É necessária uma assinatura da JoVE para visualizar este conteúdo. Faça login ou comece sua avaliação gratuita.
Method Article
Preparação de Poly(pentafluorophenyl acrylate) acrescida SiO2 grânulos para purificação de proteínas
Neste Artigo
Erratum Notice
Resumo
Um protocolo para a preparação de poli (acrilato de pentafluorophenyl) (poly(PFPA)) enxertados grânulos de sílica é apresentado. A superfície funcionalizados poly(PFPA) é então imobilizada com anticorpos e usada com sucesso para a separação de proteínas através da imunoprecipitação.
Resumo
Vamos demonstrar um método simples para preparar poli (acrilato de pentafluorophenyl) (poly(PFPA)) enxertados grânulos de sílica para imobilização de anticorpo e aplicação subsequente da imunoprecipitação (IP). A superfície enxertados poly(PFPA) é preparada através de um processo de duas etapas simples. Na primeira etapa, 3-aminopropyltriethoxysilane (APTES) é depositado como uma molécula de vinculador na superfície da sílica. Na segunda etapa, poly(PFPA) homopolímero, sintetizado através da adição reversível e polimerização de transferência (balsa) de cadeia de fragmentação, é enxertado à molécula de vinculador através da reação de troca entre as unidades de pentafluorophenyl (PFP) sobre o polímero e os grupos amina na APTES. A deposição de APTES e poly(PFPA) sobre a sílica, partículas são confirmadas por espectroscopia de fotoelétron de raios x (XPS), bem como monitoradas pela alteração de tamanho de partículas medido através de difusão dinâmica da luz (DLS). Para melhorar a superfície Hidrofilia dos grânulos, substituição parcial de poly(PFPA) com poly(ethylene glycol) acrescida de amina (amino-PEG) também é executada. O PEG-substituídos poly(PFPA) enxertados sílica grânulos então são imobilizados com anticorpos para aplicação de IP. Para demonstração, um anticorpo contra uma proteína quinase RNA-ativado (PKR) é empregado, e eficiência IP é determinada pela mancha ocidental. Os resultados da análise mostram que os grânulos de anticorpo imobilizado na verdade podem ser usados para enriquecer PKR enquanto interações não específicas da proteína são mínimas.
Introdução
Escovas de polímero reativo tem recebido muito interesse nos últimos anos. Eles podem ser usados para imobilizar moléculas funcionais em materiais orgânicos ou inorgânicos para criar superfícies registradas com aplicações em áreas como a deteção e a separação1,2,3,4, 5. Entre os polímeros reativos relatados, aqueles que contêm unidades de éster de pentafluorophenyl são particularmente útil devido a sua alta reatividade com aminas e resistência em direção a hidrólise6. Um tal polímero é poly(PFPA), e pode ser prontamente funcionalizado pós-polimerização com moléculas contendo aminas primárias ou secundárias7,8,9,10. Em um exemplo, escovas de poly(PFPA) foram reagiu com amino-spiropyrans para criar superfícies luz-responsivo7.
A preparação do poly(PFPA) e suas aplicações têm sido descritos em algumas das anteriores Publicações6,7,8,9,10,11,12 ,13,14,15,16,17. Em particular, Theato e colaboradores relataram a síntese de escovas poly(PFPA) via tanto "enxertia para" e "enxertia de" métodos7,8,10,11,12 . Na "enxertia para" abordagem, um poli (methylsilsesquioxane)-poli (acrilato de pentafluorophenyl) (polímero híbrido de poly(MSSQ-PFPA)) foi sintetizado8,10,11,12. O componente de poly(MSSQ) foi capaz de adesão forte de formulário com um número de diferentes superfícies orgânicas e inorgânicas, permitindo assim o componente poly(PFPA) formar uma camada de pincel sobre a superfície revestida de material. Na "enxertia de" abordagem, superfície iniciada adição reversível e polimerização de transferência (SI-JANGADA) fragmentação da cadeia foi empregada para preparar poly(PFPA) pincéis7. Neste caso, um agente de transferência de corrente da superfície de imobilizado (SI-CTA) primeiro era ligado covalentemente ao substrato através de reação sílica-silano. SI-CTA imobilizado em seguida, participou a polimerização de SI-JANGADA de monômeros de espionagem, gerando densamente poly(PFPA) escovas com ligação covalente estável ao substrato.
Utilizando as escovas poly(PFPA) sintetizadas através da polimerização de SI-JANGADA, recentemente Demonstramos a imobilização dos anticorpos sobre partículas de sílica poly(PFPA) enxertados e sua subsequente aplicação na purificação de proteína18. O uso de escovas de poly(PFPA) para a imobilização de anticorpo foi encontrado para resolver uma série de problemas associados a atual separação de proteínas através de IP. IP convencional baseia-se na utilização de proteína A/G como um vinculador para anticorpo imobilização19,20,21. Desde que o uso de proteína A/G permite que os anticorpos ser anexado com uma orientação específica, eficiência de recuperação de antígeno alvo alto é alcançada. No entanto, o uso de proteína A/G sofre de interação de proteínas não específicas, bem como a perda de anticorpos durante a recuperação de proteína, os quais contribuem para um elevado nível de ruído de fundo. Para resolver essas deficiências, ligando direto dos anticorpos a um sólido apoio tem sido explorada22,23,24. A eficiência dessas técnicas é geralmente baixa devido a orientação aleatória dos anticorpos quitosana. Para o substrato poly(PFPA) enxertadas, a imobilização de anticorpos é permanente, conseguida através da reação de troca entre unidades PFP e funcionalidades de amina em anticorpos. Embora a orientação de anticorpo é ainda aleatória, o sistema beneficia-se de ter muitos sites PFP reativas, controláveis pelo grau de polimerização. Além disso, mostramos que, por substituição parcial das unidades PFP com amino-PEG, Hidrofilia superfície pode ser ajustada, melhorando assim a eficiência de recuperação de proteínas do sistema18. Em geral, as partículas de sílica poly(PFPA) enxertadas foram mostradas para ser uma alternativa eficaz ao IP tradicional com razoável eficiência, bem como a experiência mais limpa.
Esta contribuição, nós relatamos um método alternativo para preparar poly(PFPA) superfície enxertados para imobilização de anticorpo e aplicação de IP. Em um processo de duas etapas simples, conforme ilustrado na Figura 1, uma molécula de vinculador APTES é primeiro depositada na superfície da sílica, em seguida, o polímero de poly(PFPA) está covalentemente ligado à molécula de vinculador através da reação entre as unidades PFP a polímero e as funções Amina na APTES. Esse método de preparação permite a reticulação permanente de poly(PFPA) a uma superfície de substrato, mas evita as muitas complicações associadas à síntese de SI-CTA e polimerização de SI-JANGADA de escovas poly(PFPA). Substituição parcial das unidades com amino-PEG PFP ainda pode ser executada, permitindo o ajuste fino de propriedades escova da superfície do polímero. Mostramos que os grânulos de sílica poly(PFPA) enxertado assim preparados podem ser imobilizados com anticorpos e utilizados para o enriquecimento da proteína através do IP. O procedimento de preparação detalhada do grânulo, imobilização do anticorpo e teste de IP estão documentadas neste artigo, para os leitores interessados na busca de uma alternativa à convencional proteína A/G com base em IP.
Access restricted. Please log in or start a trial to view this content.
Protocolo
1. preparação de homopolímero de Poly(PFPA)
- Recristalização de AIBN
- Combine 5 g de 2,2'-azobis(2-methylpropionitrile) (AIBN) com 25 mL de metanol em um copo de 250 mL. Mergulhe o béquer em banho de óleo 60 ° C e, em seguida, agitar vigorosamente a mistura com uma barra de agitação até AIBN é totalmente dissolvido.
- Filtrar a solução quente através de papel de filtro (5-8 μm retenção de partículas) e armazene o filtrado a 4 ° C, para permitir que os cristais de forma lenta.
- Recolha o AIBN recrystallized por filtração. Combinar o produto coletado com 25 mL de metanol fresco e repetir o processo de recristalização.
- O 2 x recrystallized AIBN em um forno a vácuo à temperatura ambiente (RT) seca durante a noite. Armazenar o produto no escuro a <-10 ° C.
- Síntese de benzil dithiobenzoate25
- Prepare um balão de fundo redondo 500 mL três-pescoço equipado com uma barra de agitação magnética, um condensador de refluxo, um funil e um septo de borracha. Ligar o balão para a linha de gás nitrogênio através do condensador de refluxo e expulsar o interior ar com nitrogênio. Inserir um termômetro através do septo. Adicione 41 mL (0,041 mol) de uma solução 1 M de brometo de fenilmagnésio em tetrahidrofurano (THF) através de uma seringa através do septo mesmo.
- Aquecer a solução de brometo de fenilmagnésio a 40 ° C em banho de óleo. Em seguida, adicione 3,1 g (0,041 mol) de dissulfeto de carbono através do funil caindo lentamente, mantendo a temperatura da solução a 40 ° C.
- Adicione 7,1 g (0.042 mol) de brometo de benzila à mistura resultante através do funil mais 15 min. aumento da temperatura de reação de 50 ° C. Continue mexendo a esta temperatura durante 45 min.
- Transferir a mistura reacional para um funil de separação e diluir com 15 mL de água gelada. Extrair o produto adicionando-se 15 mL de éter dietílico e remover a camada inferior de água. Repita a extração com éter dietílico mais duas vezes.
- Lave as fases orgânicas combinadas com abundante quantidade de água e, em seguida, salmoura (solução de 50% (p/v) NaCl em água) e secagem do produto sobre o sulfato de magnésio anidro.
- Remova o solvente no vácuo a 35 ° C, usando um evaporador rotativo.
- Purifica o produto por cromatografia em coluna utilizando 400 mL de gel de silicone (tamanho do pore 60 Å, tamanho de partícula de malha de 63-200) e éter de petróleo como o eluente, rendendo 5 g de benzil dithiobenzoate (BDB) como óleo vermelho. Confirmar a pureza do produto por 1H NMR (400 MHz, CDCl3): δ 8.02-7.99 (2 H, m), 7,55-7,50 (1h, m), 7,41-7.29 (7 H, m), 4,60 (2 H, s).
- Síntese de poly(PFPA) via balsa polimerização9,26
- Monômero de espionagem disponível comercialmente contém pequena quantidade de inibidores. Antes de polimerização, retire os inibidores, passando o monômero, através de uma seringa descartável, embalada com alumina básica.
- Adicione 0,4 mg (0.0024 mmol) de AIBN recrystallized, 4,3 mg (0,018 mmol) de BDB, 1012 mg (4,25 mmol) de espionagem livre de inibidor e 0,7 mL de anidro anisole de Schlenk de 20 mL.
- Ligar o balão ao Schlenk linha e desgaseificar a mistura pelo menos três ciclos de gelo-degelo-bomba. Brevemente, congele a mistura reacional em um banho de nitrogênio líquido. Aplica o vácuo para remover o gás no headspace. Selo do frasco, em seguida, remover longe de nitrogênio líquido para permitir que o conteúdo descongelar no RT
- Colocar o balão em banho de óleo de 70 ° C e reagir durante 4 h sob expurgo de2 N.
- Para finalizar a reação, retirar o frasco do banho de óleo e expor o conteúdo de reação ao ar.
- Precipitar o polímero em metanol a frio e, em seguida, secar o polímero recuperado em um forno de vácuo a 40 ° C durante a noite.
- Para medir o peso molecular do polímero, use cromatografia de permeação de gel (GPC). Use o THF como taxa de fluxo da fase móvel a 35 ° C, com um 1 mL/min e construir a curva de calibração usando padrões de poliestireno monodisperso. Para adquirir a medição do GPC, dissolva o polímero em THF (1-2 mg/mL) e filtro através de filtro de descartáveis politetrafluoretileno (PTFE) 0,2 μm. Injete 100 μL da amostra dentro do instrumento do GPC. Converta o tempo de retenção de amostra medido em peso molecular utilizando a curva de calibração de poliestireno.
2. preparação de Poly(PFPA) acrescida de SiO2 grânulos
- Tratamento de SiO2 contas com APTES
- SiO2 partículas estão disponíveis sob a forma de uma suspensão aquosa de 5% (p/v). Combine a 0,8 mL de SiO2 suspensão com 40 mg de APTES e 8 mL de metanol em um frasco de 20 mL cintilação equipado com uma barra de agitação.
- Permitir que a reação prosseguir no RT para 5 h com agitação vigorosa.
- Transferi a solução para um tubo cônico. Para isolar o APTES acrescida SiO2 grânulos, centrifugar a solução a 10.000 x g por 5 min e, em seguida, remover o sobrenadante. Lave os grânulos re-dispersando-os em 3 mL de metanol fresco. Agitar o tubo com a mão para a mistura, mas se necessário, melhorar a dispersão pelo sonication em banho-maria por alguns segundos. Centrifugue os grânulos a 10.000 x g durante 5 min. Retire o sobrenadante e repetir a etapa de lavagem mais uma vez.
- Combine o metanol lavado SiO2 grânulos com 3 mL de Dimetilsulfóxido (DMSO). Agitar a mistura com a mão, ou se necessário proceda à sonicação durante alguns segundos, até que os grânulos são totalmente dispersos em DMSO. Centrifugue os grânulos a 10.000 x g por 5 min e, em seguida, remover o sobrenadante. Repita a etapa para assegurar a completa troca solvente metanol de DMSO.
Nota: A suspensão final contém os APTES acrescida SiO2 grânulos dispersaram em 4 mL de DMSO. - Para verificar a distribuição de tamanho de partícula, realizar análise DLS. Tome uma gota da suspensão preparada no passo 2.1.4 e lugar em uma cubeta UV descartável. Diluir a amostra preenchendo a cubeta com DMSO fresco até que é 2/3 cheio. Introduza a amostra no suporte de célula para começar a aquisição de dados. Para medição de tamanho de partícula, use os seguintes parâmetros de configuração: temperatura: 25 ° C; Tempo da equilibração: 120 s; Duração da medição: automático.
- Para verificar a composição da superfície, realizar análise XPS. Seca uma pequena amostra da suspensão preparada na etapa 2.1.4 na estufa de vácuo a 40 ° C durante a noite. Pegue o polímero seco e pacote uniformente um titular de amostra de 0,5 x 0,5 cm. Carregar a amostra na câmara de alto vácuo (10-8 torr) e começar a aquisição de dados. Para o instrumento XPS particular usado, gerar os photoelectrons usando um monocromático Al Kα raios-x operado em 15 kV e mA 6,7 e coletar usando ampliação de modo híbrido com o analisador em um eV 50 passam energia para espectros de alta resolução e um eV 100 passar energia para inquéritos elementares.
- Enxertia de poly(PFPA) para APTES acrescida de SiO2 grânulos
- Prepare a solução de poly(PFPA) dissolvendo-se 20 mg de poly(PFPA) em 2 mL de DMSO em um frasco de cintilação de 20 mL.
Nota: Neste estudo, é usado um poly(PFPA) relativamente baixo peso molecular (20 kg/mol). Assim, apesar da concentração do polímero elevado (10 mg/mL), sem evidência de reticulação do polímero é observada. Se for usado um polímero de peso molecular mais elevado, concentração da solução de polímero pode precisar ser ajustada para evitar possível reticulação. - Adicione 1 mL de APTES acrescida de SiO2 grânulos suspendidos em DMSO (da etapa 2.1.4) para a solução de poly(PFPA). Reagem na RT para 1 h com agitação vigorosa.
- Isole os grânulos de2 SiO poly(PFPA) enxertados por centrifugação a 10.000 x g durante 5 min, seguido pela remoção do sobrenadante. Lave os grânulos adicionando-se 3 mL de DMSO e misturar ou agitando com a mão ou alguns segundos de sonication. Centrifugue os grânulos a 10.000 x g por 5 min e, em seguida, remover o sobrenadante. Repeti a lavagem dos poly(PFPA) enxertados SiO2 grânulos com DMSO duas vezes.
- Lave os grânulos mais duas vezes com água destilada triplo (TDW). Nesta etapa, combine os grânulos com 3 mL de TDW e, em seguida, misturar por agitação com a mão ou alguns segundos de sonication. Centrifugue os grânulos a 10.000 x g por 5 min e, em seguida, remover o sobrenadante.
- Para verificar a distribuição de tamanho de partícula, execute DLS seguindo o procedimento descrito na etapa 2.1.5. Para verificar a química de superfície, execute XPS seguindo o procedimento descrito na etapa 2.1.6.
- Prepare a solução de poly(PFPA) dissolvendo-se 20 mg de poly(PFPA) em 2 mL de DMSO em um frasco de cintilação de 20 mL.
3. preparação de SiO2 grânulos enxertado com Poly(PFPA) PEG-substituídos
- Para preparar a solução de poly(PFPA), dissolva 20 mg de poly(PFPA) em 2 mL de DMSO em um frasco de cintilação de 20 mL.
- Para preparar a solução de PEG, dissolva PEG amina acrescida em 1 mL de DMSO. A quantidade exata de PEG usado é determinado pelo grau desejado de substituição de PFP, determinada pela equação mostrada abaixo:
Quantidade de amino-PEG (g/g-poly(PFPA)) = (N_poly(PFPA) x % PEG-Sub) x (MW_PEG / MW_poly(PFPA))
onde N_poly(PFPA) = poly(PFPA) grau de polimerização
% De PEG-Sub = porcentagem PEG de substituição
MW_PEG = peso molecular do amino-PEG
MW_ poly(PFPA) = peso molecular de poly(PFPA) - Transferi a solução de PEG para a solução de poly(PFPA). Reagem na RT para 1 h com agitação vigorosa.
- Para preparar APTES acrescida SiO2 grânulos suspensos em DMSO, siga os mesmos passos mostrados no passo 2.1. Transferi 1 mL da suspensão do grânulo para a solução de PEG-substituídos poly(PFPA) preparada no passo 3.3. Permitir que a enxertia entre poly(PFPA) e APTES acrescida de SiO2 grânulos para prosseguir na RT para 1 h com agitação vigorosa.
- Isole os grânulos por centrifugação a 10.000 x g durante 5 min, seguido pela remoção do sobrenadante. Lave os grânulos adicionando-se 3 mL de DMSO e misturar ou agitando com a mão ou alguns segundos de sonication. Centrifugue os grânulos a 10.000 x g por 5 min e, em seguida, remover o sobrenadante. Repita a lavagem de DMSO duas vezes.
- Lave os grânulos duas vezes mais com TDW. Nesta etapa, combine os grânulos com 3ml TDW e, em seguida, misturar por agitação com a mão ou alguns segundos de sonication. Centrifugue os grânulos a 10.000 x g por 5 min e, em seguida, remover o sobrenadante.
- Os grânulos a 40 ° C em um forno a vácuo seca durante a noite.
4. anticorpo imobilização na Poly(PFPA) enxertados SiO2 grânulos
Nota: O mesmo procedimento é usado independentemente de substituição de PEG por cento na poly(PFPA). Prepare salina tamponada fosfato (PBS), dissolvendo o tablet de PBS em TDW. Prepare o soro de tampão fosfato 0,1% (v/v) com Tween-20 (PBST) adicionando 1/1000 de Tween-20 a PBS.
- Adicione 5 mg de poly(PFPA) enxertados SiO2 grânulos para um tubo de microcentrifugadora de 1,5 mL.
- Lave os grânulos adicionando 800 µ l de PBS e misture bem vortexing. Centrifugue os grânulos a 10.000 x g em RT para 1 min. Retire o sobrenadante e repetir a etapa de lavagem três vezes.
- Adicione 350 µ l de PBS fresco e 50 µ l 0,1% (v/v) PBST µ g 6,67 do anticorpo. Incube ~ 20 h em rotacao a 4 ° C.
- Lave os grânulos para remover anticorpos não acoplados. Centrifugue os grânulos a 400 x g e 4 ° C, durante 1 min. Retire o sobrenadante e adicionar 400 µ l de tampão de Lise cuidadosamente. Delicadamente, re-suspenda os grânulos pipetando para cima e para baixo por cinco vezes.
Nota: Tampão de Lise costumava lavar os grânulos deve ser o mesmo usado durante a lise celular e IP, exceto que a adição de ditiotreitol, inibidor de protease são opcionais, (Veja passo 5). - Repita essa etapa de lavagem três vezes. Após a lavagem final, remova o máximo possível de sobrenadante.
5. pilha Lysis e imunoprecipitação
- Preparação de Lise e tampão de lavagem
- Prepare a lise (50 mM Tris-HCl (pH 8.0), 100 mM KCl, 0,5% (v/v) NP-40, 10% (v/v) glicerol, 1mm ditiotreitol (DTT) e protease inibidor cocktail).
- Prepare o tampão de lavagem (50 mM Tris-HCl (pH 8.0), 100 mM KCl, 0,1% (v/v) NP-40 e 10% (v/v) glicerol).
- Armazenar as soluções-tampão a 4 ° C.
- Preparação das células
- As células (células HeLa) de sementes de um ou dois dias antes do experimento IP e crescem as células a 37 ° C e 5% de CO2.
- Colete cerca de 1.4 x 107 células com um raspador de célula e transferência em um tubo cônico de 15 mL. Centrifugação, as células a 380 x g em RT por 3 min. Retire o sobrenadante e ressuspender com 1 mL de frio PBS e transferir para um tubo de microcentrifugadora de 1,5 mL.
- Centrifugar as células a 10.000 x g a 4 ° C durante 30 s. remover o sobrenadante limpo. Aglomerados de células podem ser armazenados a-80 ° C depois de retirar o sobrenadante.
- Preparação de lisados celulares
- Ressuspender o centrifugado com 400 µ l da lise. Proceda à sonicação as células usando um ultrasonicator.
- Após o sonication, vórtice brevemente e centrifugar o lisado a 20.000 x g a 4 ° C por 10 min.
- Transferi o sobrenadante para um novo tubo de centrifugação de 1,5 mL.
- Imunoprecipitação
- Transferência de 300 µ l de lisado celular anticorpo previamente preparado incubado poly(PFPA) enxertados SiO2 talões. Reter 30 µ l da célula lisada como o exemplo de entrada em um novo tubo de microcentrifugadora. Armazenar o exemplo de entrada a 4 ° C.
Nota: A quantidade total de proteína no lisado celular deve ser de aproximadamente 4 mg. - Incubar a mistura de lisado/grânulos para 3h em rotacao a 4 ° C.
- Centrifugue a mistura a 400 x g a 4 ° C, durante 1 min. Retire o sobrenadante e adicionar 400 µ l de tampão de lavagem com cuidado. Delicadamente, re-suspenda os grânulos pipetando para cima e para baixo cerca de cinco vezes.
- Repita essa etapa de lavagem três vezes. Após a lavagem final, remova o máximo possível de sobrenadante.
- Prepare 2 x sódio Dodecil sulfato de sódio (SDS) carregando tintura (glicerol de 25% (v/v), 0,1% (p/v) bromo fenol azul (BPB), 60 mM Tris-HCl (pH 6,8), 2% (p/v) SDS e 2,75 mM 2-Mercaptoetanol). Loja 2 x tintura de carregamento SDS a-20 ° C. Adicionar 30 µ l de 2 x tintura de carregamento SDS para as contas e o exemplo de entrada armazenado e aquecê-los durante 10 minutos a 95 ° C.
- Após o aquecimento, analisar a amostra usando a mancha27ocidental, ou armazenar a amostra a-20 ° C.
- Transferência de 300 µ l de lisado celular anticorpo previamente preparado incubado poly(PFPA) enxertados SiO2 talões. Reter 30 µ l da célula lisada como o exemplo de entrada em um novo tubo de microcentrifugadora. Armazenar o exemplo de entrada a 4 ° C.
Access restricted. Please log in or start a trial to view this content.
Resultados
Um esquema para a preparação de poly(PFPA) enxertado SiO2 grânulos, com ou sem PEG substituição é mostrada na Figura 1. Para monitorar o APTES e poly(PFPA) enxertia processo, desencapado SiO2 grânulos, APTES acrescida de SiO2 grânulos, e poly(PFPA) enxertados SiO2 miçangas caracterizam-se por DLS (Figura 2) e XPS (Figura 3). Eficiências IP dos gr?...
Access restricted. Please log in or start a trial to view this content.
Discussão
A síntese de poly(PFPA) enxertados SiO2 grânulos é ilustrado na Figura 1. Empregando APTES como uma molécula de vinculador, escovas poly(PFPA) covalentemente enxertadas de SiO2 substrato podem ser preparadas através de um processo de duas etapas simples. Embora algumas das unidades de PFP são sacrificadas para a reação com APTES, um grande número de unidades a PFP deverão permanecer disponível para posterior reação com anticorpos ou amino-PEG. Os grupos PFP...
Access restricted. Please log in or start a trial to view this content.
Divulgações
Os autores não têm nada para divulgar.
Agradecimentos
Este trabalho foi financiado pela Agência para o desenvolvimento de defesa (Grant no. UD170039ID).
Access restricted. Please log in or start a trial to view this content.
Materiais
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
Referências
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
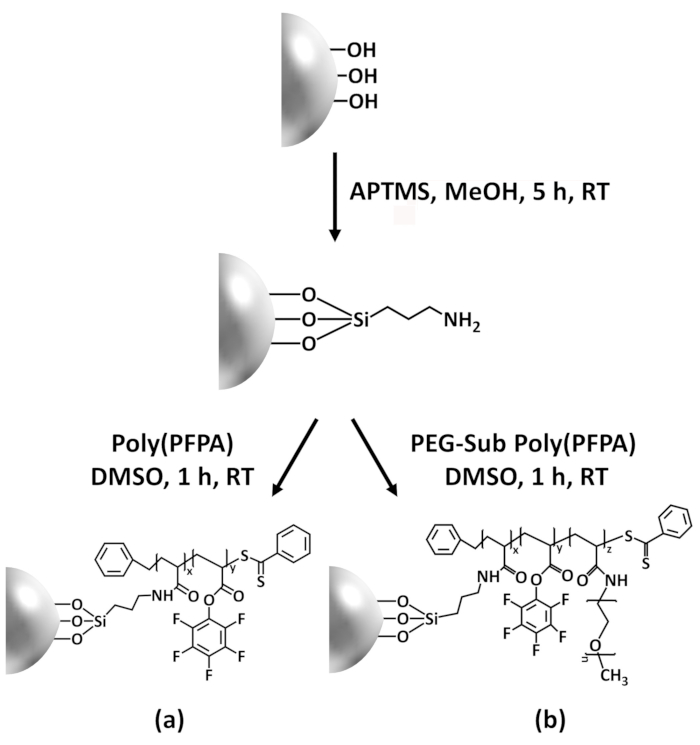
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
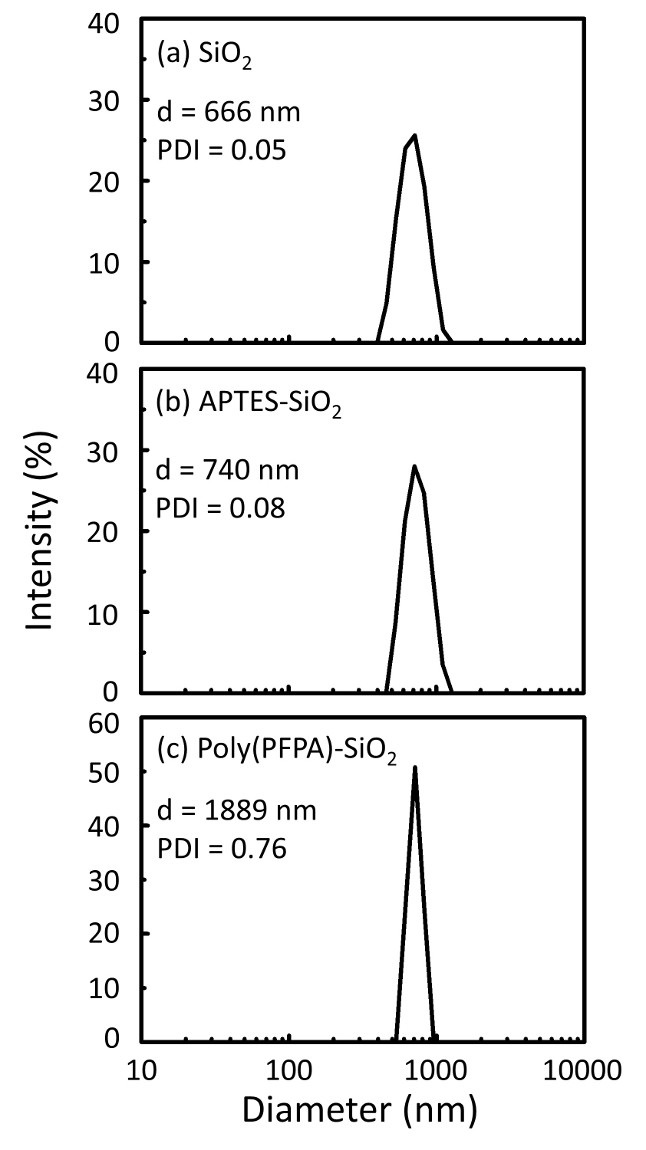
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
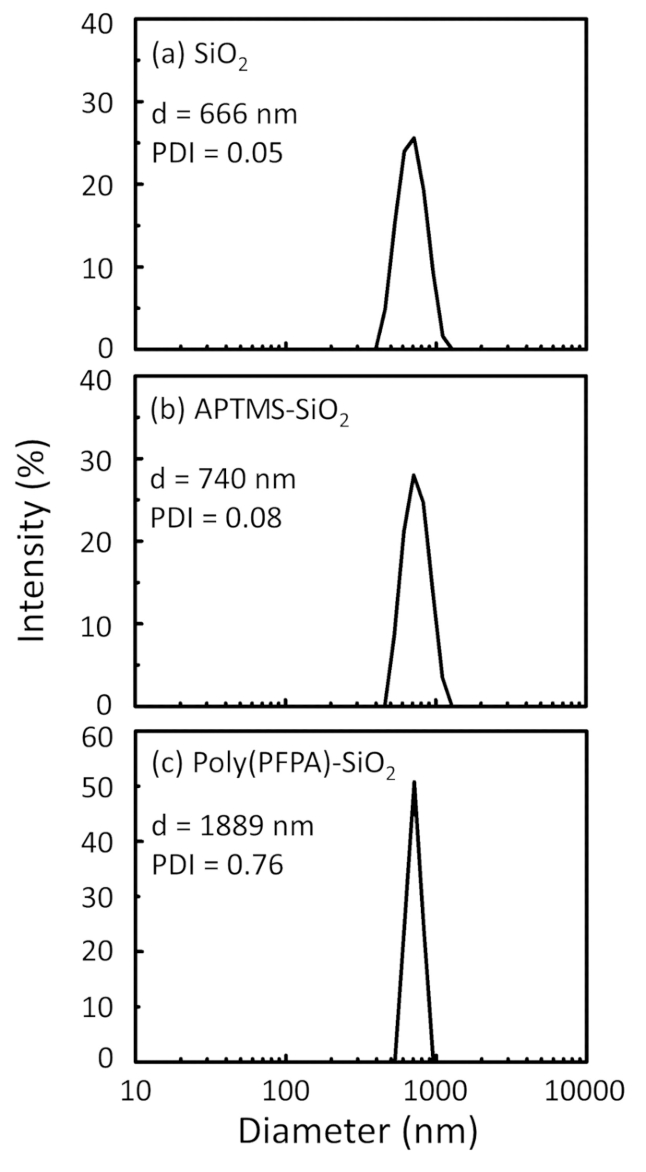
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
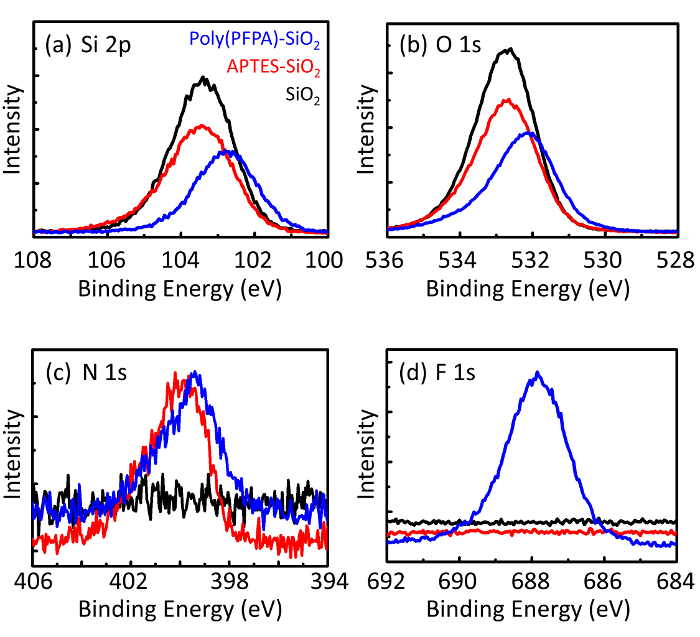
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
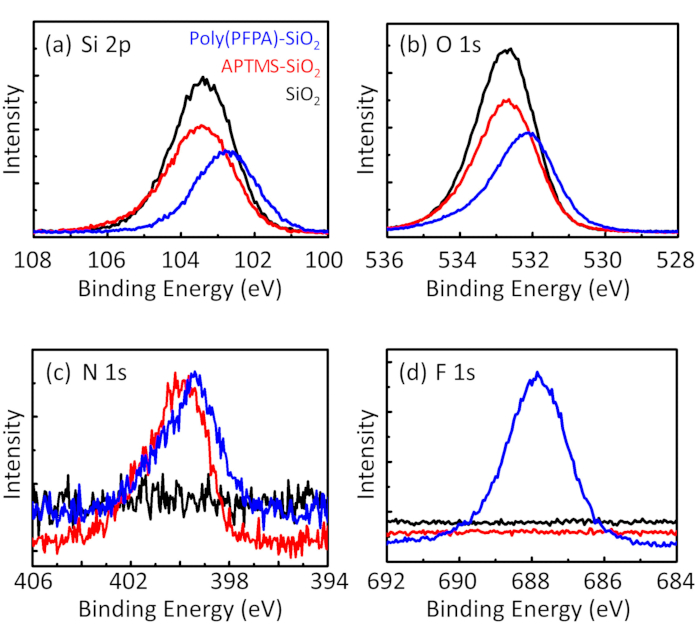
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
Reimpressões e Permissões
Solicitar permissão para reutilizar o texto ou figuras deste artigo JoVE
Solicitar PermissãoThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados