A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Digital Planimetry for Assessing Wound Closure Kinetics in a Mouse Model
In This Article
Summary
Wounds represent a global health challenge. This study developed a standardized photo booth utilizing digital planimetry to minimize wound measurement variability. Monitoring wounds in mice over 14 days revealed an initial increase in wound area and perimeter, followed by gradual closure. This methodology may aid in evaluating wound closure kinetics in pre-clinical models.
Abstract
Chronic wounds, due to their high prevalence, are a serious global health concern. Effective therapeutic strategies can significantly accelerate healing, thereby reducing the risk of complications and alleviating the economic burden on healthcare systems. Although numerous experimental studies have investigated wound healing, most rely on qualitative observations or quantitative direct measurements. The objective of this study was to standardize an indirect wound measurement method using digital planimetry, incorporating digital scaling and segmentation. This approach addresses the lack of detailed, step-by-step methodologies for accurate wound assessment. A photodocumentation booth was designed and constructed, and computer-assisted digital planimetry tools were employed to minimize variability in measurements of the wound area, perimeter, and the distance from the wound center to its edges. A circular traumatic wound (5 mm in diameter) was created on the dorsal midline at the shoulder blade level of male CD1 mice (n = 4, 10 weeks old, 30-35 g). Wound evolution was photodocumented for 14 days using the custom-designed photo booth, which controlled lighting conditions, focal distance, and subject positioning. Scaling and wound measurements were performed using segmentation in ImageJ software, and statistical analysis was conducted using statistical analysis software. The kinetics of wound closure showed a slight increase in wound size and perimeter between day 0 and day 2, followed by a gradual decrease until complete closure by day 14. The photodocumentation booth and computer-assisted digital planimetry enabled quantitative measurements with minimal variability. In conclusion, these tools provide a reliable and reproducible method for evaluating wound closure kinetics in pre-clinical models.
Introduction
Traumatic wound healing takes approximately 21 days and has a well-defined sequence of four distinct phases: (1) hemostasis, (2) inflammation, (3) proliferation, and (4) remodelling1. If any phase of wound healing is prolonged, it can lead to the development of chronic wounds1. Due to their high prevalence, potential complications2, and significant economic burden, they are considered a global health problem.
Pre-clinical studies aim to achieve faster healing by promoting comprehensive wound re-epithelialization3,4,5, preventing complications, and reducing treatment costs. These studies evaluate various strategies, including the development of biomaterials, pharmacological interventions, and other regenerative medicine procedures6,7,8,9.
Multiple experimental models have been developed for the study of traumatic wounds. Some focus on macroscopically visible qualitative characteristics such as size, inflammation indicators, presence of granulation tissue, secretions, and scab formation5. Others analyze quantitative data, including area, perimeter, radius, diameter, color, depth, and distances from the center to the edges of wounds.
In this regard, most in vivo investigations directly measure wound radius and depth. However, manual delineation of wound edges in a macroscopic image can introduce biases in the measurement10. Other studies use mechanical planimetry, using transparent gridded plastic sheets, where the wound edges are previously delineated; in both cases, obtaining the area or perimeter requires manual instruments such as rulers or digital planimeters. Nowadays, computer-assisted digital planimetry allows computerized analysis of macroscopic images of wounds or plastic sheets. In situ manipulation and quality of macroscopic image are a limitation, however, this tool11,12,13,14 considerably reduces the variability between area and perimeter measurements.
This proposed methodology offers significant advantages over existing techniques for evaluating wound closure in mice15,16,17,18,19,20. While photo documentation has been considered an accurate and consistent tool for assessing wound closure kinetics, previous studies21,22 have highlighted the limitations of manual wound measurement, such as observer bias and variability due to inconsistent lighting and camera positioning. The current approach addresses these issues by standardizing imaging conditions through a custom-built booth, improving reproducibility and precision. Furthermore, computerized digital planimetry enables more accurate quantitative assessments, enhancing the evaluation of therapeutic interventions and minimizing measurement errors, as evidenced in other studies comparing manual and digital techniques12,22 making it particularly suited for studies of wound closure kinetics in murine models, allowing precise evaluation of treatments by maintaining strict control over image acquisition conditions.
Protocol
All experimental procedures involving laboratory mice were conducted in accordance with the ethical standards and regulations established in the Official Mexican Standard (NOM-062-ZOO-1999) for the handling and care of laboratory animals. The protocol was reviewed and approved by the Internal Committee for the Care and Use of Laboratory Animals (CICUAL) of the National Institute for Nuclear Research (ININ) under reference number CICUAL-01-23. Male CD1 mice (n = 4), 10 weeks old, with a body weight ranging from 28-32 g, were used in this study. All animals were selected to ensure uniformity in strain, age, sex, and body weight, minimizing variability in the experimental outcomes. Details of the reagents and the equipment used are listed in the Table of Materials.
1. Photo booth construction for the acquisition of macroscopic images
NOTE: Licensed SolidWorks software (version 2015) was used to design a photo booth to eliminate external lighting sources. A 40 cm × 40 cm cube was constructed using a one-inch thick white aluminum profile. The cube consisted of three sections, assembled sequentially: the roof, side walls, and floor (Figure 1A).
- Roof construction
NOTE: The roof was regionalized as front edge (A), rear edge (B), and side edges (C and D) to orient (Figure 1B).- Fix on the inner area of the side edge of the roof (C and D) a slotted aluminum profile (designed to hold glass) 1.5 cm wide, 2.51 cm thick, and 34.9 cm long (Figure 1B).
- Place two 34.5 cm long x 13 cm wide rectangular aluminum plates with polyethylene in the center in the groove of each roof profile (Plate 1 and 2 in Figure 1C) to enable sliding through the groove of both profiles.
- Install a 20 cm RGB LED tube light with a color temperature range of 2500-9000 K (Figure 1C) at a 45° angle on the underside edge of panel 1 using double-sided tape.
- Mount on the roof panels 1 and 2 a 32 cm x 12 cm rectangular foam board panel with a central 7.82 cm diameter circular cut-out to allow the installation of the camera lens (Figure 1C).
- Construction of the floor
- Place a 40 cm x 40 cm square piece of white foam board on top of the floor area profiles. Cut squares with a side length of 2.54 cm from each corner of the foam board (Figure 1E).
- Construction of side walls
- Cut four 40 cm x 40 cm quadrangular foam board panels.
- Glue two panels with silicone on sides C and D to form the left and right side walls (Figure 1F).
- Attach hook and loop fastener strips to the aluminum profiles A and B for easy manipulation of internal photo booth elements. Additionally, attach 1 cm from the edge of a line of adhesive fasteners to two foam cardboard squares on one side, and place them on the front and rear walls (Figure 1F).
- Construction of the reference base
NOTE: A reference base was necessary to maintain the mouse in the prone position during anesthesia and throughout the documentation of wound evolution. 3D components were designed and fabricated using the Fused Deposition Modeling (FDM) technique with PLA material and a 3D printer23,24.- 3D print the mask with a base of 2 cm in width, length, and height and place it 11.5 cm from the edge of a pre-cut 40 cm x 28 cm white foam board rectangle to maintain the mice under inhalation anesthesia (Figure 1D, red color).
- 3D print the rectangular platform (9 cm long, 5 cm wide, 2.5 cm high) with four extruded areas to align the mouse dorsum during image acquisition. These areas supported the head, limbs, and abdominal region to maintain the prone position. Glue this platform to the center of the reference base (Figure 1D, green color).
- 3D print the ruler block (2 cm wide, 8 cm long, 2.5 cm high) and fix it 1 cm from the left side of the mouse base to place the measurement reference element needed for digital image processing (Figure 1D, blue color).
- Attach a 15 cm stainless steel graduated ruler to the ruler block, and install the entire reference base inside the cabin for image scaling (Figure 1D).

Figure 1: Diagram for the construction of the macroscopic image acquisition cabinet. (A) Sections of the cabin (roof, side walls, floor).(B)Orientation of the profiles forming the roof; front (A), rear (B), and sides (inner side of the profiles in red "C,D"). (C) Roof panels 1 and 2, installation of the LED light tube, camera lens plate, and floor installation. (D) Installation of the anesthesia mask (RED), mouse platform (GREEN), and rectangular platform for positioning of the measuring ruler (BLUE) on the reference base. (E) Final location for the reference base. (F) Installation of sides, front, and rear walls. Please click here to view a larger version of this figure.
2. Animal maintenance
- Obtain authorization from the institution's bioethics committee for animal testing. Use CD1 mice (n = 4), 10 weeks old, weighing 28-32 g.
- House the mice under standard conditions: maintain a temperature of 21 °C, 12/12 h light/dark cycles, 45% humidity, and provide ad libitum access to water and food, except during photodocumentation sessions.
3. Traumatic wound generation
- Fast the mice for 8 h prior to the procedure. Anaesthetize the mice with intraperitoneal sodium pentobarbital at 65 mg/kg25 (following institutionally approved protocols).
- Confirm the depth of anesthesia by checking for a lack of withdrawal reflex during an interdigital pinch. Apply ophthalmic ointment to the eyes to prevent dryness during anesthesia. Maintain the mice at a stable temperature of 37 °C during the surgery and recovery (20 min).
- Place the mouse in a ventral decubitus position and depilate a 2.5 cm x 3 cm area from the cervical region caudally using surgical blades. Stretch the skin to avoid lacerations. Perform asepsis and antisepsis using alternating povidone iodine and sterile water rinses.
- Use a sterile 5 mm biopsy punch and apply pressure with circular movements to create a dermo-epidermal incision at the shoulder blade level. Remove the flap with serrated forceps and cut with iris-tip scissors (Figure 2A).
- In case of the presence of bleeding points in the wound, apply an electrician shock with an electrocautery device to achieve hemostasis.
- Place a 1 cm diameter circular dermal film over the wound using Jeweler forceps to prevent contamination and contraction.
- Perform all survival surgeries under sterile conditions. Prepare the surgical site with disinfectants like povidone-iodine. Wear sterile gloves and a mask, and maintain sterility throughout the procedure by using sterile instruments and minimizing contact with non-sterile surfaces.
- After surgery, place the mice in individual cages at 24 °C and monitor them until they regain sufficient consciousness to maintain the sternal position. Ensure body temperature is controlled during and after surgery to prevent hypothermia by using external heat sources.
- Administer analgesia until day 4 by diluting ketorolac in drinking water at 5 mg/ kg26.
- After 14 days, when the wounds in healthy rodents typically reach advanced stages of healing, euthanize the mice by O2 replacement in a CO2 chamber following international standards. This marks the endpoint of the study.
4. Macroscopic image acquisition
- Capture images of the wounds daily for 14 consecutive days to monitor the continuous kinetics of wound closure. Use a semi-professional or professional camera; position the lens through the circular opening in the photo booth roof.
NOTE: Adjust the schedule as necessary based on experimental conditions. - Use an RGB LED light tube in Correlated Colour Temperature (CCT) mode, set to 9000 K and 100% brightness for lighting.
- Position the reference base at the center of the photo booth floor.
- Place the mouse in the inhaled anesthesia chamber for 3 min (Figure 2B).
- After inducing anesthesia, place the mouse in a prone position on the mouse platform. Secure the snout within the sevoflurane anesthesia mask, delivering 5% sevoflurane at an oxygen flow rate of 1.5 L/min (Figure 2C).
- Before capturing images, remove the dermal film from the wound using Jeweller forceps and align the reference base with the camera lens.
- Install the front wall of the photo booth, and capture macroscopic images with the following settings: aperture f/3.2, exposure time 1/200s, ISO speed 80, focal length 4mm.
- Using Jeweller forceps, place a 1 cm diameter dermal film on the wound before removing the anesthesia mask. Transfer the mice to individual cages and observe until motor recovery.
5. Image processing
- Generate a backup copy of the macroscopic images. Process the images using the ImageJ software.
- Open the backup image in ImageJ by following the path: File > Open > Search in the images, then click on Open.
- Scale the image by reference: Maximize the image, select the straight-line tool, zoom in on the ruler next to the mouse using the + key, draw a 10 mm straight line on the ruler image, then go to Analyze > Set Scale > Enter " 10" for Known distance > Set Unit of length to mm, and then click on Ok. This determines the pixels-to-distance ratio for the macroscopic image (Figure 2D).
- Separate the wound area from the image: Use the rectangle tool to select the area around the wound (w = 150, h = 150). Record the X and Y values, right-click on the rectangle > Duplicate > Name the subject > Press Enter. In the new image, press + twice to zoom in (Figure 2E).
- Save the cropped image by following the path: File > Save As > Tiff > Enter the name > Save.
- Create a duplicate of the cropped image to avoid modification during segmentation: Right-click on the cropped image > Duplicate > Name it by adding "Segmentation" to the end. Next, press Enter. In the new image, press + twice to zoom in.
- Separate the image into color channels by following this path: Image > Type > RGB Stack. In the red channel image (1/3 Red), right-click on Duplicate, and press OK in the Duplicate window.
- Segment the red channel image. Click on the red channel image, Image > Adjust > Threshold. Next, select Default > Apply (Figure 2F).
- Rectify the Region of Interest (ROI) to ensure complete wound coverage and avoid segmentation distortions.
NOTE: If areas around the wound are not included in segmentation, go to Process > Binary > Fill Holes. To remove black dots outside the wound, go to Process > Binary > Erode. If the Erode command reduces the wound size, correct it by using Process > Binary > Dilate. - Mark the perimeter of the ROI: Edit > Selection > Create Selection. The ROI will be outlined in yellow. Add it to the ROI Manager by following Edit > Selection > Add to Manager (Figure 2G).
- Validate the segmentation: Open the ROI Manager window, and select the ROI to review: More > Translate. Enter the X and Y values recorded in step 4, and press OK.
- Maximize the original image, then select the ROI in the ROI Manager. Adjust the ROI using the arrow keys until it aligns with the wound (Figure 2H). If the ROI matches the wound, the segmentation was successful. Otherwise, repeat from step 2.
- Once the segmentation is confirmed, obtain the wound measurements: Analyze > Measure. A results table will display the area, perimeter, and X/Y position values. Copy these values to SPSS software for documentation and statistical analysis(Figure 2I).
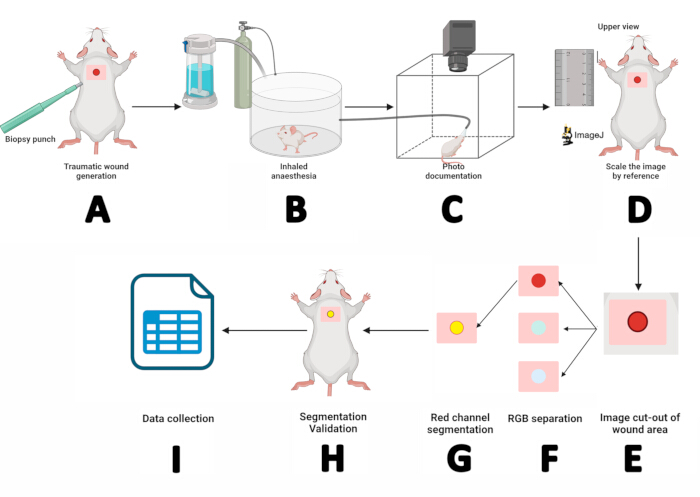
Figure 2: Workflow of wound measurement using digital planimetry and segmentation techniques. (A) Dermo-epidermal incision using a sterile 5 mm biopsy punch. (B) Placing the mouse in an inhaled anesthesia chamber for 3 min. (C) Photo documentation by positioning the anesthetized mouse in the photo booth and securing its snout within a sevoflurane mask. (D) Opening the obtained image in ImageJ and scaling it using the ruler as a reference. (E) Extracting the wound area using the rectangle tool. (F) Separating the image into RGB channels and processing the red channel. (G) Outlining and managing the region of interest (ROI). (H) Validating the segmentation by matching the ROI with the wound. (I) Measuring wound parameters and recording the results for statistical analysis. Please click here to view a larger version of this figure.
6. Post-procedural euthanasia
NOTE: The study concludes after 14 days, at which point the wounds in healthy rodents typically reach advanced stages of healing. At this stage, the mice were humanely euthanized following the established institutionally approved euthanasia procedure.
- Prepare the equipment. Ensure the euthanasia chamber is clean, dry, and of appropriate size to accommodate the mice without overcrowding. Connect the chamber to a medical-grade CO2 supply with a flow regulator.
- Gently transfer the mice into the chamber to minimize stress. Ensure they are in a calm environment with minimal external disturbances.
- Set the CO2 flow rate to 20%-30% of the chamber's volume per minute, as recommended by the AVMA Guidelines for the Euthanasia of Animals. Gradually increase the CO2 concentration to avoid inducing distress or discomfort in the mice.
- Observe the mice closely during the procedure. Look for signs of gradual loss of consciousness, including reduced activity and breathing, followed by apnea and cardiac arrest.
- Confirm the death once the mice are no longer breathing and show no signs of movement; wait an additional 1-2 min to ensure a complete cessation of vital functions. Verify death by confirming the absence of reflexes (e.g., corneal reflex) and cardiac activity.
- Transfer the carcasses to designated Biological Hazardous Waste containers. Follow institutional protocols and national guidelines for the safe disposal of animal remains.
- Record the number of animals euthanized, the date, and any relevant observations to ensure compliance with ethical and institutional standards.
Results
After scaling the images in ImageJ software, the average perimeter (Table 1) and area (Table 2) of the wounds, along with their respective standard deviations, were obtained through digital segmentation. These values were recorded from day zero to day fourteen (D0-D14).
| Day | Perimeter (mm) |
| 0 | 22.75 ± 0.8900 |
Discussion
In pre-clinical models, quantitatively analyzing the evolution of traumatic wounds in pre-clinical models faces challenges due to factors like wound size, localized inflammatory response34, location, and/or manipulation. Direct manual36 and indirect digital11,16,37,38 planimetry methods exist for these measurements. In contrast to studies using ma...
Disclosures
The authors declare that there are no conflicts of interest related to this research.
Acknowledgements
The authors would like to acknowledge the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCyT, CVU: 933600) through the grant for providing funding, and the Laboratorio Nacional de Investigación y Desarrollo de Radiofármacos del Instituto Nacional de Investigaciones Nucleares (LANIDER-ININ) for their support. Additionally, Figure 2 was prepared with the assistance of BioRender software (2020), available at BioRender.com/p67z056.
Materials
| Name | Company | Catalog Number | Comments |
| 5 mm Biopsy Punch | MILTEX, USA | 33-35 | To mark the wound edges |
| Aluminum with polyethylene core | Alucobond,USA | Bright Silver 119 | For the construction of the macroscopic Image Acquisition Booth |
| Camera Lens | Sony, Japan | SEL2470Z | To focus the images to photograph |
| Electrocautery | Bonart, USA | ART-E1 | To eliminates bleeding points in the wound if present. |
| Hook and loop fastener strips | VELCRO | ||
| IBM SPSS Statistics Version 22 | IBM Corporation, USA | https://www.ibm.com/analytics/spss-statistics | Used for statistical analysis of wound measurements, including area and perimeter data. |
| ImageJ Version 1.53t | National Institutes of Health, USA | https://imagej.nih.gov/ij/ | Used for processing macroscopic images, including scaling, segmentation, and measurement of wound parameters. |
| Ketorolac | SIEGFRIED RHEIN, Mexico | 493977 | For postoperative pain management |
| Miltex Iris Scissors, 4-1/8" Curved | MILTEX, USA | V95-306 | To cut the wound flap generated with the biopsy punch |
| RGB LED Light Tube | ANDOER, China | B09F8RLMSY | To illuminate the Macroscopic Image Acquisition Booth |
| Semi profesional camera | Sony, Japan | DSC-HX300 | To take the photos |
| Serrated Forceps | MILTEX, USA | V96-118 | To hold the flap during the cut |
| Sevoflurane | Baxter, USA | AMX2L9117PR | For inhaled anaesthesia |
| Sodium Pentobarbital | Aranda, Mexico | 734.448.001.212 | For intraperitoneal anaesthesia |
| SolidWorks Version 2015 | Dassault Systèmes, France | https://www.solidworks.com/ | Used to design and create 3D models for constructing accessories for the photodocumentation booth. |
| Surgical blades | HERGOM, Mexico | H10 | To shave the hair in the area where the wound will be created |
| Transparent Adhesive Dressing | 3M, USA | F51CA07 | To cover the traumatic wound |
References
- Martinengo, L., et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 29, 8-15 (2019).
- Chen, X., Shi, X., Xiao, H., Xiao, D., Xu, X. Research hotspot and trend of chronic wounds: A bibliometric analysis from 2013 to 2022. Wound Repair Regen. 31 (5), 597-612 (2013).
- Gould, L., Li, W. W. Defining complete wound closure: Closing the gap in clinical trials and practice. Wound Repair Regen. 27 (3), 201-224 (2019).
- Baron, J. M., Glatz, M., Proksch, E. Optimal support of wound healing: New insights. Dermatology. 236 (6), 593-600 (2020).
- Tiwari, R., Pathak, K. Local drug delivery strategies towards wound healing. Pharmaceutics. 15 (2), 634 (2023).
- Gupta, B., Agarwal, R., Alam, M. S. Hydrogels for wound healing applications. Biomed Hydrogels. , 184-227 (2011).
- Pastar, I., et al. Pre-clinical models for wound-healing studies. Skin Tissue Models Regen Med. , 223-253 (2018).
- Shen, Z., Zhang, C., Wang, T., Xu, J. Advances in functional hydrogel wound dressings: A Review. Polymers (Basel). 15 (9), 2000 (2000).
- Wong, V. W., Sorkin, M., Glotzbach, J. P., Longaker, M. T., Gurtner, G. C. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol. 2011, 1-8 (2011).
- Peterson, N., Stevenson, H., Sahni, V. Size matters: How accurate is clinical estimation of traumatic wound size. Injury. 45 (1), 232-236 (2014).
- Öien, R. F., Håkansson, A., Hansen, B. U., Bjellerup, M. Measuring the size of ulcers by planimetry: a useful method in the clinical setting. J Wound Care. 11 (5), 165-168 (2002).
- Rogers, L. C., Bevilacqua, N. J., Armstrong, D. G., Andros, G. Digital planimetry results in more accurate wound measurements: a comparison to standard ruler measurements. J Diabetes Sci Technol. 4 (4), 799-802 (2010).
- Shamata, A., Thompson, T. Documentation and analysis of traumatic injuries in clinical forensic medicine involving structured light three-dimensional surface scanning versus photography. J Forensic Leg Med. 58, 93-100 (2018).
- Foltynski, P., Ladyzynski, P., Ciechanowska, A., Migalska-Musial, K., Judzewicz, G., Sabalinska, S. Wound area measurement with digital planimetry: Improved accuracy and precision with calibration based on 2 rulers. PLoS One. 10 (8), e0134622 (2015).
- Frank, S., Kämpfer, H. Excisional wound healing. Wound Healing: Methods Protoc. , 3-15 (2003).
- Dunn, L., et al. Murine model of wound healing. J Vis Exp. (75), e50265 (2013).
- Barakat, M., DiPietro, L. A., Chen, L. Limited Treatment options for diabetic wounds: Barriers to clinical translation despite therapeutic success in murine models. Adv Wound Care (New Rochelle). 10 (8), 436-460 (2021).
- Goh, C. C., et al. Inducing ischemia-reperfusion injury in the mouse ear skin for intravital multiphoton imaging of immune responses. J Vis Exp. (118), e54956 (2016).
- Wang, Y., et al. Autologous fat grafting promotes macrophage infiltration to increase secretion of growth factors and revascularization, thereby treating diabetic rat skin defect. Diabetes, Metab Syndr Obes: Targets and Therapy. 13, 4897-4908 (2020).
- Notodihardjo, S. C., et al. A comparison of the wound healing process after the application of three dermal substitutes with or without basic fibroblast growth factor impregnation in diabetic mice. J Plast Reconstr Aesthet Surg. 73 (8), 1547-1555 (2020).
- Thawer, H. A., Houghton, P. E., Woodbury, M. G., Keast, D., Campbell, K. A comparison of computer-assisted and manual wound size measurement. Ostomy Wound Manage. 48 (10), 46-53 (2002).
- Huang, C. X., et al. Comparison of digital and traditional skin wound closure assessment methods in mice. Lab Anim Res. 39 (1), 25 (2023).
- Subramaniam, S. R., et al. 3D printing: Overview of PLA progress. AIP Conf Proc. , 020015 (2019).
- Oleksy, M., Dynarowicz, K., Aebisher, D. Rapid prototyping technologies: 3D printing applied in medicine. Pharmaceutics. 15 (8), 2169 (2023).
- Gómez de Segura, &. #. 1. 9. 3. ;. I. Métodos de anestesia, analgesia y eutanasia. Métodos de anestesia, analgesia y eutanasia. 14, 97-127 (2016).
- Romero-Fernandez, W., et al. El 1, 2, 3 de la experimentación con animales de laboratorio. Rev Peru Med Exp Salud Publica. 33 (2), 288 (2016).
- Cukjati, D., Reberšek, S., Miklavčič, D. A reliable method of determining wound healing rate. Med Biol Eng Comput. 39 (2), 263-271 (2001).
- Payne, W. G., Bhalla, R., Hill, D. P., Pierpont, Y. N., Robson, M. C. Wound healing trajectories to determine pressure ulcer treatment efficacy. Wound Repair Regen. 11, e1 (2011).
- Cardinal, M., Eisenbud, D. E., Phillips, T., Harding, K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 16 (1), 19-22 (2008).
- Tallman, P. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol. 133 (10), 1231 (1997).
- Hopkins, N. F. G., Jamieson, C. W. Antibiotic concentration in the exudate of venous ulcers: The prediction of ulcer healing rate. Br J Surg. 70 (9), 532-534 (2005).
- Robson, M. C. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg. 135 (7), 773 (2000).
- Jessup, R. L. What is the best method for assessing the rate of wound healing? A comparison of 3 mathematical formulas. Wounds. 19 (3), 138-147 (2006).
- Landén, N. X., Li, D., Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol Life Sci. 73 (20), 3861-3885 (2016).
- Szpaderska, A. M., DiPietro, L. A. Inflammation in surgical wound healing: Friend or foe. Surgery. 137 (5), 571-573 (2005).
- Hamed, E., Al Balah, O. F. A., Refaat, M., Badr, A. M., Afifi, A. Photodynamic therapy mediated by methylene blue-loaded PEG accelerates skin mouse wound healing: An immune response. Lasers in Med Sci. 39 (1), 141 (2024).
- Ibraheem, W. I., et al. Comparison of digital planimetry and ruler methods for the measurement of extraction socket wounds. Medicina (Kaunas). 59 (1), 135 (2023).
- Mayrovitz, H. N., Soontupe, L. B. Wound areas by computerized planimetry of digital images: accuracy and reliability. Wounds. 22 (5), 222-229 (2009).
- Galiano, R. D., Michaels, J., Dobryansky, M., Levine, J. P., Gurtner, G. C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 12 (4), 485-495 (2004).
- Price, A., Grey, J. E., Patel, G. K., Cbe, K. G. H. . ABC of wound healing. , 0470658975 (2022).
- Eming, S. A., Krieg, T., Davidson, J. M. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 127 (3), 514-525 (2007).
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature. 438 (7070), 932-936 (2005).
- López, N., Cervero, S., Jiménez, M. J., Sanchez, J. F. Cellular characterization of wound exudate as a predictor of wound healing phases. Wounds. 26 (4), 101-107 (2014).
- Liu, N. T., et al. Quantifying the effects of wound healing risk and potential on clinical measurements and outcomes of severely burned patients: A data-driven approach. Burns. 46 (2), 303-313 (2020).
- Han, C., Barakat, M., DiPietro, L. A. Angiogenesis in wound repair: Too much of a good thing. Cold Spring Harb Perspect Biol. 14 (10), a041225 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved