Bu içeriği görüntülemek için JoVE aboneliği gereklidir. Oturum açın veya ücretsiz deneme sürümünü başlatın.
Method Article
Poly(pentafluorophenyl acrylate) Functionalized SiO2 boncuk hazırlanması için Protein Saflaştırma
Bu Makalede
Erratum Notice
Özet
Poli (pentafluorophenyl akrilat) hazırlanması için bir protokol (aşılı poly(PFPA)) silika boncuk sunulur. Poly(PFPA) functionalized yüzey o zaman ile antikorlar immobilize ve başarıyla immunoprecipitation yoluyla protein ayrılması için kullanılır.
Özet
Biz poli (pentafluorophenyl akrilat) hazırlamak için basit bir yöntem göstermek (poly(PFPA)) aşılı silis boncuk antikor immobilizasyon ve sonraki immunoprecipitation (IP) uygulama için. Poly(PFPA) aşılı yüzey yolu ile basit bir işlemdir hazırlanır. İlk adımda, 3-aminopropyltriethoxysilane (APTES) silis yüzeyine bir bağlayıcı molekül olarak yatırılır. İkinci adımda, poly(PFPA) homopolymer, tersine çevrilebilir toplama ve parçalanma zincir transfer (Sal) polimerizasyonu, yolu ile sentezlenmiş aracılığıyla exchange tepki pentafluorophenyl (Bio) birimler arasında bağlayıcı molekül için üzerine aşılı Polimer ve APTES Amin gruplarında. Devrilmesinden sonra APTES ve poly(PFPA) silika partikülleri x-ışını photoelectron spektroskopisi (XPS) tarafından teyit yanı sıra parçacık Boyut değişikliği tarafından izlenen üzerinde dinamik ışık saçılma (DL) ile ölçülür. Boncuklar, kısmi ikame poly(PFPA) Amin functionalized poly(ethylene glycol) ile yüzey hydrophilicity geliştirmek için (amino-PEG) de gerçekleştirilir. PEG yerine poly(PFPA) boncuk sonra ile IP uygulama için antikorlar immobilize silis aşılı. Gösteri, bir antikor protein kinaz RNA-harekete geçirmek (PKR) karşı istihdam ve IP etkinliği Western blot tarafından belirlenir. Çözümleme sonuçlarını immobilize antikor boncuk gerçekten de non-spesifik protein etkileşimleri çok az olmakla birlikte PKR zenginleştirmek için kullanılabileceğini göstermektedir.
Giriş
Reaktif polimer fırçaları çok ilgi son yıllarda almış. Algılama ve ayırma1,2,3,4gibi alanlarda uygulamaları ile aktif yüzey oluşturmak için organik veya inorganik malzemeler üzerinde fonksiyonel molekülleri hareketsiz için kullanılabilir, 5. Rapor reaktif polimerler arasında pentafluorophenyl ester birimleri içeren aminler ve direnç hidroliz6doğru onların yüksek reaktivite nedeniyle özellikle yararlı olur. Böyle bir polimer poly(PFPA) ve birincil veya İkincil aminler7,8,9,10içeren moleküller ile kolayca functionalized sonrası polimerizasyon olabilir. Bir örnekte, poly(PFPA) fırçalar amino-ışık duyarlı yüzeyler7oluşturmak için spiropyrans ile tepki.
Poly(PFPA) ve uygulamaları hazırlanması, önceki yayınları6,7,8,9,10,11,12 sayısında açıklanan ,13,14,15,16,17. Özellikle, Theato ve iş poly(PFPA) fırçalar "aşılama" hem de "dan aşılama yöntemleri7,8,10,11,12" ile sentezi bildirdi . "İçin yaklaşım, bir poli (methylsilsesquioxane) aşılama" içinde-poli (pentafluorophenyl akrilat) (poly(MSSQ-PFPA)) Hibrit Polimer sentez8,10,11,12oldu. Poly(MSSQ) bileşeni ile bir dizi farklı organik ve inorganik yüzeyler, böylece kaplanmış malzeme yüzeyinde bir fırça katman oluşturmak poly(PFPA) bileşen izin formu güçlü yapışma başardı. "Üzerinden yaklaşım aşılama", tersinir ek yüzey başlatılan ve parçalanma zincir transfer (SI-sal) polimerizasyon poly(PFPA) fırçalar7hazırlamak için kullanıldı. Bu durumda, bir yüzey immobilize zinciri Aktarım Aracısı (SI-CTA) ilk kovalent substrat silis-silane reaksiyon ile bağlı. İmmobilize SI-CTA substrat için kararlı kovalent bağ ile yoğun paketlenmiş poly(PFPA) fırçalar üreten PFPA monomerleri, SI-Sal polimerizasyon daha sonra katıldı.
SI-Sal polimerizasyonu sentez poly(PFPA) fırçalar kullanarak, biz son zamanlarda antikorlar aşılı poly(PFPA) silika partikülleri ve protein saflaştırma18sonraki uygulama onların immobilizasyon gösterdi. Poly(PFPA) fırçalar kullanıma antikor immobilizasyon IP üzerinden geçerli protein ayırma ile ilgili konularda bir dizi çözmek için bulundu. Geleneksel IP antikor immobilizasyon19,20,21Protein A/bir bağlayıcı G kullanımına dayanır. Belirli bir yönlendirme ile bağlanmak antikor Protein A/G kullanımı sağlar beri yüksek hedef antijen kurtarma verimliliği elde edilir. Ancak, Protein A/G kullanımı ikisi de arka plan gürültü yüksek bir düzeyde katkıda protein kurtarma sırasında--dan non-spesifik protein etkileşim hem hem antikorlar kaybına uğrar. Bu eksiklikleri gidermek için sağlam bir destek için antikorlar doğrudan polietilenin keşfedilmeyi22,23,24oldu. Bu tür teknikler verimliliğini çapraz antikorlar rasgele yönelimi nedeniyle genellikle düşüktür. Aşılı poly(PFPA) substrat için antikorlar immobilizasyon Satım tepki Bio birimleri ve amin functionalities antikorlar tarih arasında yoluyla elde, kalıcıdır. Antikor yönelimi hala rasgele olsa da, sistem çok reaktif Bio sitelerinde, polimerizasyon derecesine tarafından kontrol edilebilir olan faydaları. Ayrıca, biz gösterdi ki Bio birimleri ile amino-PEG, kısmi ikame tarafından yüzey hydrophilicity, daha fazla sistem18protein kurtarma verimliliğini artırmak ayarlanabilir. Genel olarak, aşılı poly(PFPA) silika partikülleri makul verimliliği hem de çok temiz arka plan ile geleneksel IP etkili bir alternatif gösterilmiştir.
Bu katkı antikor immobilizasyon ve IP uygulama için poly(PFPA) aşılı yüzey hazırlamak için alternatif bir yöntem rapor. Basit bir işlemdir Şekil 1' de gösterildiği gibi bir APTES bağlayıcı molekül ilk silis yüzey yatırılır, sonra poly(PFPA) polimer kovalent Bio birimler arasında tepki ile bağlayıcı molekül üzerinde bağlı olduğu Polimer ve APTES Amin işlevleri. Bu hazırlama yöntemi poly(PFPA) bir substrat yüzeye kalıcı polietilenin izin veren ancak SI-CTA sentezi ve SI-Sal polimerizasyon poly(PFPA) fırça ile ilgili birçok komplikasyonları önler. Amino-PEG Bio birimleriyle kısmi ikame hala, polimer fırça yüzey özelliklerini ince ayar sağlayan gerçekleştirilebilir. Biz böylece hazırlanan aşılı poly(PFPA) silika boncuk ile antikorlar immobilize ve protein zenginleştirme IP üzerinden için kullanılan gösterir. Detaylı boncuk hazırlık prosedürü, antikor immobilizasyon ve IP test bu makalede belgelenen, okuyucular arayışında baktılar için geleneksel Protein A/G alternatif IP tabanlı.
Access restricted. Please log in or start a trial to view this content.
Protokol
1. Poly(PFPA) Homopolymer hazırlanması
- AIBN in rekristalizasyon
- 2,2'-azobis(2-methylpropionitrile) (AIBN) 5 g 25 mL metanol bir 250 mL ölçek ile birleştirir. 60 ° C yağ banyosu kabı bırakın, sonra AIBN tamamen eriyene kadar karışımı heyecan bar ile karistirin.
- Filtre kağıdı (5-8 mikron partikül tutma) aracılığıyla sıcak çözüm filtre ve filtrate 4 ° C'de forma kristalleri yavaş yavaş izin vermek için saklayın.
- Recrystallized AIBN filtrasyon tarafından toplamak. Toplanan ürün 25 mL taze metanol ile birleştirin ve rekristalizasyon işlemi yineleyin.
- 2 adet oda sıcaklığında (RT) vakum bir fırında recrystallized AIBN gecede kuru. Mağaza ürün <-10 ° C'de karanlıkta
- Benzil dithiobenzoate25 sentezi
- Bir manyetik heyecan bar, refluxing bir kondansatör, bırakma huni ve kauçuk septum ile donatılmış bir 500 mL üç-boyun yuvarlak alt şişesi hazırla. Şişeye refluxing kondenser azot gaz hattýna baðlayýn ve içten dışa sifonu azot ile hava. Septum aracılığıyla bir termometre yerleştirin. 41 mL (0,041 mol) 1 M lik phenylmagnesium bromür tetrahydrofuran (THF) bir şırınga ile aynı septum ile ekleyin.
- Phenylmagnesium bromür çözüm bir yağ banyosu içinde 40 ° c sıcak. O zaman 3.1 g (0,041 mol) karbon disülfür bırakarak huni yavaş yavaş, çözüm sıcaklık 40 ° C'de muhafaza ekleyin
- 7.1 g (0,042 mol) Benzil bromür bırakarak huni üzerinde 15 dk. artış tepki sıcaklığı 50 ° c için sonuç karışıma ekleyin 45 dk için bu sıcaklıkta karıştırmaya devam.
- Reaksiyon karışımı HCI'yi huni aktarmak ve buz soğuk su ile 15 mL seyreltik. 15 mL dietil eter ekleyerek ürün ayıklamak ve daha düşük su katmanı kaldırın. Ayıklama dietil eter ile iki kez daha tekrarlayın.
- Bol miktarda su, daha sonra tuzlu su (çözüm % 50 (w/v) suda NaCl) kombine organik aşamaları yıkayıp ürün üzerinde susuz magnezyum sülfat kurulayın.
- Çözücü vakum 35 ° c döner buharlaştırıcı kullanarak kaldırın.
- Ürün Petrol eter ve silika jel (gözenek boyutu 60 Å, 63-200 mesh partikül büyüklüğü) 400 mL 5 g Benzil dithiobenzoate (BDB) kırmızı yağ olarak verimli eluent olarak kullanarak sütun Kromatografi tarafından arındırmak. 1tarafından H NMR (400 MHz, CDCl3) ürün saflık onaylayın: δ 8.02-7,99 (2 H, m), 7.55-7,50 (1 H, m), 7,41-7,29 (7 H, m), 4,60 (2 H, s).
- Sal polimerizasyon9,26 via poly(PFPA) sentezi
- Piyasada bulunan PFPA monomer inhibitörleri az miktarda içerir. Polimerizasyon önce inhibitörleri ile temel Alümina Paketli bir tek kullanımlık şırınga monomer geçerek kaldırın.
- 0.4 mg (0.0024 mmol) recrystallized AIBN, 4,3 mg (0,018 mmol) BDB, 1012 mg (4,25 mmol) PFPA inhibitörü-alerjik ve susuz ANISOL 0.7 mL 20 mL Schlenk şişesi ekleyin.
- Şişeye Schlenk hattýna baðlayýn ve en az üç donma-pompa-çözülme çevrimleri ile karışımı degas. Kısaca, reaksiyon karışımı bir sıvı azot banyoda dondur. Gaz headspace içinde kaldırmak için vakum uygulanır. Mühür şişeye RT. çözülme içeriğe izin vermek için sıvı azot uzak kaldır
- Şişeye bir 70 ° C yağ banyosunda yerleştirin ve 4 h N2 temizliği altında için tepki.
- Reaksiyon sona erdirmek için balonun yağ banyosu kaldırmak ve hava tepki içeriğe maruz.
- Polimer soğuk metanol içinde çökelti, sonra gece kurtarılan polimer 40 ° C'de vakum bir fırında kuru.
- Polimer molekül ağırlığı ölçmek için jel Permeasyon Kromatografi (GPC) kullanın. Mobil faz 1 mL/dk ile 35 ° c debi ve monodisperse polistren standartlar kullanılarak kalibrasyon eğrisi oluşturmak gibi THF kullanın. GPC ölçü elde etmek için THF (1-2 mg/mL) ve filtre polimer 0,2 mikron tek kullanımlık politetrafloroetilin (PTFE) filtre üzerinden geçiyoruz. 100 μL örnek GPC araç enjekte. Ölçülen örnek tutma zamanı moleküler ağırlık için polistren kalibrasyon eğrisi kullanarak dönüştürmek.
2. Poly(PFPA) hazırlanması SiO2 boncuk Functionalized
- SiO2 boncuk APTES ile tedavi
- SiO2 parçacıklar % 5 (w/v) sulu süspansiyon şeklinde mevcuttur. SiO2 süspansiyon 0.8 mL 40 mg APTES ve 8 mL metanol içinde bir heyecan bar ile donatılmış bir 20 mL mercek şişe ile birleştirir.
- Reaksiyon RT dinç karıştırma ile 5 h için devam etmek izin verir.
- Çözüm için konik bir tüp aktarın. APTES yalıtmak için SiO2 boncuk functionalized, 10.000 x g 5 min için de çözüm santrifüj kapasitesi sonra süpernatant kaldırın. Boncuk onları tarafından 3 mL taze metanol yeniden malzemeleri yıkayın. Tüp elle karıştırma için sallamak, ama gerekirse, birkaç saniye için bir su banyosunda sonication dispersiyon geliştirin. Boncuk 5 dk. Kaldır için 10.000 x g de süpernatant santrifüj kapasitesi ve yıkama adım bir kez daha tekrarlayın.
- SiO2 boncuk dimetil sülfoksit (DMSO) 3 mL ile yıkanmış metanol birleştirin. Karışımı el ile sallamak ya da boncuk DMSO içinde tümüyle dağıldı kadar birkaç saniye için gerekli solüsyon içeren temizleyicide. Boncuk 5 min için 10.000 x g, santrifüj kapasitesi, sonra süpernatant kaldırın. Metanol tam solvent değişiminden DMSO için emin olmak için adımı yineleyin.
Not: Son süspansiyon functionalized APTES içerir SiO2 boncuk dağınık DMSO 4 mL. - Parçacık boyutu dağıtım denetlemek için DL çözümlemesi gerçekleştirin. Adım 2.1.4 ve yer bir tek kullanımlık UV küvet hazırlanan süspansiyon bir damla alın. Örnek 2/3 kadar taze DMSO ile küvet doldurarak seyreltik tam. Örnek veri toplama başlatmak için hücre yuvasına takın. Parçacık boyutu ölçüm için aşağıdaki kurulum parametrelerini kullanın: sıcaklık: 25 ° C; Denge Saat: 120 s; Ölçüm Süresi: otomatik.
- Yüzey kompozisyon denetlemek için XPS çözümlemesi gerçekleştirin. Küçük bir örnek üzerinden adım 2.1.4 40 ° C'de vakum fırında gecede hazırlanan süspansiyon kuru. Kurutulmuş polimer ve 0.5 cm x 0.5 cm örnek sahibi eşit Paketi'ni alın. Örnek yüksek vakum odası (10-8 torr) yük ve veri toplama başlar. Kullanılan belirli XPS enstrüman için bir monokromatik Al Kα 15'te işletilen x-ışını kullanarak photoelectrons oluşturmak kV ve 6,7 mA ve toplamak, 50 eV analyzer ile hybrid modu büyütme kullanarak geçmek için yüksek çözünürlüklü spectra enerji ve 100 eV enerji geçmek Elemental anketler için.
- Poly(PFPA) için APTES aşılama SiO2 boncuk functionalized
- Poly(PFPA) çözüm poly(PFPA) 20 mL mercek şişe DMSO 2 ml 20 mg çözülerek hazırlayın.
Not: Bu çalışmada, nispeten düşük molekül ağırlıklı poly(PFPA) (20 kg/mol) kullanılır. Böylece, yüksek polimer konsantrasyon (10 mg/mL) rağmen polimer çapraz izine rastlanmadı görülmektedir. Bir daha yüksek molekül ağırlıklı polimer kullandıysanız, Polimer çözüm konsantrasyonu mümkün crosslinking önlemek için ayarlanması gerekebilir. - APTES 1 mL (Kimden adım 2.1.4) DMSO poly(PFPA) çözüm için askıya SiO2 boncuk functionalized ekleyin. RT dinç karıştırma ile 1 h için tepki.
- Poly(PFPA) aşılı SiO2 boncuk Santrifüjü süpernatant kaldırılması tarafından takip 5 min için 10.000 x g de tarafından izole et. Boncuk ya el ya da kaç saniye sonication sallayarak DMSO ve mix 3 mL ekleyerek yıkayın. Boncuk 5 min için 10.000 x g, santrifüj kapasitesi, sonra süpernatant kaldırın. Çamaşır poly(PFPA) aşılı SiO2 boncuk DMSO ile iki kez tekrarlayın.
- Boncuk üç kişilik distile su (TDW) ile iki kat daha fazla yıkamak. Bu adımda, boncuk TDW 3 mL ile birleştirmek, sonra el ya da kaç saniye sonication sallayarak karışımı. Boncuk 5 min için 10.000 x g, santrifüj kapasitesi, sonra süpernatant kaldırın.
- Parçacık boyutu dağıtım denetlemek için aşağıdaki 2.1.5 adımda açıklanan yordamı DLS gerçekleştirin. Yüzey kimyası denetlemek için XPS aşağıdaki 2.1.6. adımda açıklanan yordamı gerçekleştirin.
- Poly(PFPA) çözüm poly(PFPA) 20 mL mercek şişe DMSO 2 ml 20 mg çözülerek hazırlayın.
3. hazırlık SiO2 boncuk PEG yerine Poly(PFPA) ile aşılı.
- Poly(PFPA) çözüm hazırlamak için 20 mg 2 mL DMSO 20 mL mercek şişe içinde poly(PFPA) geçiyoruz.
- PEG çözüm hazırlamak için Amin functionalized PEG DMSO 1 ml geçiyoruz. PEG tam miktarı kullanılan Bio ikame, aşağıda gösterilen denklemi tarafından belirlenen istenilen ölçüde tarafından belirlenir:
Amino-PEG miktarı (g/g-poly(PFPA)) = (N_poly(PFPA) x % PEG-Sub) x (MW_PEG / MW_poly(PFPA))
nerede N_poly(PFPA) polimerizasyon derecesine poly(PFPA) =
% PEG-Sub yüzde PEG ikame =
MW_PEG amino-PEG molekül ağırlığı =
MW_ poly(PFPA) poly(PFPA) molekül ağırlığı = - PEG çözüm poly(PFPA) çözüm aktarın. RT dinç karıştırma ile 1 h için tepki.
- APTES hazırlamak için SiO2 boncuk DMSO içinde askıya functionalized, aynı adım 2.1 gösterilen adımları izleyin. Boncuk süspansiyon 1 mL adım 3.3 hazırlanan PEG yerine poly(PFPA) çözüm içine aktarın. Poly(PFPA) ve APTES arasında aşılama functionalized SiO2 boncuk RT dinç karıştırma ile 1 h için devam etmek için izin verir.
- Boncuk Santrifüjü süpernatant kaldırılması tarafından takip 5 min için 10.000 x g de tarafından izole et. Boncuk ya el ya da kaç saniye sonication sallayarak DMSO ve mix 3 mL ekleyerek yıkayın. Boncuk 5 min için 10.000 x g, santrifüj kapasitesi, sonra süpernatant kaldırın. DMSO yıkama iki kez tekrarlayın.
- Boncuk iki kat daha fazla TDW ile yıkayın. Bu adımda, boncuk 3 mL TDW ile birleştirmek, sonra el ya da kaç saniye sonication sallayarak karışımı. Boncuk 5 min için 10.000 x g, santrifüj kapasitesi, sonra süpernatant kaldırın.
- Bir vakum fırında 40 ° C'de boncuk gecede kuru.
4. antikor immobilizasyon Poly(PFPA) üzerinde SiO2 boncuk aşılı
Not: Aynı yordam poly(PFPA) üzerinde yüzde PEG ikame bağımsız olarak kullanılır. Fosfat tamponlu tuz (PBS) TDW PBS tablet çözülerek hazırlayın. %0,1 (v/v) fosfat tamponlu tuz ile ara-20 (PBST) PBS için 1/1000 ara-20 ekleyerek hazırlayın.
- Poly(PFPA) 5 mg SiO2 boncuk 1.5 mL microcentrifuge Tube aşılı ekleyin.
- Boncuk de vortexing tarafından PBS ve mix 800 µL ekleyerek yıkayın. 1 dk. Kaldır RT de 10.000 x g, boncuk süpernatant santrifüj kapasitesi ve yıkama adım üç kez tekrarlayın.
- Taze PBS, 50 µL % 0,1 (v/v) PBST ve antikor 6.67 µg 350 µL ekleyin. ~ 20 h 4 ° C'de bir rotator üzerinde kuluçkaya
- İlişkisiz antikorlar kaldırmak için boncuk yıkayın. 400 x g de boncuk santrifüj kapasitesi ve 4 ° C 1 dk. süpernatant kaldırın ve 400 µL lizis arabellek dikkatle ekleyin. Yavaşça boncuk yukarı ve aşağı için beş kez pipetting tarafından yeniden askıya alma.
Not: dithiothreitol ve proteaz inhibitörü eklenmesi dışında isteğe bağlı, (bkz. Adım 5) boncuk yıkamak için kullanılan lizis arabellek hücre lizis ve IP, sırasında kullanılan olmalıdır. - Bu yıkama üç kez tekrarlayın. Son yıkama sonra süpernatant mümkün olduğunca kaldırın.
5. hücre lizis ve Immunoprecipitation
- Lizis arabellek ve yıkama arabellek hazırlanması
- Lizis arabellek (50 mM Tris-HCl (pH 8.0), 100 mM KCl, %0,5 (v/v) NP-40, % 10 (v/v) gliserol, 1 mM dithiothreitol (DTT) ve proteaz inhibitörü kokteyl) hazırlayın.
- Yıkama arabellek (50 mM Tris-HCl (pH 8.0), 100 mM KCl, % 0,1 (v/v) NP-40 ve %10 (v/v) gliserol) hazırlayın.
- Tampon çözeltiler 4 ° C'de depolayın
- Hücreleri hazırlanması
- Bir veya iki gün öncesine IP deney (HeLa hücreleri) tohum ve 37 ° C ve % 5 CO2hücrelerin büyümesine.
- Yaklaşık 1.4 x 107 hücreleri hücre kazıyıcı ve transfer 15 mL konik tüp içine toplamak. Senaryo Özeti 3 dakika süreyle RT de 380 x g süpernatant kaldırmak ve yeniden 1 mL soğuk PBS ve transfer içine 1.5 mL microcentrifuge tüp ile askıya alma santrifüj.
- Hücreleri, 10.000 x g 4 ° C'de 30 s. Kaldır süpernatant temiz için santrifüj kapasitesi. Hücre granül-80 ° C'de süpernatant çıkardıktan sonra saklanır.
- Hücre lysates hazırlanması
- Hücre Pelet 400 µL lizis arabelleği ile yeniden askıya alma. Bir ultrasonicator kullanarak hücreleri solüsyon içeren temizleyicide.
- Sonication, girdap kısaca ve santrifüj lysate 20.000 x g 4 ° c de 10 dakika sonra.
- Süpernatant yeni bir 1,5 mL santrifüj tüpü aktarın.
- Immunoprecipitation
- Hücrenin lysate 300 µL önceden hazırlanmış antikor inkübe poly(PFPA) aşılı SiO2 boncuk için transfer. Yeni bir microcentrifuge tüp giriş örnek olarak lysate hücrenin 30 µL korur. Mağaza giriş örneği 4 ° c '
Not: Protein hücredeki lysate toplam miktarı yaklaşık 4 mg olmalıdır. - Lysate/boncuk karışımı bir rotator 4 ° C'de 3 h için kuluçkaya
- 400 x g 1 dk. Kaldır 4 ° C'de karisimin süpernatant santrifüj kapasitesi ve yıkama arabelleği 400 µL dikkatle ekleyin. Yavaşça boncuk yukarı ve aşağı yaklaşık beş kat pipetting tarafından yeniden askıya alma.
- Bu yıkama üç kez tekrarlayın. Son yıkama sonra süpernatant mümkün olduğunca kaldırın.
- 2 boya (%25 (v/v) gliserol, 2-mercaptoethanol %2 (w/v) SDS ve 2.75 mM (w/v) % 0,1 bromo fenol mavi (BPB), 60 mM Tris-HCl (pH 6.8)) yükleme Sodyum Lauryl Sülfat (SDS) x hazırlamak. 2 x SDS yükleme boya-20 ° C'de depolayın 2 x SDS yükleme boya 30 µL boncuklar ve saklı giriş örnek eklemek ve onları 95 ° C'de 10 dakika için ısı
- Isıtma sonra Western blot27kullanarak örneğini analiz veya örnek-20 ° C'de depolayın
- Hücrenin lysate 300 µL önceden hazırlanmış antikor inkübe poly(PFPA) aşılı SiO2 boncuk için transfer. Yeni bir microcentrifuge tüp giriş örnek olarak lysate hücrenin 30 µL korur. Mağaza giriş örneği 4 ° c '
Access restricted. Please log in or start a trial to view this content.
Sonuçlar
Poly(PFPA) hazırlanması için bir şema ile SiO2 boncuklar, aşılı veya PEG ikame Şekil 1' de gösterilen. Poly(PFPA) işlemi, çıplak SiO2 boncuklar, aşılama ve APTES izlemek için APTES SiO2 boncuk functionalized ve poly(PFPA) aşılı SiO2 boncuk DLS (Şekil 2) ve XPS (Şekil 3) ile karakterizedir. IP verimliliği boncuk Western blot tarafından ...
Access restricted. Please log in or start a trial to view this content.
Tartışmalar
Poly(PFPA) sentezi SiO2 boncuk Şekil 1' de gösterildiği aşılı. APTES bir bağlayıcı molekül istihdam ederek, kovalent SiO2 substrat için aşılı poly(PFPA) fırçalar basit bir işlemdir yolu ile hazırlanabilir. Her ne kadar bazı Bio birimlerinin APTES ile reaksiyonu için feda, Bio birimleri çok sayıda amino-PEG veya antikorlar ile daha sonra tepki için kullanılabilir kalması beklenir. Bio grupları poly(PFPA) fırçalar değil solvate su
Access restricted. Please log in or start a trial to view this content.
Açıklamalar
Yazarlar ifşa gerek yok.
Teşekkürler
Bu eser için savunma geliştirme (Grant No ajansı tarafından desteklenmiştir UD170039ID).
Access restricted. Please log in or start a trial to view this content.
Malzemeler
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
Referanslar
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
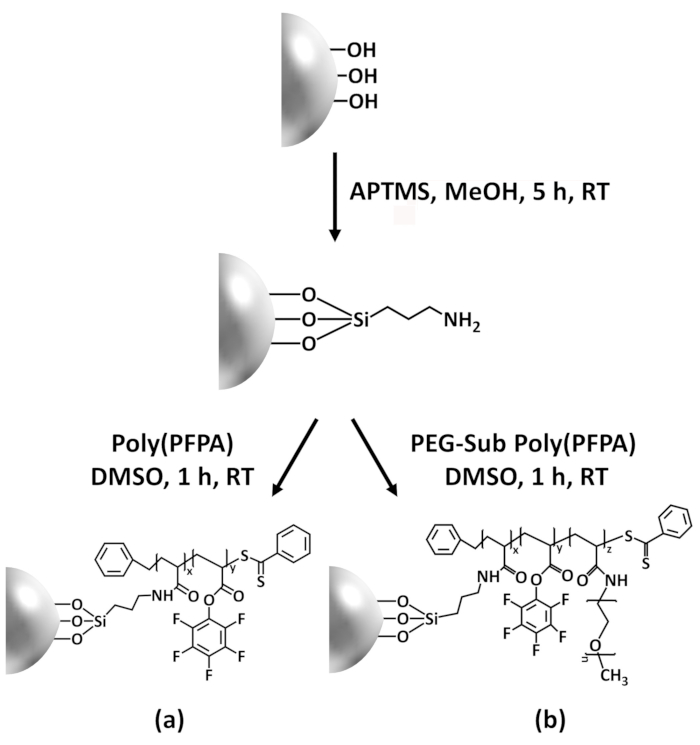
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
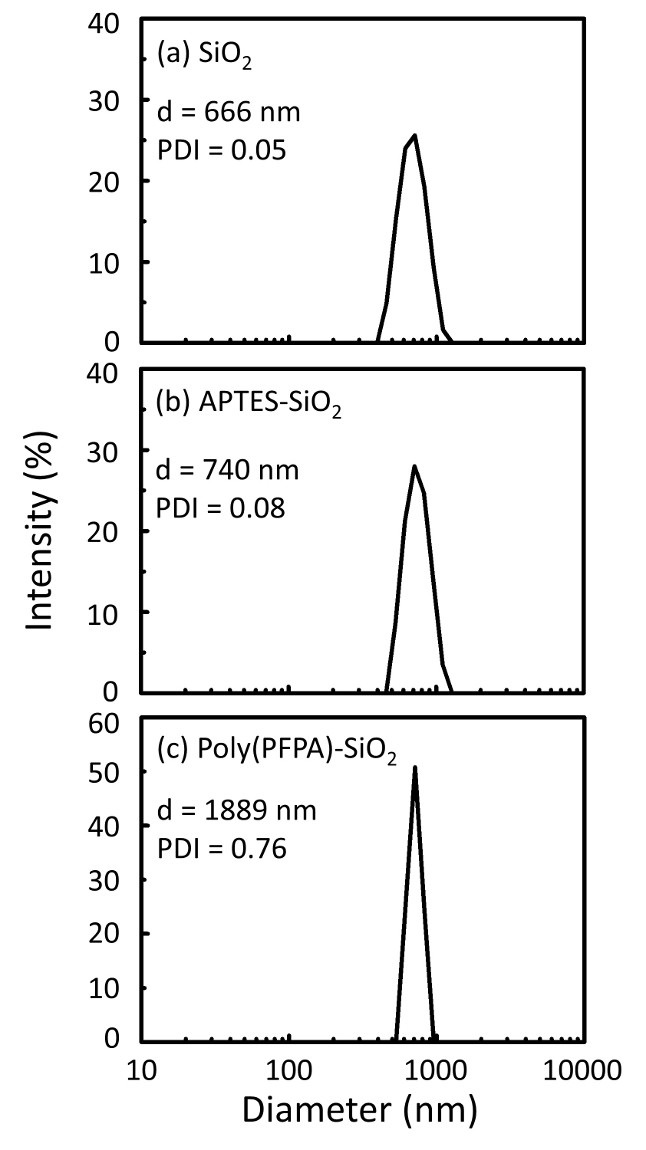
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
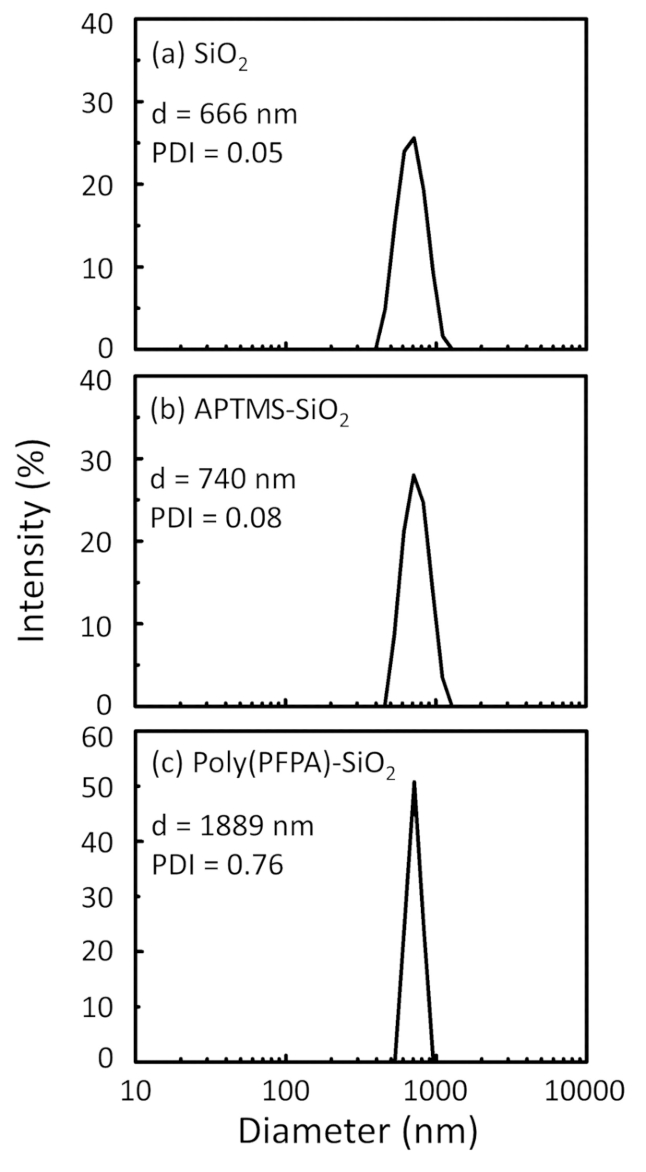
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
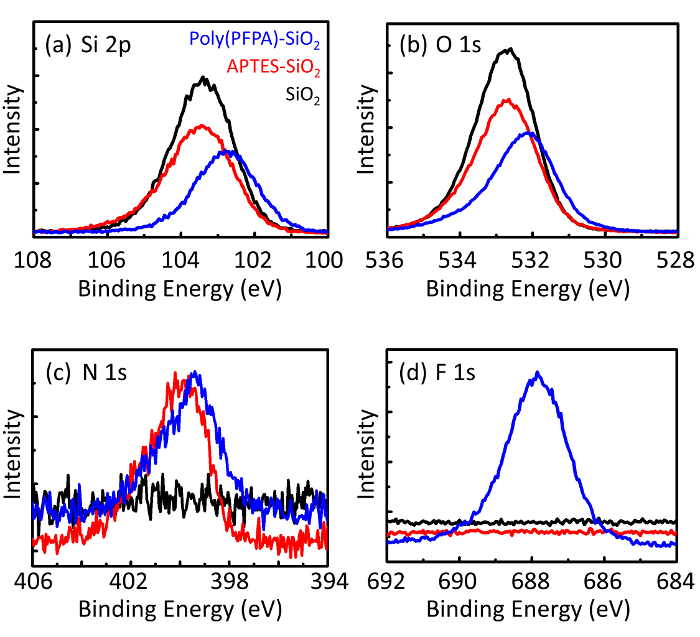
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
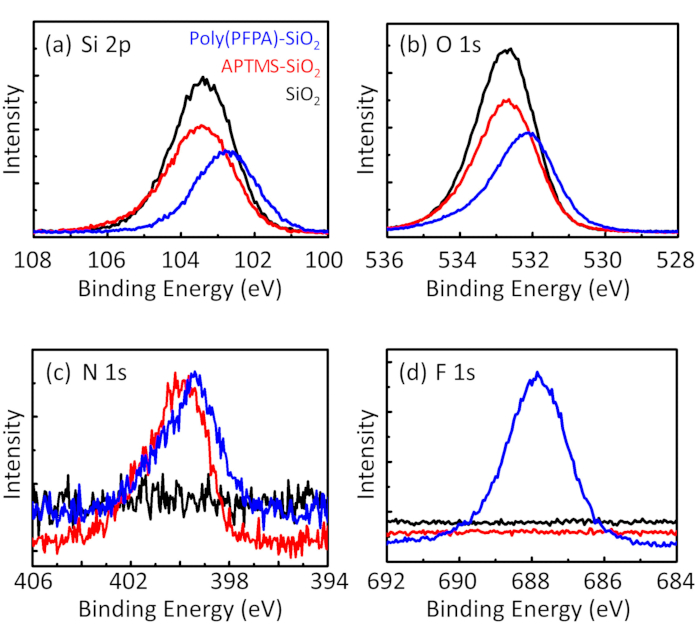
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
Yeniden Basımlar ve İzinler
Bu JoVE makalesinin metnini veya resimlerini yeniden kullanma izni talebi
Izin talebiThis article has been published
Video Coming Soon
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır