このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
蛋白質の浄化のため Poly(pentafluorophenyl acrylate) 機能 SiO2ビーズの準備
Erratum Notice
要約
ポリ (アクリル酸ペンタフルオロフェニル) の準備のためのプロトコル (poly(PFPA)) 接木シリカビーズを提示。Poly(PFPA) 機能性表面は抗体を固定化し、正常に免疫沈降法による蛋白質の分離のために使用します。
要約
ポリ (アクリル酸ペンタフルオロフェニル) を準備する簡単な方法を示す (poly(PFPA)) は、抗体の固定化とそれに続く免疫沈降 (IP) アプリケーションのシリカビーズを移植しました。Poly(PFPA) グラフト表面は、単純な 2 段階のプロセスを介して用意しています。最初のステップで 3-アミノプロピルトリエトキシシラン (APTES) は、シリカ表面にリンカー分子として預けられます。2 番目のステップでペンタフルオロフェニル (PFP) 単位間の交換反応によってリンカー分子に可逆的付加と断片化チェーン転送 (いかだ) 重合により合成した poly(PFPA) ホモポリマーを移植で、ポリマーと APTES のアミン グループ。APTES とシリカ粒子は x 線光電子分光法 (XPS) により確認し同様、粒子サイズの変更によって監視 poly(PFPA) の沈着は、動的光散乱 (DLS) を介して測定。ビーズ、アミン官能基化 poly(ethylene glycol) と poly(PFPA) の一部代替の表面の親水性を向上させる (アミノ PEG) が行われます。ペグ-置換 poly(PFPA) は、ビーズが IP アプリケーションのための抗体を固定化し、シリカを移植しました。デモで、タンパク質キナーゼ (PKR) rna 抗体を採用、IP 効率は西部にしみが付くことによって決定されます。分析の結果, 抗体固定化ビーズを無指定蛋白質の相互作用は最小限、PKR を豊かに使用確かにできます。
概要
反応性ポリマー ブラシは、近年多くの関心を受けています。検出と分離1,2,3,4などの分野でのアプリケーションと非アクティブなサーフェスを作成する有機又は無機の物質に機能分子を固定化する使用することができます。 5。報告された反応性高分子の中でペンタフルオロフェニル エステル単位を含む、アミンと加水分解6に向けて耐反応性が高いため特に便利です。1 つのようなポリマーは poly(PFPA) と、それはプライマリまたはセカンダリのアミン7,8,9,10を含む分子と容易に官能の後重合をすることができます。1 つの例で、poly(PFPA) ブラシした光応答性表面7を作成するアミノ スピロピランと反応しました。
Poly(PFPA) とその応用の準備は前出版物6,7,8,9,10、11,12 の番号に記載されています。 ,13,14,15,16,17。特に、Theato と同僚は報告「移植」と""方法7,8,10、11,12から移植の両方を介して poly(PFPA) ブラシの合成.「接木する」アプローチは、ポリ (メチルシルセスキオキサン) の-ポリ (アクリル酸ペンタフルオロフェニル) (poly(MSSQ-PFPA)) ハイブリッド ・ ポリマーは合成8,10、11,12。Poly(MSSQ) コンポーネントはフォーム数材料表面上ブラシ層を形成するコンポーネントの poly(PFPA) をように別の有機・無機表面と密着できた。「接木から」アプローチ、表面は可逆的付加を開始され、断片化チェーン転送 (SI ・ ラフト) 重合を用いて poly(PFPA) ブラシ7を準備します。この場合、表面固定化連鎖移動剤 (SI CTA) 最初共有についたシリカ シラン反応による基板。固定化 SI CTA は、基板に安定した共有結合のリンケージを持つ密集 poly(PFPA) ブラシを生成する PFPA モノマーの SI ・ ラフト重合し参加しました。
SI ・ ラフト重合により合成した poly(PFPA) ブラシを活用し、最近移植 poly(PFPA) シリカ粒子やタンパク質精製18で、後続のアプリケーションにおける抗体の固定化を示した。IP で現在の蛋白質の分離に関連する問題の数を解決する抗体固定化のための poly(PFPA) ブラシの使用が見つかりました。従来の IP は、抗体固定化19,20,21のタンパク質 A/G リンカーとしての使用に依存します。タンパク質 A/G の特定の方向で接続する抗体を使用、高いターゲット抗原回復効率が達成されました。ただし、タンパク質 A/G の使用苦しんでいる抗体の損失と同様、非特異的タンパク質間相互作用タンパク質の回収、どちらもはバック グラウンド ノイズの高レベルに貢献中。これらの欠点を解決するには、しっかりサポートする抗体の直接架橋検討22,23,24をされています。このような技術の効率は架橋抗体のランダムな向きがあるため通常は低くなります。接木 poly(PFPA) 基板の抗体の固定化は永久、PFP ユニットと抗体のアミン機能間の交換反応によって達成されます。抗体の向きはまだランダムが、システム多く反応 PFP サイト、重合度によって制御を持っていることから寄与します。さらに、我々 はアミノ止め釘と PFP 単位の部分的な置換によることを示した表面親水性調整できるさらにシステム18のタンパク質回収効率を向上します。全体的にみて、多くのクリーナーの背景と同様、合理的な効率で従来の IP へ有効な代わりをする接木 poly(PFPA) シリカ粒子が示されていた。
この貢献抗体固定化と IP アプリケーションを poly(PFPA) グラフト表面を準備する方法を報告する.単純な 2 段階のプロセスで図 1に示すように、APTES のリンカー分子最初に到着したシリカ表面にし、poly(PFPA) 高分子に PFP ユニット間反応によってリンカー分子に接続した、ポリマーと APTES のアミン機能。本調製法は基板表面に poly(PFPA) の永久的な架橋が可能が SI CTA 合成と poly(PFPA) ブラシの SI ・ ラフト重合に関連する多くの合併症を回避します。アミノ ペグの PFP ユニットの部分置換実行できますが、ポリマー ブラシ表面特性の微調整可能します。従って準備 poly(PFPA) 接木シリカビーズを抗体を固定化して IP を介したタンパク質の濃縮に使用できますを示します。詳細なビード作製手順、抗体固定化と IP テストは、この資料に記載されて、読者を求めて興味従来タンパク質 A/G に代わるベースの IP。
Access restricted. Please log in or start a trial to view this content.
プロトコル
1. Poly(PFPA) ホモポリマーの準備
- AIBN の再結晶
- 250 mL のビーカーにメタノール 25 ml 2,2'-azobis(2-methylpropionitrile) (AIBN) の 5 g を組み合わせます。60 ° C のオイルバスでビーカーに没頭し、精力的に AIBN は完全に溶解するまで攪拌棒との混合物をかき混ぜます。
- ろ紙 (5-8 μ m 粒子保持) を通じて暖かいソリューションをフィルター処理し、ゆっくりと結晶の形成を許可する 4 ° C で濾液を格納します。
- ろ過して再結晶 AIBN を収集します。新鮮なメタノール 25 mL で収集した製品を組み合わせるし、再結晶化プロセスを繰り返します。
- 真空オーブン中室温 (RT) での再結晶 AIBN x 2 を一晩乾かします。<-10 ° C で暗闇の中で保管してください。
- ベンジル dithiobenzoate25の合成
- 電磁攪拌棒、還流凝縮器、滴下漏斗ゴムキャップと 500 mL 三首丸底フラスコを準備します。還流凝縮器を通じて窒素ガスラインにフラスコを接続、内部を洗い流す窒素と空気。中隔から温度計を挿入します。同じ隔壁を通して注射器テトラヒドロ フラン (THF) に臭化フェニル 1 M 溶液 41 mL (0.041 mol) を追加します。
- オイルバスで 40 ° C にフェニル臭化解決を暖めます。40 ° C で溶液の温度を維持すること、ゆっくりと滴下漏斗を通してカーボン二硫化物の 3.1 g (0.041 mol) を追加します。
- 滴下漏斗以上 15 分 50 ° c. に反応温度の増加を通じて得られた混合物に臭化ベンジルの 7.1 g (0.042 mol) を追加します。45 分間この温度で攪拌を続けます。
- 漏斗に反応混合物を転送し、15 mL の氷冷水で希釈します。ジエチル エーテル 15 mL を追加することによって、製品を抽出し、下の水層を除去します。ジエチル エーテルで抽出、さらに 2 回を繰り返します。
- 水とし、かん水 (50% (w/v) 水に塩化ナトリウム溶液) のおびただしい量の合わせた有機相を洗浄し、無水硫酸マグネシウム上製品の乾燥します。
- 35 ° c 回転蒸発器を用いて真空溶媒を削除します。
- 赤色の油としてベンジル dithiobenzoate (BDB) の 5 グラム、溶離液としてシリカゲル (細孔径 60 Å、63 200 メッシュ パーティクル サイズ) 400 mL、石油エーテルを用いたクロマトグラフィーによって製品を浄化します。1H NMR (400 MHz、CDCl3) 製品純度を確認: δ 8.02 7.99 (2 H, m) 7.55 7.50 (1 H, m)、7.41 7.29 (7 H、m) 4.60 (2 H, s)。
- ラフト重合9,26を介して poly(PFPA) の合成
- 市販 PFPA モノマーには、阻害剤の少量が含まれています。重合前にモノマーを塩基性アルミナ満載使い捨て注射器に通すことによって阻害剤を削除します。
- 20 mL Schlenk フラスコに無水アニソールの 0.7 mL、1012 mg (4.25 モル) 阻害剤無料 PFPA、BDB の再結晶 AIBN、4.3 mg (0.018 ミリ モル) の 0.4 mg (0.0024 mmol) を追加します。
- シュレンク管にフラスコを接続し、ドガの少なくとも 3 つ凍結ポンプ融解との混合物。簡単に言えば、液体窒素風呂で反応混合物を凍結します。ヘッド スペースにガスを除去するために真空を適用します。フラスコを密封し、室温解凍するコンテンツを許可する液体窒素から削除
- 70 ° C のオイルバスにフラスコを置き、N2パージの下で 4 h の反応します。
- 反応を終了、油浴からフラスコを削除し、空気に反応内容を公開します。
- 冷メタノール中でポリマーを沈殿し、40 ° C で真空オーブンで回復したポリマーを一晩乾燥します。
- ポリマーの分子量を測定するには、ゲル浸透クロマトグラフィー (GPC) を使用します。1 mL/min と 35 ° C で移動相流量し、単分散ポリスチレン標準を使用して検量線の作成は、THF を使用します。GPC 測定を取得するには、0.2 μ m 使い捨てポリテトラフルオロ エチレン (PTFE) フィルターを通じて THF (1-2 mg/mL) とフィルターでポリマーを溶解します。GPC 装置に 100 μ L のサンプルを挿入します。測定サンプルの保持時間を分子量ポリスチレンの検量線を使用してに変換します。
2. Poly(PFPA) の準備機能 SiO2ビーズ
- APTES SiO2ビーズの治療
- 5% (w/v) 水性懸濁液の形では、SiO2粒子があります。APTES と 8 mL のメタノール シンチレーションの 20 mL バイアル攪拌棒装備で 40 mg と SiO2懸濁液 0.8 mL を組み合わせます。
- 激しく攪拌しながら 5 h の RT で続行する反応を許可します。
- 円錐管にソリューションを転送します。APTES を分離する SiO2ビーズを官能基化、5 分間、10,000 × g で遠心分離し、上澄みを除去します。3 mL の新鮮なメタノールでそれらを再分散することで、ビーズを洗浄します。混合チューブを手で振るが、必要に応じて、数秒間水お風呂で超音波処理による分散を改善します。遠心 10,000 × g で 5 分間削除でビーズ上清および洗浄ステップをあと 1 回繰り返します。
- ジメチルスルホキシド (DMSO) 3 mL と SiO2ビーズを洗浄メタノールを組み合わせます。混合物を手で振るかビーズが DMSO で完全に分散するまで、数秒の超音波照射必要です。ビーズ 5 分間、10,000 × g で遠心分離し、上澄みを除去します。DMSO にメタノールから完全な溶媒交換を確保するための手順を繰り返します。
メモ: 最終的な懸濁液に含まれる官能基化 APTES 4 ml の DMSO の SiO2ビーズ分散します。 - 粒度分布を調べるには、DLS の分析を実行します。使い捨て UV キュベットにステップ 2.1.4 および場所の準備懸濁液の一滴を取る。2/3 まで、新鮮な dmso キュヴェットに記入サンプルを希釈による。サンプルをデータ集録を開始する電池ホルダーに挿入します。粒度測定、次のセットアップ パラメーターを使用して: 気温: 25 ° C平衡の時間: 120 s;測定時間: 自動。
- 表面の組成を確認するには、XPS 分析を実行します。一晩 2.1.4 真空オーブンで 40 ° C の手順で作製した懸濁液からの小さいサンプルを乾燥させます。乾燥ポリマーと 0.5 × 0.5 cm の試料ホルダーに均等にパックを取る。高真空 (10-8 torr) にサンプルをロードし、データ集録を開始します。特定の XPS の楽器使用、生成 15 で単色アル α x 線を用いた光電子 kV と 6.7 mA、および収集 50 eV でアナライザーでハイブリッド モードの倍率を使用して高分解能スペクトルのエネルギーを渡すし、100 eV はエネルギーを渡す元素の調査。
- APTES に poly(PFPA) を移植、SiO2ビーズを官能基化
- Poly(PFPA) ソリューションを準備するには、シンチレーション バイアル 20 mL の DMSO の 2 mL で poly(PFPA) の 20 mg を溶解します。
メモ: この研究では、比較的低分子量 poly(PFPA) (20 kg/mol) が使用されます。したがって、にもかかわらず、高分子濃度 (10 mg/mL)、高分子架橋の証拠は認められなかった。高分子量ポリマーを使用すると、ポリマー溶液濃度が可能な架橋結合を避けるために調整する必要があります。 - APTES の 1 mL は SiO2ビーズ poly(PFPA) ソリューションに (からステップ 2.1.4) DMSO で中断機能の集積化を追加します。激しく攪拌しながら 1 時間室温で反応します。
- 5 分、上澄みの除去が続く 10,000 × g で遠心分離によって接木 poly(PFPA) SiO2ビーズを分離します。どちらかの手や超音波処理の数秒間揺れによって DMSO とミックスの 3 mL を加えることによってビーズを洗浄します。ビーズ 5 分間、10,000 × g で遠心分離し、上澄みを除去します。DMSO と接木 poly(PFPA) SiO2ビーズの 2 回洗濯を繰り返します。
- トリプル蒸留水 (TDW) の 2 倍以上のビーズを洗浄します。この手順ではインスタレーション、3 mL でビーズを組み合わせるし、手や超音波照射の数秒の揺れでミックスします。ビーズ 5 分間、10,000 × g で遠心分離し、上澄みを除去します。
- 粒度分布を調べるには、DLS 2.1.5 の手順で説明されている手順を次を実行します。表面化学を確認するには、XPS 2.1.6 のステップで説明されている手順を実行します。
- Poly(PFPA) ソリューションを準備するには、シンチレーション バイアル 20 mL の DMSO の 2 mL で poly(PFPA) の 20 mg を溶解します。
3. SiO2の準備ペグ-置換 Poly(PFPA) をグラフトしたビーズ
- Poly(PFPA) ソリューションを準備するには、poly(PFPA) DMSO 20 mL シンチレーション バイアル 2 mL に 20 mg を溶解します。
- 止め釘の解決を準備するには、1 ml の DMSO のアミン官能基化 PEG を溶解します。ペグの正確な量を使用は、PFP 置換では、以下の式によって決定の所望の程度によって決まります。
ペグ アミノ酸量 (g/g-poly(PFPA)) = (N_poly(PFPA) x %peg サブ) x (MW_PEG/MW_poly(PFPA))
どこ N_poly(PFPA) = poly(PFPA) 重合度
%Peg サブ = %peg 置換
MW_PEG アミノ peg 分子量を =
MW_ poly(PFPA) = poly(PFPA) の分子量 - Poly(PFPA) ソリューションに止め釘の解決を転送します。激しく攪拌しながら 1 時間室温で反応します。
- APTES を準備する機能 SiO2ビーズ DMSO で中断、同じステップ 2.1 に示されている手順に従ってください。ステップ 3.3 で、ペグ-置換 poly(PFPA) 溶液にビーズ懸濁液の 1 mL を移します。Poly(PFPA) と APTES の間の接木を激しく攪拌しながら 1 時間常温続行 SiO2ビーズ官能基化を許可します。
- 5 分、上澄みの除去が続く 10,000 × g で遠心分離によってビーズを分離します。どちらかの手や超音波処理の数秒間揺れによって DMSO とミックスの 3 mL を加えることによってビーズを洗浄します。ビーズ 5 分間、10,000 × g で遠心分離し、上澄みを除去します。DMSO 洗浄を 2 回繰り返します。
- TDW で 2 倍以上のビーズを洗浄します。この手順で 3 ml のインスタレーション、ビーズを組み合わせるし、手や超音波照射の数秒の揺れでミックスします。ビーズ 5 分間、10,000 × g で遠心分離し、上澄みを除去します。
- 真空オーブンで 40 ° C でビーズを一晩乾かします。
4. 抗体固定化 Poly(PFPA) の接ぎ木 SiO2ビーズ
注: 同じ手順は、poly(PFPA) %peg 置換に関係なく用いられます。TDW で PBS タブレットを溶解することにより、リン酸緩衝生理食塩水 (PBS) を準備します。0.1% (v/v) リン酸緩衝生理食塩水でトゥイーン 20 (pbst;) を準備するには、PBS に Tween 20 1/1000 を追加します。
- Poly(PFPA) の 5 mg 接木 1.5 mL 遠心チューブに SiO2ビーズを追加します。
- ボルテックスでよく 800 μ L の PBS とミックスを追加することによって、ビーズを洗浄します。上清 1 分削除の RT で 10,000 x g でビーズを遠心し、洗浄手順を 3 回繰り返します。
- 新鮮な PBS、50 μ L 0.1% (v/v) PBST、抗体の 6.67 μ ・ 350 μ L を追加します。4 ° C でローテーター上 ~ 20 h を孵化させなさい
- 非連結の抗体を削除するためにビーズを洗浄します。400 x g でビーズを遠心し、4 ° C で 1 分間上澄みを除去し、慎重に換散バッファーの 400 μ L を追加します。優しく上下に 5 回のピペッティングによる再ビーズを中断します。
注: 換散バッファー、ビーズを洗浄するために使用される必要がありますセル換散および IP、使用されるものと同じ除いてジチオトレイトールとプロテアーゼ阻害剤の添加は省略可能、(ステップ 5 を参照してください)。 - このステップを 3 回繰り返します。最終的な洗浄の後可能な限り、上清を削除します。
5. 細胞換散および免疫沈降
- 換散バッファーおよび洗浄バッファーの準備
- 換散バッファー (50 mM トリス-HCl (pH 8.0)、100 mM KCl、0.5% (v/v) NP 40、10% (v/v) グリセロール、カクテルの 1 mM ジチオトレイトール (DTT)、プロテアーゼ阻害剤) を準備します。
- 洗浄バッファー (50 mM トリス塩酸 (pH 8.0)、100 mM KCl、0.1% (v/v) NP-40、および 10% (v/v) グリセロール) を準備します。
- 4 ° C で緩衝溶液を保存します。
- セルの作製
- シードの細胞 (HeLa 細胞) IP 実験の前に 1 つまたは 2 日間、37 ° C、5% CO2で細胞を成長します。
- 15 mL の円錐管に約 1.4 × 107細胞細胞スクレーパーと転送を収集します。遠心 380 x g の RT で 3 分で細胞の上澄みを除去し、再 1 ml の PBS 寒くて 1.5 mL 遠心チューブに転送を中断します。
- 30 s. 削除きれいに上清の 4 ° C で 10,000 x g で細胞を遠心分離機します。細胞ペレットは、上清を除去後-80 ° C で保存できます。
- セル lysates の準備
- 換散バッファーの 400 μ L で細胞ペレットを再停止します。Ultrasonicator を用いた細胞を超音波照射します。
- 超音波、渦を簡潔に、遠心分離機ライセート 4 ° C で 20,000 × g で 10 分後。
- 上清を新しい 1.5 mL 遠心チューブに転送します。
- 免疫沈降
- 事前に準備された抗体培養移植 poly(PFPA) SiO2ビーズに細胞ライセートの 300 μ L を転送します。細胞の新しい微量遠心チューブに入力サンプルとしてライセートの 30 μ L を保持します。4 ° C で入力のサンプルを保存します。
注: 細胞ライセート中のタンパク質の総量は約 4 mg をする必要があります。 - 4 ° C で回転子に 3 h ライセート/ビーズ混合物を孵化させなさい
- 4 ° c で 1 分間削除 400 x g で混合物の上澄みを遠心し、慎重に洗浄バッファーの 400 μ L を追加します。軽くピペッティングを上下約 5 倍で再ビーズを中断します。
- このステップを 3 回繰り返します。最終的な洗浄の後可能な限り、上清を削除します。
- ドデシル硫酸ナトリウム (SDS) ローディングの染料 (25% (v/v) グリセロール、0.1% (w/v) ブロモ フェノール ブルー (BPB) 60 mM トリス-HCl (Ph6.8) 2% (w/v) SDS と 2.75 mM 2-メルカプトエタノール) x 2 を準備します。ストア 2 × SDS 読み込み染料-20 ° C でビーズとストアドの入力サンプルの 2 × SDS ローディングの染料の 30 μ L を追加し、95 ° C で 10 分間加熱します。
- 加熱した後、27ウエスタンブロットを使用してサンプルを分析したり、-20 ° C でサンプルを格納
- 事前に準備された抗体培養移植 poly(PFPA) SiO2ビーズに細胞ライセートの 300 μ L を転送します。細胞の新しい微量遠心チューブに入力サンプルとしてライセートの 30 μ L を保持します。4 ° C で入力のサンプルを保存します。
Access restricted. Please log in or start a trial to view this content.
結果
Poly(PFPA) の準備のための回路図と SiO2ビーズおよびペグなし置換は図 1に示します。APTES と poly(PFPA) プロセス、裸の SiO2ビーズを移植を監視する APTES 機能 SiO2ビーズと poly(PFPA) 接木 SiO2ビーズは DLS (図 2) と XPS (図 3) によって特徴付けられます。ビーズの IP 効率は西部に?...
Access restricted. Please log in or start a trial to view this content.
ディスカッション
Poly(PFPA) の合成は接ぎ木 SiO2ビーズは図 1に示します。リンカー分子として APTES を採用し、単純な 2 段階のプロセスを介して SiO2基板に移植した poly(PFPA) ブラシを用意できます。PFP の単位のいくつかが APTES との反応を犠牲に、多数の PFP ユニットがアミノ ペグまたは抗体のいずれかで後の反応に利用可能なままと予想します。PFP グループは、poly(PFPA) ブ?...
Access restricted. Please log in or start a trial to view this content.
開示事項
著者が明らかに何もありません。
謝辞
この作品は、防衛開発 (グラント号の代理店によって支えられました。UD170039ID)。
Access restricted. Please log in or start a trial to view this content.
資料
| Name | Company | Catalog Number | Comments |
| 2,2-Azobisisobutyronitrile, 99% | Daejung Chemicals | 1102-4405 | |
| Methyl alcohol for HPLC, 99.9% | Duksan Pure Chemicals | d62 | |
| Phenylmagnesium bromide solution 1.0 M in THF | Sigma-Aldrich | 331376 | |
| Carbon disulfide anhydrous, ≥99% | Sigma-Aldrich | 335266 | |
| Benzyl bromide, 98% | Sigma-Aldrich | B17905 | |
| Petroleum ether, 90% | Samchun Chemicals | P0220 | |
| Ethyl ether, 99% | Daejung Chemicals | 4025-4404 | |
| Magnesium sulfate anhydrous, powder, 99% | Daejung Chemicals | 5514-4405 | |
| Pentafluorophenyl acrylate | Santa Cruz Biotechnology | sc-264001 | contains inhibitor |
| Aluminium oxide, activated, basic, Brockmann I | Sigma-Aldrich | 199443 | |
| Sodium Chloride (NaCl) | Daejung Chemicals | 7548-4400 | |
| Anisole anhydrous, 99.7% | Sigma-Aldrich | 296295 | |
| Silica nanoparticle | Microparticles GmbH | SiO2-R-0.7 | 5% w/v aqueous suspension |
| 3-Aminopropyltrimethoxysilane, >96.0% | Tokyo Chemical Industry | T1255 | |
| Dimethyl sulfoxide for HPLC, ≥99.7% | Sigma-Aldrich | 34869 | |
| Amino-terminated poly(ethylene glycol) methyl ether | Polymer Source | P16082-EGOCH3NH2 | |
| Phosphate buffered saline tablet | Takara | T9181 | |
| Tween-20 | Calbiochem | 9480 | |
| Tris-HCl (pH 8.0) | Invitrogen | AM9855G | |
| KCl | Invitrogen | AM9640G | |
| NP-40 | VWR | E109-50ML | |
| Glycerol | Invitrogen | 15514-011 | |
| Dithiothreitol | Biosesang | D1037 | |
| Protease inhibitor | Merck | 535140-1MLCN | |
| Bromo phenol blue | Sigma-Aldrich | B5525-5G | |
| Tris-HCl (pH 6.8) | Biosolution | BT033 | |
| Sodium dodecyl sulfate | Biosolution | BS003 | |
| 2-Mercaptoethanol | Gibco | 21985-023 | |
| PKR Antibody | Cell Signaling Technology | 12297S | |
| GAPDH Antibody | Santa Cruz Biotechnology | sc-32233 | |
| Normal Rabbit IgG | Cell Signaling Technology | 2729S | |
| HeLa | Korea Cell Line Bank | 10002 | |
| Sonicator | DAIHAN Scientific | WUC-D10H | |
| Ultrasonicator | BMBio | BR2006A | |
| Centrifuge I | Eppendorf | 5424 R | |
| Centrifuge II | LABOGENE | 1736R | |
| Rotator | FINEPCR | ROTATOR/AG | |
| Vacuum oven | DAIHAN Scientific | ThermoStable OV-30 | |
| Gel permeation chromatography (THF) | Agilent Technologies | 1260 Infinity II | |
| X-ray photoelectron spectrometer | Thermo VG Scientific | Sigma Probe | |
| Dynamic light scattering | Malvern Instruments | ZEN 3690 |
参考文献
- Johnsson, B., Löfås, S., Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry. 198 (2), 268-277 (1991).
- Kurzawa, C., Hengstenberg, A., Schuhmann, W. Immobilization method for the preparation of biosensors based on pH shift-induced deposition of biomolecule-containing polymer films. Analytical Chemistry. 74 (2), 355-361 (2002).
- You, C. C., et al. Detection and identification of proteins using nanoparticle-fluorescent polymer 'chemical nose' sensors. Nature Nanotechnology. 2 (5), 318-323 (2007).
- Roberts, M. W., Ongkudon, C. M., Forde, G. M., Danquah, M. K. Versatility of polymethacrylate monoliths for chromatographic purification of biomolecules. Journal of Separation Science. 32 (15-16), 2485-2494 (2009).
- Sandison, M. E., Cumming, S. A., Kolch, W., Pitt, A. R. On-chip immunoprecipitation for protein purification. Lab on a Chip. 10 (20), 2805-2813 (2010).
- Das, A., Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chemical Reviews. 116 (3), 1434-1495 (2016).
- Choi, J., et al. Functionalization and patterning of reactive polymer brushes based on surface reversible addition and fragmentation chain transfer polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 50 (19), 4010-4018 (2012).
- Kessler, D., Jochum, F. D., Choi, J., Char, K., Theato, P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light-responsive surfaces. ACS Applied Materials & Interfaces. 3 (2), 124-128 (2011).
- Son, H., et al. Penetration and exchange kinetics of primary alkyl amines applied to reactive poly(pentafluorophenyl acrylate) thin films. Polymer Journal. 48 (4), 487-495 (2016).
- Kessler, D., Roth, P. J., Theato, P. Reactive surface coatings based on polysilsesquioxanes: controlled functionalization for specific protein immobilization. Langmuir. 25 (17), 10068-10076 (2009).
- Kessler, D., Theato, P. Reactive surface coatings based on polysilsesquioxanes: defined adjustment of surface wettability. Langmuir. 25 (24), 14200-14206 (2009).
- Kessler, D., Nilles, K., Theato, P. Modular approach towards multi-functional surfaces with adjustable and dual-responsive wettability using a hybrid polymer toolbox. Journal of Materials Chemistry. 19 (43), 8184-8189 (2009).
- Eberhardt, M., Mruk, R., Zentel, R., Theato, P. Synthesis of pentafluorophenyl(meth)acrylate polymers: new precursor polymers for the synthesis of multifunctional materials. European Polymer Journal. 41 (7), 1569-1575 (2005).
- Jochum, F. D., Forst, F. R., Theato, P. PNIPAM copolymers containing light-responsive chromophores: a method toward molecular logic gates. Macromolecular Rapid Communications. 31 (16), 1456-1461 (2010).
- Schattling, P., Pollmann, I., Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. Reactive & Functional Polymers. 75, 16-21 (2014).
- He, L., Szameit, K., Zhao, H., Hahn, U., Theato, P. Postpolymerization modification using less cytotoxic activated ester polymers for the synthesis of biological active polymers. Biomacromolecules. 15 (8), 3197-3205 (2014).
- Arnold, R. M., McNitt, C. D., Popik, V. V., Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: versatile substrates for reactive microcapillary printing and self-sorting modification. Chemical Communications. 50 (40), 5307-5309 (2014).
- Son, H., Ku, J., Kim, Y., Li, S., Char, K. Amine-Reactive Poly(pentafluorophenyl acrylate) Brush Platforms for Cleaner Protein Purification. Biomacromolecules. 19 (3), 951-961 (2018).
- Cullen, S. E., Schwartz, B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. The Journal of Immunology. 117 (1), 136-142 (1976).
- Sisson, T. H., Castor, C. W. An improved method for immobilizing IgG antibodies on protein A-agarose. Journal of Immunology Methods. 127 (2), 215-220 (1990).
- Peritz, T., et al. Immunoprecipitation of mRNA-protein complexes. Nature Protocols. 1 (2), 577-580 (2006).
- Zhang, Z., Chen, S., Jiang, S. Dual-functional biomimetic materials: nonfouling poly (carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 7 (12), 3311-3315 (2006).
- Yao, Y., et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids and Surfaces B: Biointerfaces. 66 (2), 233-239 (2008).
- Ma, J., et al. Facile fabrication of microsphere-polymer brush hierarchically three-dimensional (3D) substrates for immunoassays. Chemical Communications. 51 (31), 6749-6752 (2015).
- Chong, Y., et al. Thiocarbonylthio compounds [SC (Ph) S− R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT Polymerization). Role of the free-radical leaving group (R). Macromolecules. 36 (7), 2256-2272 (2003).
- Jochum, F. D., Theato, P. Temperature- and Light-Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Polymers. Macromolecules. 42 (16), 5941-5945 (2009).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. JoVE. , Cambridge, MA. (2018).
- Chua, G. B. H., Roth, P. J., Duong, H. T. T., Davis, T. P., Lowe, A. B. Synthesis and Thermoresponsive Solution Properties of Poly[oligo(ethylene glycol) (meth)acrylamide]s: Biocompatible PEG Analogues. Macromolecules. 45 (3), 1362-1374 (2012).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification
Posted by JoVE Editors on 4/30/2019. Citeable Link.
An erratum was issued for: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification. Throughout the article, the term "3-aminopropyltriethoxysilane" has been replaced with "3-aminopropyltrimethoxysilane", and "APTES" with "APTMS".
The Keywords were updated from:
Poly(pentafluorophenyl acrylate), 3-aminopropyltriethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
to:
Poly(pentafluorophenyl acrylate), 3-aminopropyltrimethoxysilane, reactive polymer brush, post-polymerization functionalization, antibody immobilization, immunoprecipitation
The Abstract was updated from:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltriethoxysilane (APTES) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTES. The deposition of APTES and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
to:
We demonstrate a simple method to prepare poly(pentafluorophenyl acrylate) (poly(PFPA)) grafted silica beads for antibody immobilization and subsequent immunoprecipitation (IP) application. The poly(PFPA) grafted surface is prepared via a simple two-step process. In the first step, 3-aminopropyltrimethoxysilane (APTMS) is deposited as a linker molecule onto the silica surface. In the second step, poly(PFPA) homopolymer, synthesized via the reversible addition and fragmentation chain transfer (RAFT) polymerization, is grafted to the linker molecule through the exchange reaction between the pentafluorophenyl (PFP) units on the polymer and the amine groups on APTMS. The deposition of APTMS and poly(PFPA) on the silica particles are confirmed by X-ray photoelectron spectroscopy (XPS), as well as monitored by the particle size change measured via dynamic light scattering (DLS). To improve the surface hydrophilicity of the beads, partial substitution of poly(PFPA) with amine-functionalized poly(ethylene glycol) (amino-PEG) is also performed. The PEG-substituted poly(PFPA) grafted silica beads are then immobilized with antibodies for IP application. For demonstration, an antibody against protein kinase RNA-activated (PKR) is employed, and IP efficiency is determined by Western blotting. The analysis results show that the antibody immobilized beads can indeed be used to enrich PKR while non-specific protein interactions are minimal.
The fourth paragraph of the Introduction was updated from:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTES linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTES. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
to:
In this contribution, we report an alternative method to prepare poly(PFPA) grafted surface for antibody immobilization and IP application. In a simple two-step process, as illustrated in Figure 1, an APTMS linker molecule is first deposited onto the silica surface, then the poly(PFPA) polymer is covalently attached to the linker molecule through the reaction between the PFP units on the polymer and the amine functions on APTMS. This preparation method allows for the permanent crosslinking of poly(PFPA) to a substrate surface, but avoids the many complications associated with SI-CTA synthesis and SI-RAFT polymerization of poly(PFPA) brushes. Partial substitution of the PFP units with amino-PEG can still be performed, allowing fine-tuning of the polymer brush surface properties. We show the poly(PFPA) grafted silica beads thus prepared can be immobilized with antibodies and used for protein enrichment via IP. The detailed bead preparation procedure, antibody immobilization, and IP testing are documented in this article, for readers interested in seeking an alternative to conventional Protein A/G based IP.
Step 2.1 of the Protocol was updated from:
Treatment of SiO2 beads with APTES
to:
Treatment of SiO2 beads with APTMS
Step 2.1.1 of the Protocol was updated from:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTES and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
to:
SiO2 particles are available in the form of a 5% (w/v) aqueous suspension. Combine 0.8 mL of SiO2 suspension with 40 mg of APTMS and 8 mL of methanol in a 20 mL scintillation vial equipped with a stir bar.
Step 2.1.3 of the Protocol was updated from:
Transfer the solution to a conical tube. To isolate the APTES functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
to:
Transfer the solution to a conical tube. To isolate the APTMS functionalized SiO2 beads, centrifuge the solution at 10,000 x g for 5 min, then remove the supernatant. Wash the beads by re-dispersing them in 3 mL of fresh methanol. Shake the tube by hand for mixing, but if necessary, improve the dispersion by sonication in a water bath for a few seconds. Centrifuge the beads at 10,000 x g for 5 min. Remove the supernatant and repeat the wash step one more time.
Step 2.1.4 of the Protocol was updated from:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.NOTE: The final suspension contains the APTES functionalized SiO2 beads dispersed in 4 mL of DMSO.
to:
Combine the methanol washed SiO2 beads with 3 mL of dimethyl sulfoxide (DMSO). Shake the mixture by hand, or if necessary sonicate for a few seconds, until the beads are fully dispersed in DMSO. Centrifuge the beads at 10,000 x g for 5 min, then remove the supernatant. Repeat the step to ensure complete solvent exchange from methanol to DMSO.
NOTE: The final suspension contains the APTMS functionalized SiO2 beads dispersed in 4 mL of DMSO.
Step 2.2 of the Protocol was updated from:
Grafting poly(PFPA) to APTES functionalized SiO2 beads
to:
Grafting poly(PFPA) to APTMS functionalized SiO2 beads
Step 2.2.2 of the Protocol was updated from:
Add 1 mL of APTES functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
to:
Add 1 mL of APTMS functionalized SiO2 beads suspended in DMSO (from Step 2.1.4) to the poly(PFPA) solution. React at RT for 1 h with vigorous stirring.
Step 3.4 of the Protocol was updated from:
To prepare APTES functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTES functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
to:
To prepare APTMS functionalized SiO2 beads suspended in DMSO, follow the same steps shown in Step 2.1. Transfer 1 mL of the bead suspension into the PEG-substituted poly(PFPA) solution prepared in Step 3.3. Allow the grafting between poly(PFPA) and APTMS functionalized SiO2 beads to proceed at RT for 1 h with vigorous stirring.
The first paragraph of the Representative Results was updated from:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTES and poly(PFPA) grafting process, bare SiO2 beads, APTES functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
to:
A schematic for the preparation of poly(PFPA) grafted SiO2 beads, with or without PEG substitution is shown in Figure 1. To monitor the APTMS and poly(PFPA) grafting process, bare SiO2 beads, APTMS functionalized SiO2 beads, and poly(PFPA) grafted SiO2 beads are characterized by both DLS (Figure 2) and XPS (Figure 3). IP efficiencies of the beads are determined by Western blotting. Figure 4 shows the Western blotting results for IP using 1% PEG-substituted poly(PFPA) grafted beads, where the beads are incubated with no antibody, a non-specific antibody, or anti-PKR antibody. Figure 5 shows the Western blotting results for IP using 0% PEG-substituted poly(PFPA) grafted beads and 1% PEG-substituted poly(PFPA) grafted beads, both incubated with anti-PKR antibodies.
Figure 1 was updated from:

Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTES as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
to:
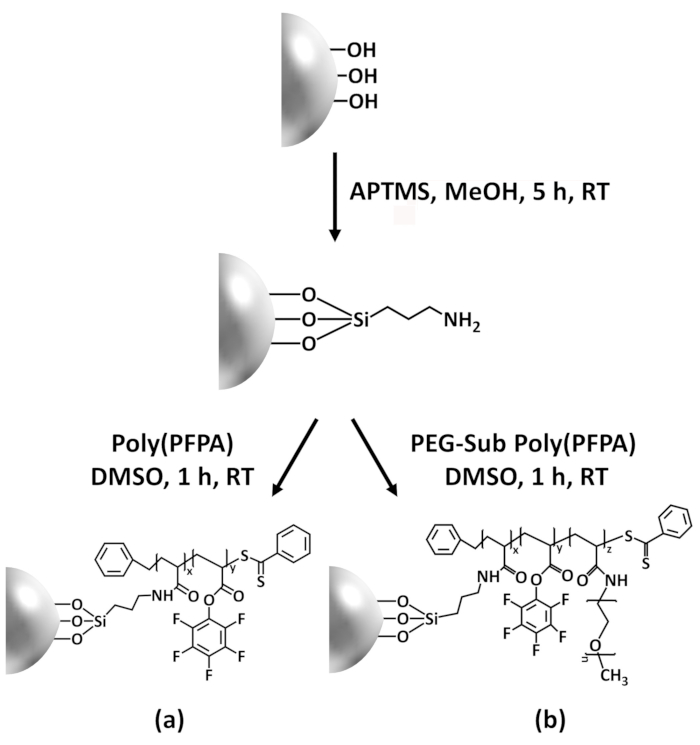
Figure 1: Schematic for the preparation of poly(PFPA) grafted SiO2 beads using APTMS as a linker molecule. (a) Poly(PFPA) grafted beads. (b) Partially PEG-substituted poly(PFPA) grafted beads.
Figure 2 was updated from:
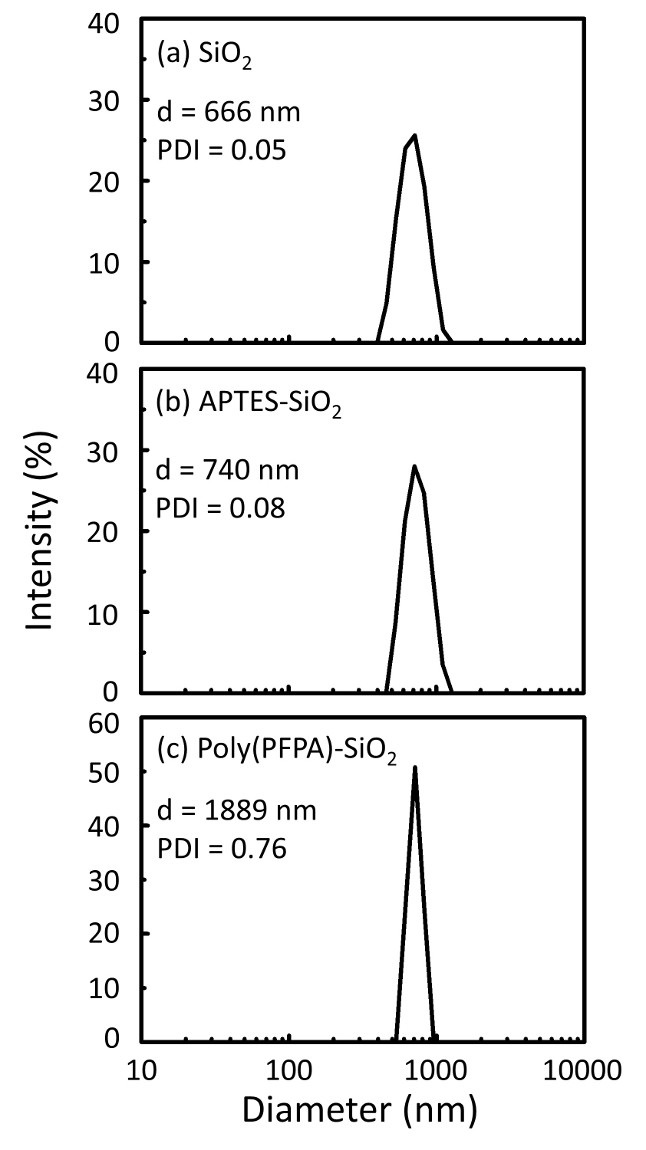
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTES functionalized SiO2 beads (APTES-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
to:
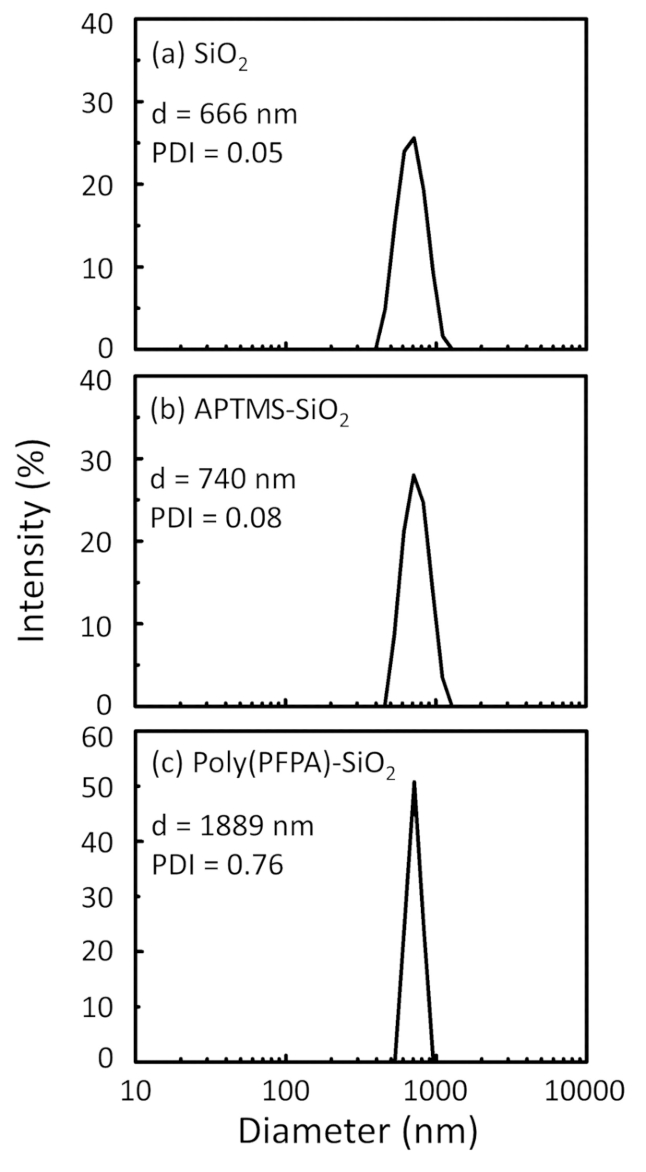
Figure 2: DLS measurements for (a) bare SiO2 beads (SiO2), (b) APTMS functionalized SiO2 beads (APTMS-SiO2), and (c) poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2), dispersed in DMSO. The Z-average diameter (d) and polydispersity index (PDI) of each sample are reported.
Figure 3 was updated from:
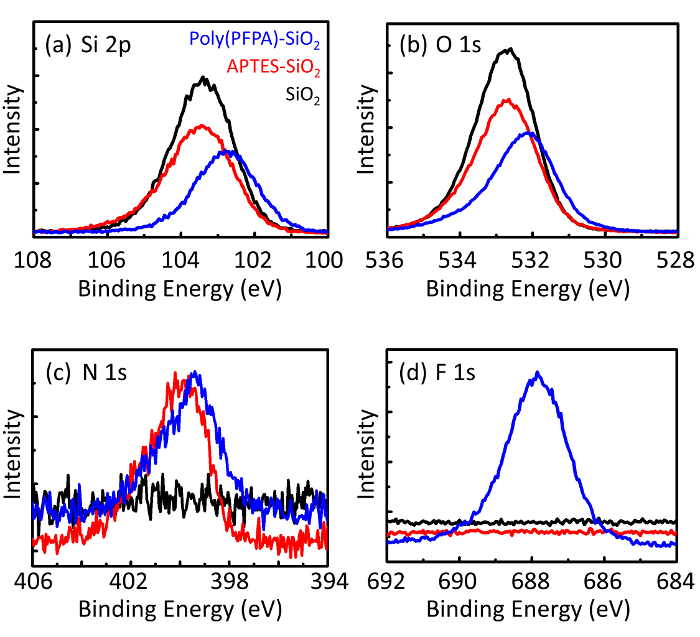
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTES functionalized SiO2 beads (APTES-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
to:
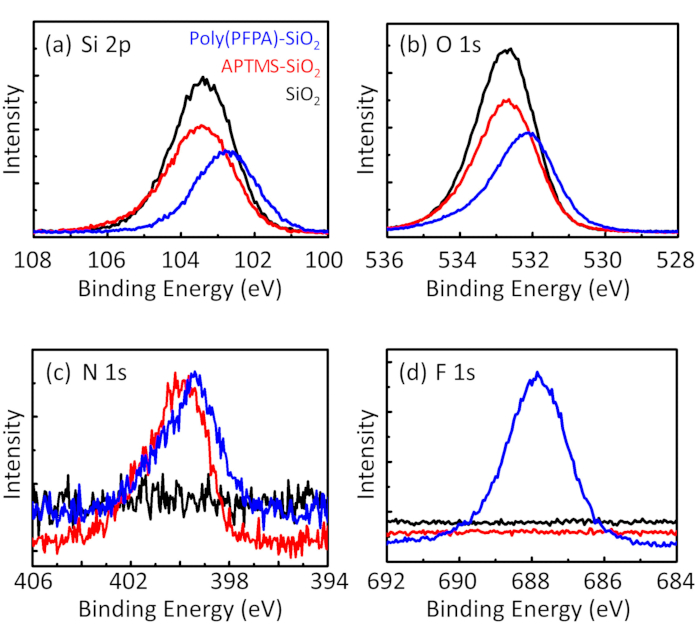
Figure 3: XPS spectra for bare SiO2 beads (SiO2), APTMS functionalized SiO2 beads (APTMS-SiO2), and poly(PFPA) grafted SiO2 beads (poly(PFPA)-SiO2). The peaks examined correspond to (a) Si 2p, (b) O 1s, (c) N 1s, and (d) F 1s.
The first and second paragraphs of the Discussion were updated from:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTES as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTES, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTES linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTES treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTES treatment, N 1s peak associated with the amine groups on APTES is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTES, then with poly(PFPA).
to:
The synthesis of poly(PFPA) grafted SiO2 beads is illustrated in Figure 1. By employing APTMS as a linker molecule, poly(PFPA) brushes covalently grafted to SiO2 substrate can be prepared via a simple two-step process. Although some of the PFP units are sacrificed for the reaction with APTMS, a large number of the PFP units are expected to remain available for later reaction with either amino-PEG or antibodies. The PFP groups are known to form low energy surfaces so poly(PFPA) brushes do not solvate well in water28. For IP application, the antibodies need to be immobilized on the poly(PFPA) brushes, and this exchange reaction is done in aqueous buffer solution in order to preserve the activity of the antibodies. As reported in our previous publication, partial substitution of the PFP units with hydrophilic molecules such as amine-functionalized PEG can improve surface hydrophilicity, leading to increased antibody immobilization efficiency18. In this study, partially PEG substituted poly(PFPA) is also prepared, then grafted to the SiO2 surface using the same APTMS linker molecule. Overall, the methods illustrated in Figure 1 allow the preparation of poly(PFPA) grafted surfaces with different degrees of PEG substitution. These polymer brushes with tunable surface properties provide an ideal platform for antibody immobilization and subsequent IP application.
The bead preparation process is monitored by both DLS and XPS. The DLS results for various functionalized SiO2 beads in DMSO are summarized in Figure 2. The bare SiO2 beads exhibit hydrodynamic diameter of 666 nm, in agreement with the manufacturer reported bead size (0.676 μm; SD = 0.03 μm). After APTMS treatment, the bead diameter increases to 740 nm; and with poly(PFPA) treatment, the bead diameter further increases to 1889 nm. It is important to point out that the polydispersity index (PDI) for the poly(PFPA) grafted beads is rather large (PDI = 0.76), which is indicative of poor quality sample containing large aggregates. Although the DLS curve only shows one nano-sized peak, small amount of aggregates may be present in the suspension. The functionalized SiO2 beads are also examined by XPS to determine surface composition (Figure 3). Following APTMS treatment, N 1s peak associated with the amine groups on APTMS is detected. And, following poly(PFPA) treatment, F 1s peak associated with the PFP units on the polymer is detected. Together these data show the successful functionalization of the SiO2 surface, first with APTMS, then with poly(PFPA).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved