur de dithiothréitol et protéase sont facultatifs, (voir étape 5). - Répétez cette étape de laver trois fois. Après le lavage final, retirez le surnageant autant que possible.
5. cellule Lysis et l’immunoprécipitation
- Préparation du tampon de lyse et tampon de lavage
- Préparer le tampon de lyse (50 mM Tris-HCl (pH 8,0), 100 mM KCl, 0,5 % (v/v) NP-40, glycérol à 10 % (v/v), 1 mM le dithiothréitol (DTT), protéase inhibiteur et cocktail).
- Préparer le tampon de lavage (50 mM Tris-HCl (pH 8,0), 100 mM KCl, 0,1 % (v/v) NP-40 et 10 % (v/v) de glycérol).
- Stocker les solutions-tampon à 4 ° C.
- Préparation des cellules
- Les cellules (cellules HeLa) des graines un ou deux jours avant l’expérience de la propriété intellectuelle et poussent les cellules à 37 ° C et 5 % de CO2.
- Recueillir environ 1,4 x 107 cellules avec un grattoir de cellules et les transférer dans un tube conique de 15 mL. Centrifuger les cellules à 380 g RT pendant 3 min. Retirez le surnageant et remettre en suspension avec 1 mL de PBS froid et le transfert dans un tube de microtubes de 1,5 mL.
- Centrifuger les cellules à 10 000 x g à 4 ° C pendant 30 s. Enlever le surnageant proprement. Boulettes de cellule peuvent être stockées à-80 ° C après avoir retiré le liquide surnageant.
- Préparation des lysats cellulaires
- Resuspendre le culot cellulaire 400 µl de tampon de la lyse. Laisser agir les cellules à l’aide d’un ultrasonicator.
- Après la sonication, vortex brièvement et la centrifugeuse le lysat à 20 000 x g à 4 ° C pendant 10 min.
- Transférer le surnageant dans un nouveau tube de centrifugation de 1,5 mL.
- Immunoprécipitation
- Transférer 300 µL de lysat cellulaire aux anticorps préalablement incubées poly(PFPA) greffés SiO2 billes. Conserver 30 µL de la cellule lysate que l’échantillon d’entrée dans un nouveau tube de microcentrifuge. Conserver l’échantillon d’entrée à 4 ° C.
Remarque : La quantité totale de protéine dans le lysat cellulaire devrait être environ de 4 mg. - Incuber le mélange de lysat/perles pendant 3 h sur un rotateur à 4 ° C.
- Centrifuger le mélange à 400 x g à 4 ° C pendant 1 min. Retirer le surnageant et ajouter 400 µL de tampon de lavage avec soin. Doucement remettre en suspension les perles en pipettant également, haut et bas, environ cinq fois.
- Répétez cette étape de laver trois fois. Après le lavage final, retirez le surnageant autant que possible.
- Préparer 2 x dodécyl sulfate de sodium (SDS) chargement de colorant (25 % (v/v) de glycérol, 0,1 % (p/v) phénol bleu (BPB), 60 mM Tris-HCl (pH 6,8), bromo 2 % (p/v) SDS et 2,75 mM 2-mercaptoéthanol). Magasin 2 x teinture chargement SDS à-20 ° C. Ajouter 30 µL de 2 x colorant de chargement de SDS dans les perles et l’échantillon d’entrée stockée et réchauffer pendant 10 min à 95 ° C.
- Après chauffage, analyser l’échantillon à l’aide de Western blotting27, ou conserver l’échantillon à-20 ° C.
- Transférer 300 µL de lysat cellulaire aux anticorps préalablement incubées poly(PFPA) greffés SiO2 billes. Conserver 30 µL de la cellule lysate que l’échantillon d’entrée dans un nouveau tube de microcentrifuge. Conserver l’échantillon d’entrée à 4 ° C.
Résultats
Un schéma pour la préparation des poly(PFPA) greffés SiO2 perles, avec ou sans PEG substitution est illustrée à la Figure 1. Pour surveiller les APTES et poly(PFPA) processus, nu SiO2 perles, la greffe selon fonctionnalisés SiO2 perles et poly(PFPA) greffés SiO2 perles sont caractérisés par des listes de distribution (Figure 2) et XPS (Figure 3). E...
Discussion
La synthèse de poly(PFPA) greffés SiO2 perles est illustrée à la Figure 1. En employant selon comme une molécule de l’éditeur de liens, brosses poly(PFPA) greffés par covalence à SiO2 substrat peuvent être préparés grâce à un processus en deux étapes simples. Même si certaines unités PPP sont sacrifiés pour la réaction avec APTES, un grand nombre des unités de PPP est censé rester disponible pour une réaction plus tard avec amino-PEG ou anticorps....
Déclarations de divulgation
Les auteurs n’ont rien à divulguer.
Remerciements
Ce travail a été soutenu par l’Agence pour le développement de défense (Grant No. UD170039ID).
matériels
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
Références
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
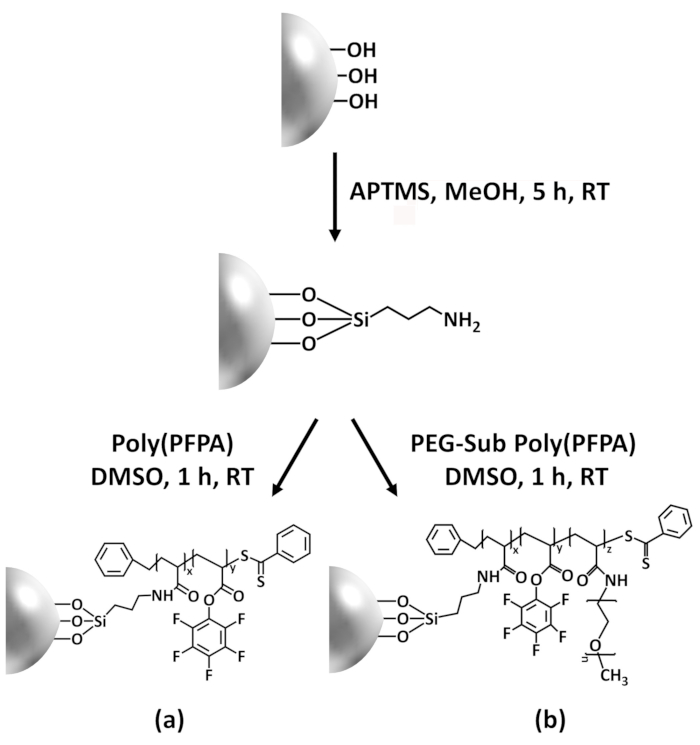
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
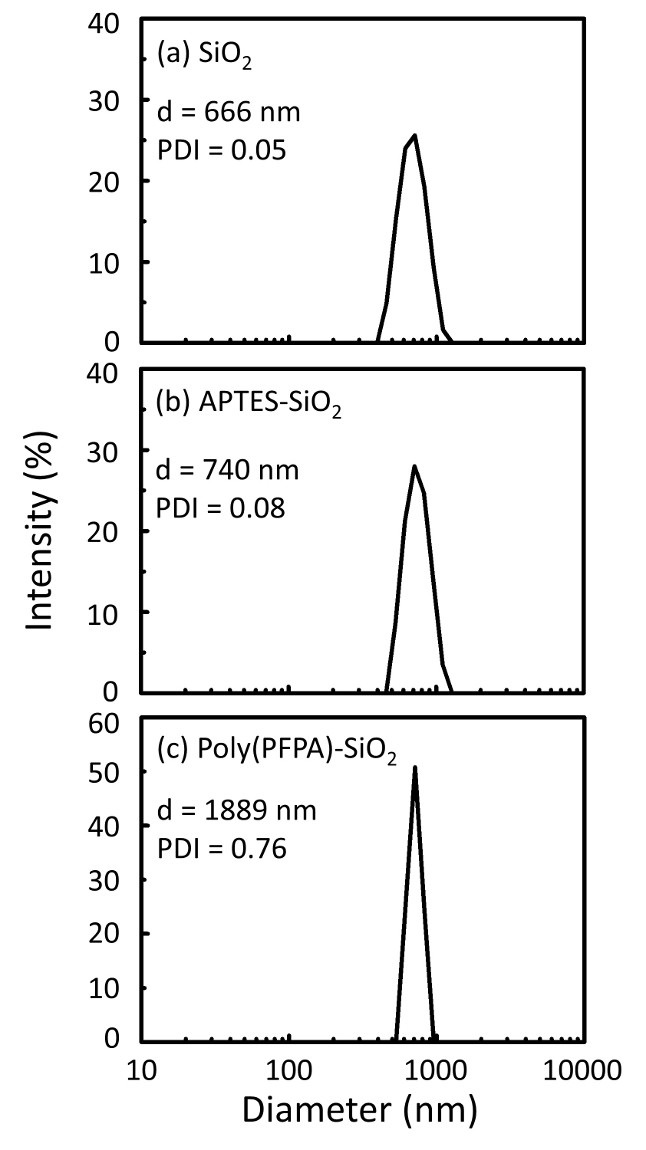
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
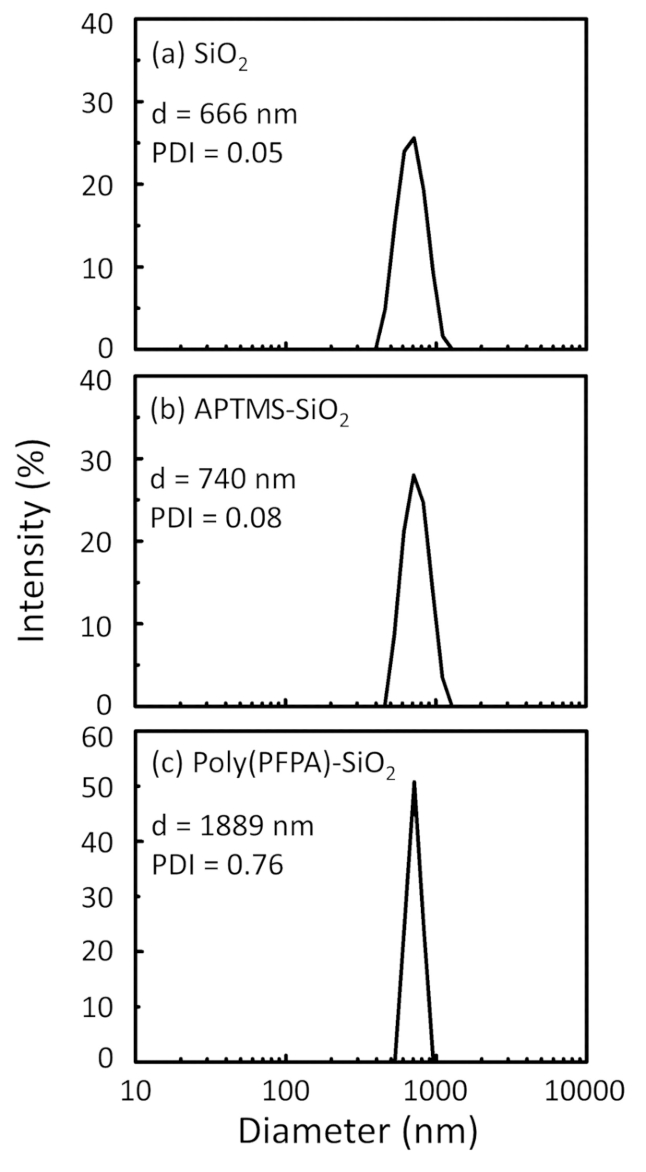
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
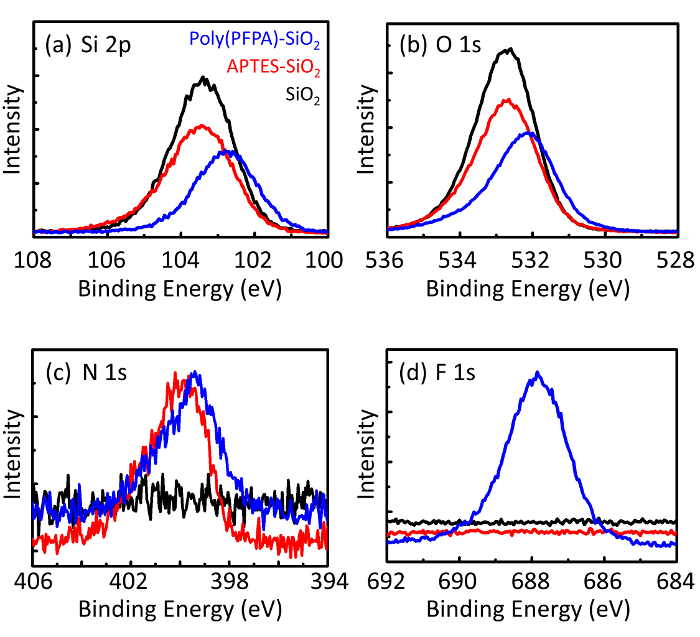
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
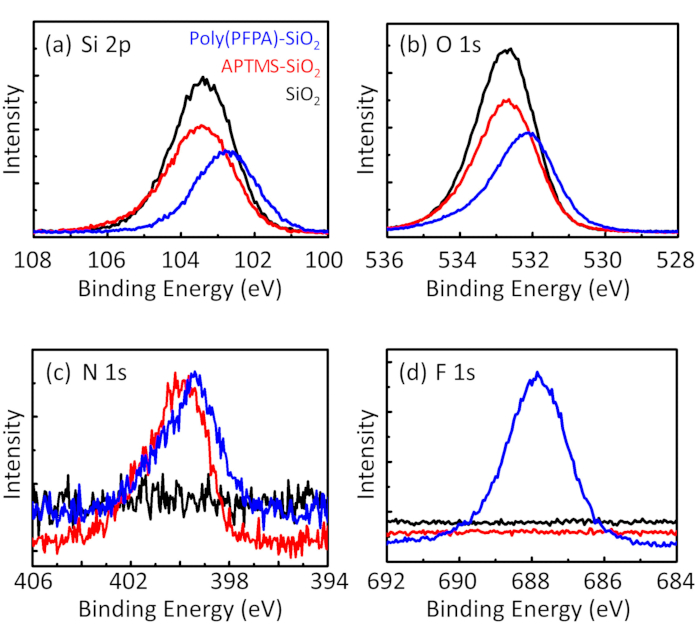
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon