Zum Anzeigen dieser Inhalte ist ein JoVE-Abonnement erforderlich. Melden Sie sich an oder starten Sie Ihre kostenlose Testversion.
Method Article
Vorbereitung der Poly(pentafluorophenyl acrylate) funktionalisiert SiO2 Perlen Proteinreinigung
In diesem Artikel
Erratum Notice
Zusammenfassung
Ein Protokoll für die Zubereitung von Poly (Pentafluorophenyl Acrylat) (poly(PFPA)) gepfropft Kieselsäure Perlen wird vorgestellt. Die poly(PFPA) funktionalisierten Oberfläche ist dann mit Antikörper immobilisiert und die Protein-Trennung durch Immunopräzipitation erfolgreich eingesetzt.
Zusammenfassung
Wir zeigen eine einfache Methode um Poly (Pentafluorophenyl Acrylat) vorzubereiten (poly(PFPA)) gepfropft Kieselsäure Perlen für Antikörper Immobilisierung und nachfolgende Immunopräzipitation (IP) Anwendung. Die poly(PFPA) veredelte Oberfläche ist über einen einfachen Schritten bereit. Im ersten Schritt wird 3-Aminopropyltriethoxysilane (APTES) wie ein Linker-Molekül auf der Oberfläche Kieselsäure hinterlegt. In einem zweiten Schritt poly(PFPA) Homopolymer, über die reversible Addition und Fragmentierung Kette Transfer (FLOß) Polymerisation synthetisiert ist gepfropft an die Linker-Molekül durch die Reaktion der Austausch zwischen den Pentafluorophenyl (PFP) Einheiten auf die Polymer und Amin Gruppen auf APTES. Die Abscheidung des APTES und poly(PFPA) auf die Kieselsäure Teilchen sind durch Röntgen-Photoelektronen-Spektroskopie (XPS) bestätigt, sowie durch die Änderung der Größe der Partikel überwacht über dynamische Lichtstreuung (DLS) gemessen. Verbesserung der Oberfläche Hydrophilie der Perlen, partielle Substitution von poly(PFPA) mit Poly(ethylene glycol) Amin funktionalisiert wird auch (amino-PEG) durchgeführt. Die PEG ersetzt poly(PFPA) gepfropft Kieselsäure, die Perlen mit Antikörpern für IP-Anwendung dann immobilisiert sind. Zur Demonstration ein Antikörper gegen Proteinkinase RNA aktiviert (PKR) beschäftigt, und IP-Effizienz wird durch Western blotting bestimmt. Die Ergebnisse der Analyse zeigen, dass die Antikörper immobilisiert Perlen in der Tat lässt sich PKR zu bereichern, während unspezifische Protein-Interaktionen minimal sind.
Einleitung
Reaktive Polymer Bürsten haben viel Interesse in den letzten Jahren erhalten. Sie können verwendet werden, um funktionelle Moleküle auf organische oder anorganische Materialien für aktivierte Oberflächen mit Anwendungen in Bereichen wie Erkennung und Trennung1,2,3,4zu immobilisieren, 5. Unter die reaktive Polymere berichtet sind jene mit Pentafluorophenyl Ester Einheiten besonders aufgrund ihrer hohen Reaktivität mit Aminen und Beständigkeit gegenüber Hydrolyse6. Eine solche Polymer ist poly(PFPA) kann, und es ohne weiteres funktionalisierten nach Polymerisation mit Molekülen, die mit primären oder sekundären Aminen7,8,9,10. In einem Beispiel wurden poly(PFPA) Bürsten mit amino-Spiropyrans Licht reagierende Oberflächen7erstellen reagiert.
Die Vorbereitung der poly(PFPA) und deren Anwendungen wurden in einer Reihe von früheren Publikationen6,7,8,9,10,11,12 beschrieben ,13,14,15,16,17. Insbesondere berichtet Theato und Mitarbeiter die Synthese von poly(PFPA) Bürsten über "Pfropfen zu" und "Pfropfen aus" Methoden7,8,10,11,12 . In der "Pfropfen" Ansatz, eine Poly (Methylsilsesquioxane)-Poly (Pentafluorophenyl Acrylat) (poly(MSSQ-PFPA))-Hybrid-Polymer synthetisiert8,10,11,12war. Die poly(MSSQ)-Komponente konnte Form starke Adhäsion mit einer Reihe von verschiedenen organischen und anorganischen Oberflächen, so dass die poly(PFPA)-Komponente, die eine Bürste auf der beschichteten Oberfläche bilden. In der "Pfropfen aus" Ansatz, Oberfläche initiiert reversible Addition und Fragmentierung Kette Transfer (SI-FLOß) Polymerisation wurde eingesetzt, um poly(PFPA) Bürsten7vorzubereiten. In diesem Fall hing eine Oberfläche immobilisiert Kette Transferstelle (SI-CTA) zuerst kovalent an das Substrat durch Kieselsäure-Silan-Reaktion. Immobilisierte SI-CTA nahm dann in der SI-RAFT Polymerisation PFPA Monomere, dicht gepackten poly(PFPA) Bürsten mit stabile kovalente Anbindung an das Substrat zu erzeugen.
Durch die Nutzung der poly(PFPA) Bürsten über SI-RAFT Polymerisation synthetisiert, haben wir vor kurzem die Immobilisierung von Antikörpern auf poly(PFPA) gepfropft Silica-Partikel und deren spätere Anwendung in Protein Reinigung18gezeigt. Die Verwendung von poly(PFPA) Bürsten für Antikörper Immobilisierung erwies sich um eine Reihe von Fragen im Zusammenhang mit aktuellen Protein Trennung durch IP-zu lösen. Herkömmliche IP beruht auf der Verwendung von Protein A/G als ein Linker für Antikörper Immobilisierung19,20,21. Da die Verwendung von Protein A/G der Antikörper mit einer bestimmten Orientierung angebracht werden kann, wird eine hohe Ziel Antigen Erholung Effizienz erreicht. Allerdings leidet die Verwendung von Protein A/G unspezifische Protein-Interaktion sowie der Verlust von Antikörpern während Proteingewinnung, die ein hohes Maß an Rauschen beitragen. Um diese Mängel zu beheben, wurde die direkte Vernetzung von Antikörpern gegen eine feste Stütze erforschten22,23,24. Die Effizienz solcher Techniken ist in der Regel gering aufgrund der zufälligen Orientierung der Antikörper vernetzt. Für das Substrat poly(PFPA) gepfropft ist die Immobilisierung von Antikörpern dauerhaft, durch Austausch Reaktion zwischen PFP Einheiten und Amin Funktionalitäten auf Antikörper erreicht. Obwohl die Antikörper-Ausrichtung noch zufällig ist, profitiert das System haben viele reaktive PFP-Sites, steuerbar durch den Grad der Polymerisation. Darüber hinaus zeigten wir, dass durch partielle Substitution von PFP-Einheiten mit amino-PEG, kann Oberfläche Hydrophilie abgestimmt werden weitere Effizienzsteigerungen Protein Recovery System18. Insgesamt zeigten die poly(PFPA) gepfropft Silica-Partikel eine wirksame Alternative zu traditionellen IP mit angemessenen Effizienz sowie viel sauberer Hintergrund sein.
In diesem Beitrag berichten wir über eine alternative Methode zur poly(PFPA) veredelte Oberfläche Antikörper Immobilisierung und IP-Anwendung vorzubereiten. In zwei einfachen Schritten, wie in Abbildung 1dargestellt ein APTES Linker Molekül zuerst lagert sich auf die Silizium-Oberfläche, dann poly(PFPA) Polymer ist kovalent angeschlossen an die Linker-Molekül durch die Reaktion zwischen der PFP-Einheiten auf die Polymer und Amin-Funktionen auf APTES. Diese Vorbereitung Methode ermöglicht die permanente Vernetzung der poly(PFPA) auf einer Substratoberfläche, aber vermeidet viele Komplikationen im Zusammenhang mit SI-CTA-Synthese und SI-RAFT Polymerisation von poly(PFPA) Bürsten. Teilsubstitution der PFP-Einheiten mit amino-PEG kann noch durchgeführt werden, ermöglicht die Feinabstimmung der Oberflächeneigenschaften der Polymer-Pinsel. Wir zeigen die poly(PFPA) gepfropft Kieselsäure Perlen so vorbereitet mit Antikörper immobilisiert und zur Proteinanreicherung über IP verwendet werden können. Die detaillierte Wulst Vorbereitung Verfahren, Antikörper Immobilisierung und IP-Tests sind in diesem Artikel dokumentiert, für Leser interessierten bei der Suche nach eine Alternative zu herkömmlichen Protein A/G IP-basierte.
Access restricted. Please log in or start a trial to view this content.
Protokoll
1. Vorbereitung des Poly(PFPA) Homopolymer
- Rekristallisation der Havariekommission
- Kombinieren Sie 5 g 2,2'-azobis(2-methylpropionitrile) (Havariekommission) mit 25 mL Methanol in einem 250 mL Becherglas. Tauchen Sie den Becher in ein Ölbad 60 ° C, dann rühren Sie kräftig die Mischung mit Stir Bar bis Havariekommission vollständig aufgelöst ist.
- Filtern Sie die warme Lösung durch Filterpapier (5-8 μm Partikel Retention) und speichern Sie das Filtrat bei 4 ° C langsam erlauben die Kristalle zu bilden.
- Sammeln Sie die umkristallisiert Havariekommission durch Filtration. Kombinieren Sie das gesammelte Produkt mit 25 mL frischem Methanol und wiederholen Sie den Vorgang der Rekristallisation.
- Trocknen Sie 2 x umkristallisiert Havariekommission in einem Vakuumofen bei Raumtemperatur (RT) über Nacht. Lagern Sie das Produkt im Dunkeln bei <-10 ° C.
- Synthese von Benzyl Dithiobenzoate25
- Bereiten Sie einen 500 mL drei Hals Rundboden Kolben ausgestattet mit einer magnetischen Stir Bar, einen refluxing Kondensator, einen Tropftrichter und ein Gummiseptum. Verbinden Sie den Kolben mit der Stickstoff-Gas-Linie durch den refluxing Kondensator und spülen Sie die innere Luft mit Stickstoff. Legen Sie einen Thermometer durch das Septum. Fügen Sie 41 mL (0.041 Mol) 1 M Lösung von Phenylmagnesium Bromid in Tetrahydrofuran (THF) über eine Spritze durch das gleiche Septum.
- Wärmen Sie die Phenylmagnesium-Bromid-Lösung auf 40 ° C in einem Ölbad. Dann fügen Sie 3,1 g (0.041 Mol) Schwefelkohlenstoff durch den Tropftrichter langsam, Aufrechterhaltung der Temperatur der Lösung bei 40 ° C.
- Die resultierende Mischung durch den Tropftrichter über 15 min. Erhöhung der Reaktionstemperatur auf 50 ° c 7,1 g (0.042 Mol) Benzyl Bromid hinzufügen Bei dieser Temperatur für 45 Minuten weiterrühren.
- Das Reaktionsgemisch in einen separatory Trichter zu übertragen und mit 15 mL eiskaltes Wasser verdünnen. Extrahieren Sie das Produkt durch Zugabe von 15 mL Diethylether zu, und entfernen Sie die untere Wasserschicht. Wiederholen Sie die Extraktion mit Diethylether noch zweimal.
- Waschen Sie die kombinierten organischen Phasen mit reichlich Wasser und Sole (Lösung von 50 % (w/V) NaCl in Wasser) und trocknen Sie das Produkt über wasserfrei Magnesiumsulfat.
- Entfernen Sie das Lösungsmittel im Vakuum bei 35 ° C mit einer Drehverdampfer.
- Reinigen Sie das Produkt durch Säulenchromatographie mit 400 mL Silica-Gel (Porengröße 60 Å, 63-200 Mesh Partikelgröße) und Petrolether als Laufmittel, 5 g Benzyl Dithiobenzoate (BDB) als rotes Öl nachgeben. Bestätigen Sie die Produktreinheit von 1H NMR (400 MHz, CDCl-3): δ 8.02-7,99 (2 H, m), 7,55-7.50 (1 H, m), 7.41-7,29 (7 H, m), 4.60 (2 H, s).
- Synthese von poly(PFPA) über RAFT Polymerisation9,26
- Handelsübliche PFPA Monomer enthält kleine Menge von Inhibitoren. Entfernen Sie vor der Polymerisation die Inhibitoren indem man das Monomer durch eine Einwegspritze mit grundlegenden Aluminiumoxid verpackt.
- Ein 20 mL-Schlenk-Kolben 0,4 mg (0.0024 Mmol) umkristallisiert Havariekommission, 4,3 mg (0.018 Mmol) der BDB, 1012 mg (4,25 Mmol) Inhibitor-freie PFPA und 0,7 mL wasserfreiem Anisole hinzufügen.
- Verbinden Sie den Kolben mit Schlenk Linie und Entgasen der Mischung mit mindestens drei Gefrier-Pumpe-Tau-Zyklen. Kurz, das Reaktionsgemisch in ein Bad von flüssigem Stickstoff eingefroren. Gelten Sie Vakuum um das Gas im Gasraum zu entfernen. Verschließen Sie die Flasche dann entfernen von flüssigem Stickstoff, um den Inhalt bei RT auftauen lassen
- Legen Sie die Flasche in ein Ölbad 70 ° C und für 4 h unter N2 Säuberung reagieren.
- Um die Reaktion zu beenden, entfernen Sie die Flasche aus dem Ölbad und setzen Sie die Reaktion Inhalt in die Luft.
- Das Polymer in kaltem Methanol ausgefällt, dann das wiederhergestellte Polymer in einem Vakuumofen bei 40 ° C über Nacht trocknen.
- Um das Molekulargewicht Polymer zu messen, verwenden Sie Gel Permeation Chromatographie (GPC). Verwenden Sie THF, wie die mobile Phase bei 35 ° C mit einer 1 mL/min Durchfluss und die Eichkurve mit monodispersen Polystyrolstandards zu konstruieren. GPC-Messung zu erwerben, lösen Sie das Polymer in THF (1-2 mg/mL) und Filter durch 0,2 μm Einweg Polytetrafluorethylen (PTFE) Filter auf. Injizieren Sie 100 μl der Probe in der GPC-Instrument. Konvertieren Sie die gemessene Probe Retentionszeit in Molekulargewicht mit Polystyrol Kalibrierkurve.
2. Vorbereitung des Poly(PFPA) funktionalisiert SiO2 Perlen
- Behandlung von SiO2 Perlen mit APTES
- SiO2 Partikel gibt es in Form einer wässrigen Suspension 5 % (w/V). Kombinieren Sie 0,8 mL SiO2 Suspension mit 40 mg APTES und 8 mL Methanol in einer 20 mL funkeln Durchstechflasche mit Stir Bar ausgestattet.
- Lassen Sie die Reaktion auf das Vorgehen bei RT für 5 h mit kräftig rühren.
- Übertragen Sie die Lösung auf einem konischen Rohr. Um die APTES isolieren funktionalisiert SiO2 Perlen, die Lösung bei 10.000 x g für 5 min Zentrifugieren, dann den Überstand zu entfernen. Waschen Sie die Perlen durch Dispergieren sie erneut in 3 mL frischem Methanol. Schütteln Sie das Rohr von hand mischen, aber wenn nötig verbessern Sie die Dispersion durch Ultraschallbehandlung in einem Wasserbad für ein paar Sekunden. Zentrifugieren Sie den Perlen bei 10.000 x g für 5 min. Entfernen des Überstands und wiederholen Sie der Waschschritt noch einmal zu.
- Kombinieren Sie das Methanol gewaschen SiO2 Perlen mit 3 mL Dimethyl Sulfoxid (DMSO). Schütteln Sie die Mischung mit der hand, oder wenn nötig beschallen, für ein paar Sekunden, bis die Perlen in DMSO vollständig dispergiert sind. Zentrifugieren Sie die Perlen bei 10.000 x g für 5 min, dann entfernen Sie den überstand. Wiederholen Sie den Schritt, um komplette Lösungsmittelaustausch aus Methanol zu DMSO zu gewährleisten.
Hinweis: Enthält die endgültige Aufhebung des APTES funktionalisiert SiO2 Perlen verstreut in 4 mL DMSO. - Prüfen Sie die Partikelgrößenverteilung, durchführen Sie DLS-Analyse. Nehmen Sie einen Tropfen der Suspension in Schritt 2.1.4 und Ort in einem Einweg-UV Küvette vorbereitet. Die Probe verdünnen, indem Sie die Küvette mit frischen DMSO, bis es 2/3 ist voll. Legen Sie die Probe in der Küvettenhalter Datenerfassung beginnen. Für die Messung der Partikelgröße, verwenden Sie die folgenden Setup-Parameter: Temperatur: 25 ° C; Gleichgewichtherstellung Zeit: 120 s; Messdauer: automatische.
- Überprüfen Sie die Zusammensetzung die Oberfläche durchführen Sie XPS Analyse. Trocknen Sie eine kleine Auswahl aus der Suspension zubereitet im Schritt 2.1.4 im Vakuumofen bei 40 ° C über Nacht. Nehmen Sie die getrocknete Polymer und Packung gleichmäßig auf einem 0,5 cm x 0,5 cm-Probenhalter. Laden Sie die Probe in die hohen Vakuumkammer (10-8 Torr) und beginnen Sie Datenerfassung. Für das jeweilige XPS-Instrument verwendet, erzeugen die Photoelektronen mit einem monochromen Al Kα Röntgen betrieben um 15 kV und 6,7 mA und sammeln mit Hybrid-Modus Vergrößerung mit dem Analyzer bei 50 eV pass Energie für hochauflösende Spektren und 100 eV geben Energie für elementare Umfragen.
- Poly(PFPA), APTES Pfropfen funktionalisiert SiO2 Perlen
- Bereiten Sie die poly(PFPA)-Lösung von 20 mg poly(PFPA) in 2 mL DMSO in einer 20 mL funkeln Durchstechflasche auflösen.
Hinweis: In dieser Studie wird ein relativ niedrigem Molekulargewicht poly(PFPA) (20 kg/Mol) verwendet. So wird trotz der hochpolymeren Konzentration (10 mg/mL), keine Hinweise auf Polymer Vernetzung beobachtet. Wenn ein höheres Molekulargewicht Polymer verwendet wird, müssen Polymer Lösungskonzentration angepasst werden, um mögliche Vernetzung zu vermeiden. - Fügen Sie 1 mL APTES funktionalisiert SiO2 Perlen in DMSO (aus Schritt 2.1.4), die poly(PFPA)-Lösung suspendiert. Reagieren Sie bei RT für 1 h mit kräftig rühren.
- Isolieren Sie die poly(PFPA) gepfropft SiO2 Perlen durch Zentrifugation bei 10.000 x g für 5 min, gefolgt von der Entfernung des Überstands. Waschen Sie die Perlen durch Zugabe von 3 mL DMSO und Mischung von beiden schütteln mit der Hand oder einige Sekunden der Zellulite. Zentrifugieren Sie die Perlen bei 10.000 x g für 5 min, dann entfernen Sie den überstand. Wiederholen Sie waschen der poly(PFPA) gepfropft SiO2 Perlen mit DMSO zweimal.
- Waschen Sie die Perlen mit dreifach destilliertem Wasser (TDW) zwei Mal mehr. In diesem Schritt die Perlen mit 3 mL tdw zu kombinieren, dann durch Schütteln mit der Hand oder einige Sekunden der Zellulite mischen. Zentrifugieren Sie die Perlen bei 10.000 x g für 5 min, dann entfernen Sie den überstand.
- Um die Partikelgrößenverteilung zu überprüfen, führen Sie DLS Anschluss an das Verfahren in Schritt 2.1.5 beschrieben. Um die Oberflächenchemie zu überprüfen, führen Sie XPS nach dem Verfahren in Schritt 2.1.6 beschrieben.
- Bereiten Sie die poly(PFPA)-Lösung von 20 mg poly(PFPA) in 2 mL DMSO in einer 20 mL funkeln Durchstechflasche auflösen.
3. Vorbereitung der SiO2 Perlen mit PEG ersetzt Poly(PFPA) veredelt
- Um die poly(PFPA) Lösung vorzubereiten, lösen Sie auf, 20 mg poly(PFPA) in 2 mL DMSO in ein 20 mL-Fläschchen funkeln.
- Zur Vorbereitung der PEG-Lösung lösen sich Amin funktionalisiert PEG in 1 mL DMSO. Die genaue Höhe der PEG verwendet richtet sich nach den gewünschten Grad der PFP-Substitution, bestimmt durch die Gleichung unten gezeigt:
Betrag von amino-PEG (g/g-poly(PFPA)) = (N_poly(PFPA) x % PEG-Sub) X (MW_PEG / MW_poly(PFPA))
wo N_poly(PFPA) = poly(PFPA) Grad der Polymerisation
PEG-Sub % = Prozent PEG-Substitution
MW_PEG = Molekulargewicht von amino-PEG
MW_ poly(PFPA) = Molekulargewicht von poly(PFPA) - Übertragen Sie die PEG-Lösung auf die poly(PFPA)-Lösung. Reagieren Sie bei RT für 1 h mit kräftig rühren.
- Zur Vorbereitung des APTES funktionalisiert SiO2 Perlen in DMSO ausgesetzt, die gleichen Schritte in Schritt 2.1 gezeigt. 1 mL der Suspension Perle in die PEG ersetzt poly(PFPA) Lösung in Schritt 3.3 vorbereitet zu übertragen. Lassen Sie die Pfropfung zwischen poly(PFPA) und APTES funktionalisiert SiO2 Perlen um fortzufahren bei RT für 1 h mit kräftig rühren.
- Isolieren Sie die Perlen durch Zentrifugation bei 10.000 x g für 5 min, gefolgt von der Entfernung des Überstands. Waschen Sie die Perlen durch Zugabe von 3 mL DMSO und Mischung von beiden schütteln mit der Hand oder einige Sekunden der Zellulite. Zentrifugieren Sie die Perlen bei 10.000 x g für 5 min, dann entfernen Sie den überstand. Wiederholen Sie die DMSO waschen zweimal.
- Waschen Sie die Perlen zwei Mal mehr mit TDW. In diesem Schritt die Perlen mit 3 mL TDW zu kombinieren, dann durch Schütteln mit der Hand oder einige Sekunden der Zellulite mischen. Zentrifugieren Sie die Perlen bei 10.000 x g für 5 min, dann entfernen Sie den überstand.
- Trocknen Sie die Perlen bei 40 ° C in einem Vakuumofen über Nacht.
(4) Antikörper Immobilisierung auf Poly(PFPA) gepfropft SiO2 Perlen
Hinweis: Das gleiche Verfahren wird unabhängig von Prozent PEG-Substitution auf poly(PFPA) verwendet. Bereiten Sie Phosphat gepufferte Kochsalzlösung (PBS) durch Auflösen von PBS Tablet im TDW. Bereiten Sie 0,1 % (V/V) Phosphat gepufferte Kochsalzlösung mit Tween-20 (PBST vor) durch Zugabe von 1/1000 der Tween 20 in PBS.
- Fügen Sie 5 mg poly(PFPA) SiO2 Perlen zu einem 1,5 mL Microcentrifuge Schlauch veredelt.
- Waschen Sie die Perlen durch Hinzufügen von 800 µL PBS und Mischung gut aufschütteln. Zentrifugieren Sie den Perlen bei 10.000 x g bei RT für 1 min. Entfernen des Überstands und wiederholen Sie der Waschschritt dreimal.
- Fügen Sie 350 µL frische PBS, 50 µL 0,1 % (V/V) PBST und 6,67 µg des Antikörpers. Inkubieren Sie ~ 20 h auf ein Rotator bei 4 ° c
- Waschen Sie die Perlen um ungebundenen Antikörper zu entfernen. Zentrifugieren Sie die Perlen bei 400 X g und 4 ° C für 1 min. Überstands entfernen und hinzufügen 400 µL Lyse Puffers sorgfältig. Sanft wieder aussetzen der Perlen von fünf Mal rauf und runter pipettieren.
Hinweis: Lysis Puffer verwendet, um die Perlen zu waschen sollte die gleiche verwendet während der Zelle Lysis und IP, außer dass die Zugabe von Dithiothreitol und Protease-Hemmer sind optional, (siehe Schritt 5). - Wiederholen Sie diese Waschschritt dreimal. Entfernen Sie nach dem letzten Waschen den überstand so weit wie möglich.
(5) Zell-Lyse und Immunopräzipitation
- Vorbereitung der Lyse Puffer und Waschpuffer
- Bereiten Sie die Lyse-Puffer (50 mM Tris-HCl (pH 8,0), 100 mM KCl, 0,5 % (V/V) NP-40, 10 % (V/V) Glycerin, 1 mM Dithiothreitol (DTT) und Protease-Inhibitor cocktail).
- Bereiten Sie die Waschpuffer (50 mM Tris-HCl (pH 8,0), 100 mM KCl, 0,1 % (V/V) NP-40 und 10 % (V/V) Glycerin).
- Die Pufferlösungen bei 4 ° c lagern
- Vorbereitung der Zellen
- Saatgut (HeLa-Zellen) ein oder zwei Tage vor IP-Experiment, und wachsen die Zellen bei 37 ° C und 5 % CO2.
- Sammeln Sie etwa 1,4 x 107 Zellen mit einer Zelle Schaber und Transfer in ein 15 mL konische Röhrchen. Zentrifuge, die Zellen bei 380 X g bei RT für 3 min. Überstand zu entfernen und wieder mit 1 mL kaltem PBS und Überführung in ein 1,5 mL Microcentrifuge Schlauch auszusetzen.
- Zentrifugieren Sie die Zellen bei 10.000 x g bei 4 ° C für 30 S. Entfernen der Überstand sauber. Zelle Pellets können bei-80 ° C gelagert werden, nach dem Entfernen des Überstands.
- Vorbereitung der Zelle lysates
- Wieder aussetzen der Zelle Pellet mit 400 µL des Puffers Lyse. Beschallen Sie die Zellen mit einem Ultrasonicator.
- Nach der Beschallung, Vortex kurz und Zentrifuge lysate bei 20.000 x g bei 4 ° C für 10 Minuten.
- Übertragen Sie den Überstand auf eine neue 1,5 mL Zentrifugenröhrchen.
- Immunopräzipitation
- Übertragen Sie 300 µL der Zelle lysate auf vorbereitete Antikörper inkubiert poly(PFPA) gepfropft SiO2 Perlen. 30 µL der Zelle lysate als Eingabe Probe in einen neuen Microcentrifuge Schlauch zu behalten. Das Eingabesample bei 4 ° c lagern
Hinweis: Die Gesamtmenge an Protein in der Zelle lysate sollte etwa 4 mg. - Inkubieren Sie die lysate/Perlen-Mischung für 3 h auf ein Rotator bei 4 ° c
- Zentrifugieren Sie der Mischung auf 400 X g bei 4 ° C für 1 min. Entfernen des Überstands und fügen Sie 400 µL Waschpuffer sorgfältig hinzu. Aussetzen Sie vorsichtig wieder die Perlen durch Pipettieren etwa fünf Mal rauf und runter.
- Wiederholen Sie diese Waschschritt dreimal. Entfernen Sie nach dem letzten Waschen den überstand so weit wie möglich.
- Bereiten Sie 2 x Natrium-Dodecyl-Sulfat (SDS) laden Farbstoff (25 % (V/V) Glycerin, 0,1 % (w/V) Bromo Phenol blau (BPB), 60 mM Tris-HCl (pH 6,8), 2 % (w/V) SDS und 2,75 mM 2-Mercaptoethanol vor). Speicher 2 x SDS laden Farbstoff bei-20 ° C. Die Perlen und die gespeicherten Eingabesample 30 µL 2 x SDS laden Farbstoff hinzu, und Wärme sie für 10 min bei 95 ° C.
- Nach dem erhitzen, analysieren Sie die Probe mittels Western Blot-27oder speichern Sie das Sample bei-20 ° C.
- Übertragen Sie 300 µL der Zelle lysate auf vorbereitete Antikörper inkubiert poly(PFPA) gepfropft SiO2 Perlen. 30 µL der Zelle lysate als Eingabe Probe in einen neuen Microcentrifuge Schlauch zu behalten. Das Eingabesample bei 4 ° c lagern
Access restricted. Please log in or start a trial to view this content.
Ergebnisse
Ein Schaltplan für die Zubereitung von poly(PFPA) gepfropft SiO2 Perlen, mit oder ohne PEG Substitution ist in Abbildung 1dargestellt. Zur Überwachung der APTES und poly(PFPA) Prozess, nackten SiO2 Perlen, Pfropfung APTES funktionalisiert SiO2 Perlen und poly(PFPA) gepfropft SiO2 Perlen von DLS (Abbildung 2) und XPS (Abbildung 3) gekennzeichnet sind. IP-E...
Access restricted. Please log in or start a trial to view this content.
Diskussion
Die Synthese von poly(PFPA) gepfropft SiO2 Perlen in Abbildung 1dargestellt ist. Durch den Einsatz von APTES als Linker Molekül, können poly(PFPA) Bürsten kovalent gepfropft, SiO2 Substrat über einen einfachen Schritten zubereitet werden. Obwohl einige der PFP-Einheiten für die Reaktion mit APTES geopfert werden, sollen eine große Anzahl von PFP-Einheiten für spätere Reaktion mit amino-PEG oder Antikörper verfügbar bleiben. Die PFP-Gruppen sind dafür bekannt,...
Access restricted. Please log in or start a trial to view this content.
Offenlegungen
Die Autoren haben nichts preisgeben.
Danksagungen
Diese Arbeit wurde von der Agentur für Defense Development (Grant Nr. unterstützt. UD170039ID).
Access restricted. Please log in or start a trial to view this content.
Materialien
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
Referenzen
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
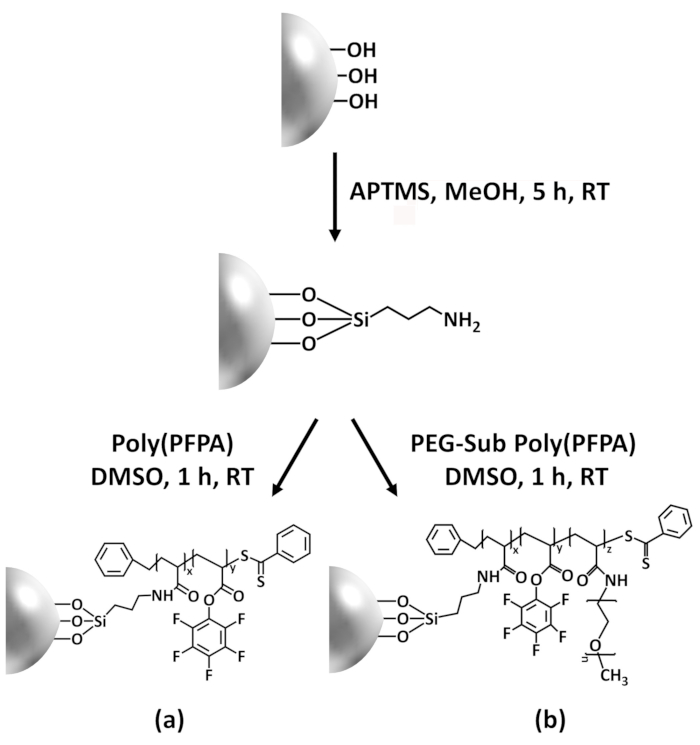
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
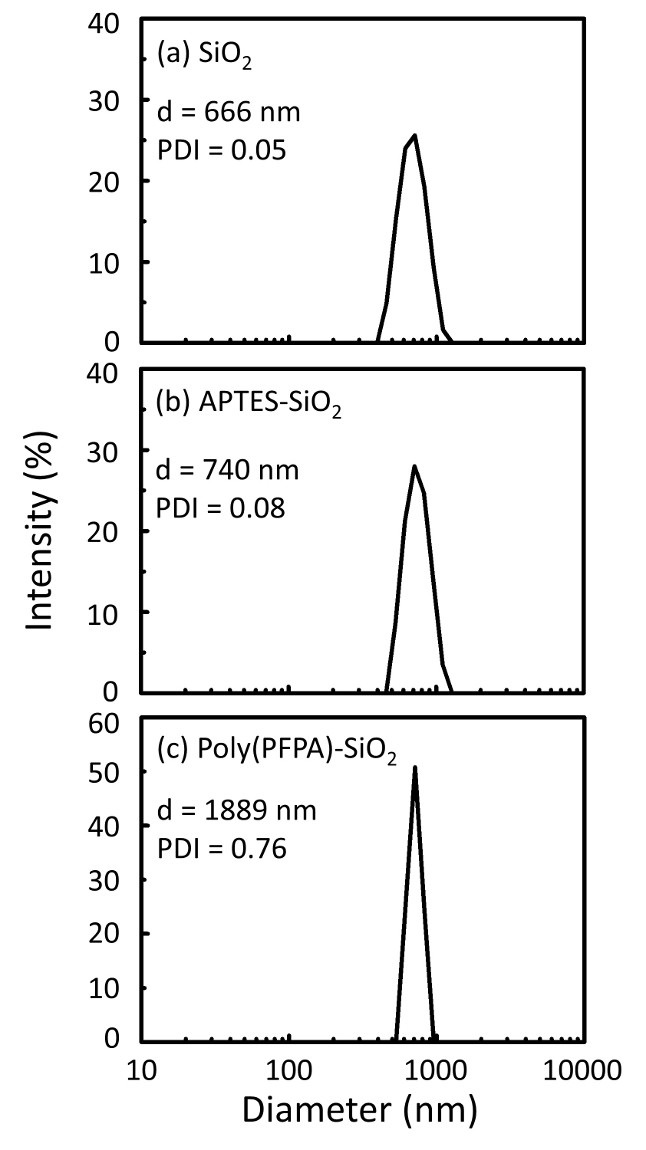
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
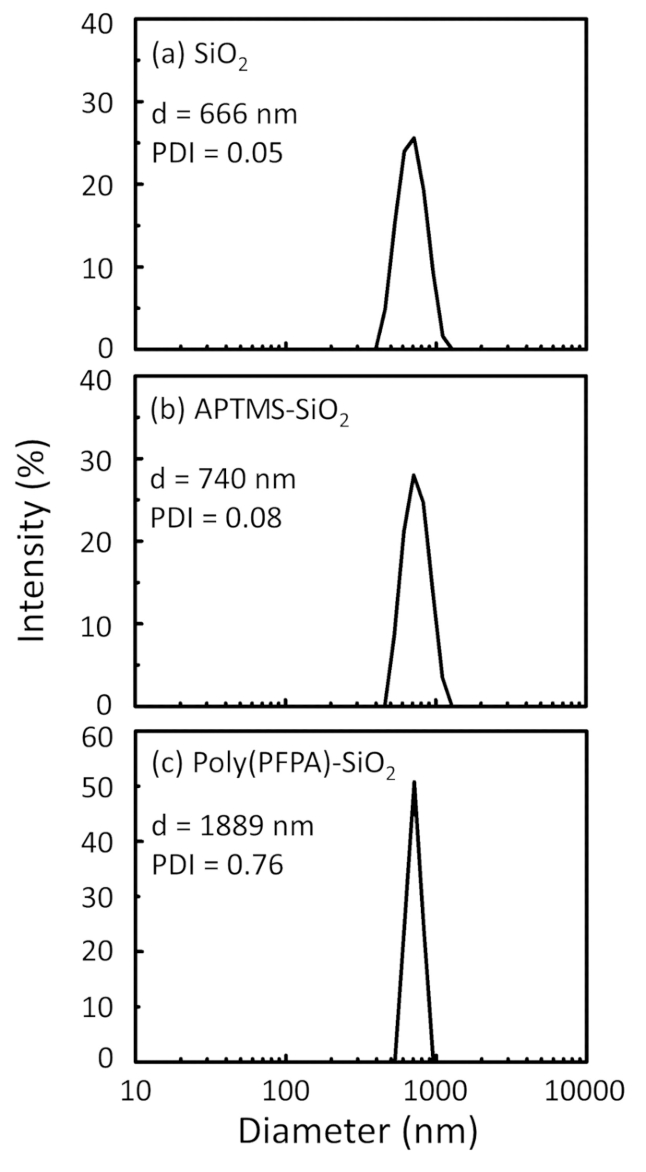
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
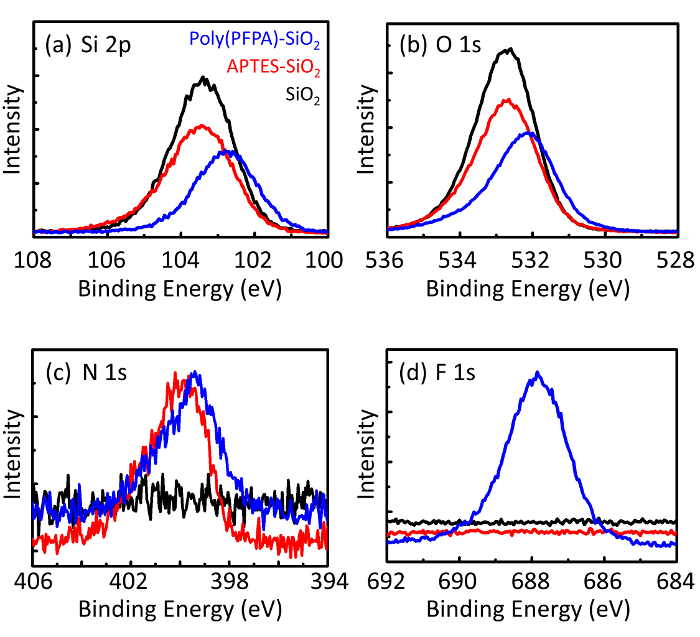
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
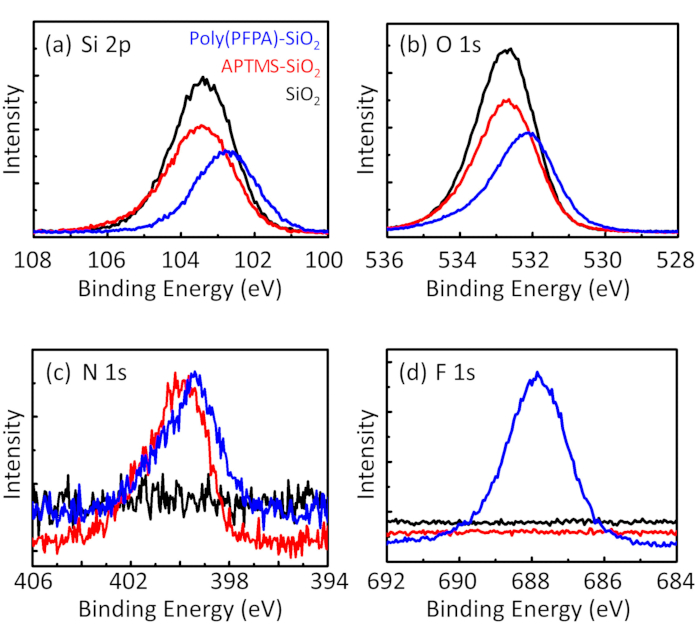
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
Nachdrucke und Genehmigungen
Genehmigung beantragen, um den Text oder die Abbildungen dieses JoVE-Artikels zu verwenden
Genehmigung beantragenThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten