需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
细胞分裂和扩张的运动学分析:量化增长和采样产业开发区的细胞学基础
摘要
Quantifying cell division and expansion is of crucial importance to the understanding of whole-plant growth. Here, we present a protocol to calculate cellular parameters determining maize leaf growth rates and highlight the use of these data for investigating molecular growth regulatory mechanisms by directing developmental stage-specific sampling strategies.
摘要
Growth analyses are often used in plant science to investigate contrasting genotypes and the effect of environmental conditions. The cellular aspect of these analyses is of crucial importance, because growth is driven by cell division and cell elongation. Kinematic analysis represents a methodology to quantify these two processes. Moreover, this technique is easy to use in non-specialized laboratories. Here, we present a protocol for performing a kinematic analysis in monocotyledonous maize (Zea mays) leaves. Two aspects are presented: (1) the quantification of cell division and expansion parameters, and (2) the determination of the location of the developmental zones. This could serve as a basis for sampling design and/or could be useful for data interpretation of biochemical and molecular measurements with high spatial resolution in the leaf growth zone. The growth zone of maize leaves is harvested during steady-state growth. Individual leaves are used for meristem length determination using a DAPI stain and cell-length profiles using DIC microscopy. The protocol is suited for emerged monocotyledonous leaves harvested during steady-state growth, with growth zones spanning at least several centimeters. To improve the understanding of plant growth regulation, data on growth and molecular studies must be combined. Therefore, an important advantage of kinematic analysis is the possibility to correlate changes at the molecular level to well-defined stages of cellular development. Furthermore, it allows for a more focused sampling of specified developmental stages, which is useful in case of limited budget or time.
引言
生长分析依赖于一组工具通常使用由植物科学家描述基因型确定生长的差异和/或表型响应于环境因素。它们包括全厂的大小和重量测量或器官和增长率的计算来探讨经济增长的内在机制。器官发育是细胞分裂和扩大在细胞水平确定。因此,包括在生长分析这两个过程的量化的关键是理解在整个器官1生长的差异。因此,关键是要具有适当的方法来确定细胞生长参数是相对容易由非专业的实验室使用。
运动学分析已经被确立为一种方法提供的器官发育模型2发展的一个强大的框架。这项技术对线性系统进行了优化,如拟南芥根和单子叶叶子,也为非线性的系统中,例如双子叶叶片3。如今,这种方法被越来越多地用于研究如何遗传,激素,发育和环境因素影响细胞的分裂和各种器官的扩展( 表1)。此外,它也提供了一个框架,以细胞过程链接到他们的潜在生化,分子和生理规章( 表2),尽管限制可以通过器官大小和空间组织为需要更高量的植物材料的技术( 例如,代谢物施加测量,蛋白质组学, 等等 )。
单子叶叶子,如玉米( 玉蜀黍 )叶,表示其中的细胞从朝向尖叶的基部移动线性系统,通过分生组织和伸长区顺序地传递到到达成熟区。这使得它的增长4的空间格局定量研究的理想模式系统。此外,玉米叶有较大的增长区域(分生组织和伸长区跨越几厘米5),并在其他组织层次的研究提供了可能性。这允许(假定)的监管机制控制细胞分裂和扩大,通过一系列的分子生物学技术,生理测量和细胞生物学的方法( 见表2)通过运动学分析定量调查。
在这里,我们提供了在单子叶树叶进行运动分析的协议。首先,我们将解释如何进行这两种细胞分裂和细胞伸长的正确分析,沿叶轴位置以及如何计算运动学参数的函数。其次,我们还显示如何可以用作用于取样设计的基础。在这里,我们讨论了两种情况:高分辨率的采样ð集中采样,从而提高了数据的解释和时间/钱储蓄,分别为。
表1.运动概述分析了细胞分裂和各种器官的扩张量化方法。
| 器官 | 参考 |
| 单子叶植物的叶子 | 16,20,21,22 |
| 根尖 | 2,23,24,25,26,27,28,29 |
| 双子叶植物的叶子 | 21,30,31 |
| 拍摄顶端分生组织 | 32 |
表1.运动概述分析了细胞分裂和各种器官的扩张量化方法。
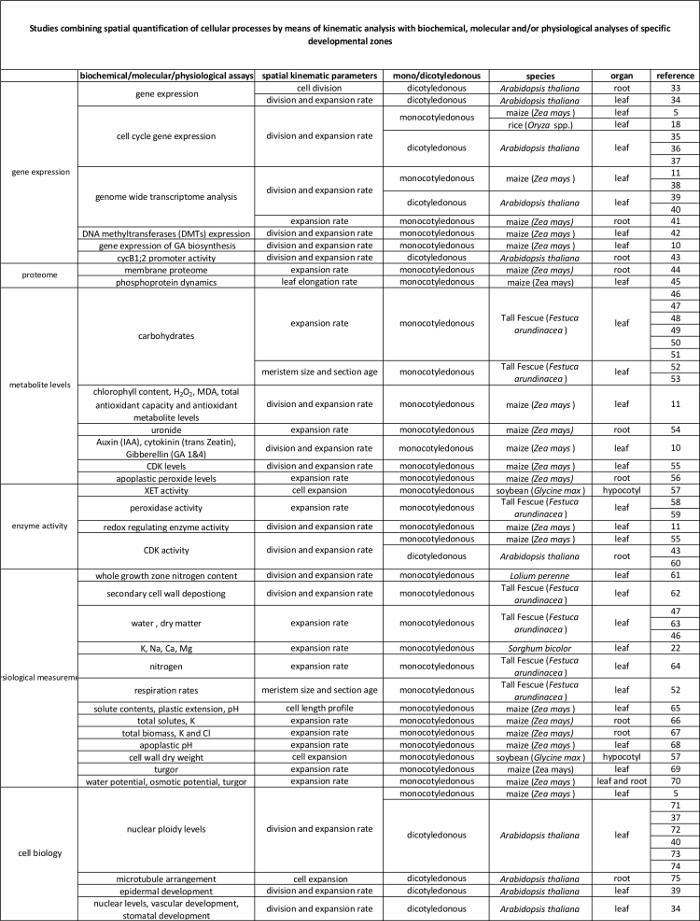
表2.由运动分析来在分子水平上的调控量化细胞过程之间的链接。参考资料细胞过程的量化链接到来自于不同物种和器官生物化学和分子分析结果的各种研究。木聚糖endotransglucosylase(XET),丙二醛(MDA),细胞周期蛋白依赖性激酶(CDK)。 请点击这里查看此表的放大版本。
Access restricted. Please log in or start a trial to view this content.
研究方案
注意:以下协议运动学分析只适用于稳态生长过程中的叶子。此期间的几天6意味着稳定叶伸长率和细胞的长度和在叶扩展空间模式。
1.植物生长和叶片伸长率的措施(LER)
- 选择在稳态增长叶片和感兴趣的发展阶段。
注意:有是稳态生长和重复的增长,这意味着对在同一轴线上的连续叶片类似空间模式之间的差。在幼苗生长的早期阶段,连续的树叶一般的生长区7的尺寸增加变得越来越快所致。虽然有少数较高的叶位置可以有类似的增长模式8,这是可以被接受调查处理的影响过渡阶段。它来比较线路和tr为因此,重要的 eatments严格在同一叶位,即使它可能在不同的时间进行开发。即使在恒定伸长率,生长速度轮廓不一定在不同发育阶段是相同的。因此,在相同的发育阶段8,典型地通过出苗后的天数定义为分析叶是重要的。 - 为了在单子叶植物叶片进行增长的全面运动学分析,至少增长15株为每个治疗和基因型生长室控制的条件下。
- 在感兴趣的叶出现(出现从围绕叶片的螺纹)的时间,开始用尺子测量叶的长度每日直至叶完全展开( 图1i)中 。叶长意味着从土壤水平长度与叶的尖端。小心不要折断或损坏叶,因为这可能会改变其生长。
重1"SRC ="/文件/ ftp_upload / 54887 / 54887fig1.jpg"/>
图1:玉米叶片的运动分析示意图感兴趣的叶子与连续三天来计算叶片伸长率(LER)尺子测量。此后,将叶收获和三厘米段被用于分生组织大小的确定。这是通过测量从基向上的长度,以DAPI染色后,最远端的有丝分裂图进行。 (A)的增殖核分裂和(B)形成核分裂的例子。从中期静脉的另一侧的叶基部第一11厘米用于切割为电池的长度测量十分之一厘米的段。这些测量提供用于创建元长度信息,其用于确定所述成熟细胞长度(L 垫 )和细胞离开分生组织(L 格 )的长度的基础。该 LER和L 垫被用于计算所述电池的生产速率(P),而L 的div和L 聚体被用来计算细胞的分生组织(N 聚体 )的数量。反过来,P和N 聚体被用来计算平均细胞分裂率(D),它是细胞周期的持续时间(T C)的倒数。相同颜色的箭头表示用于计算以下这些箭头的参数的参数。比例尺= 40微米。罗马数字被用来指在协议中所述具体的实验过程。 请点击此处查看该图的放大版本。
2.收获
- 在感兴趣的发育阶段( 例如,出苗后第三天),选择至少五个代表p从批量lants在其上进行运动学分析。继续测量植物的其余部分如在步骤1.3解释来确定最终的叶长。
- 切割植物的地上部分。为了保持分生部分完好,削减尽可能靠近根部( 图1II)。
- 从外叶开始,通过由一个轻轻展开逐一去除所有叶到感兴趣的叶子。如果需要的话,从基座移除一些额外毫米至分离的叶子。同时删除受利益( 图1iii)的叶包围的顶点和小叶子。
- 切断的3厘米的段,从基座上中期静脉的一侧开始,并把它存储在1.5ml试管填充有3:1(体积比)的绝对乙醇:乙酸溶液(小心:戴手套)在4℃放置24小时至数月( 图1iv)。本段以后将用来确定分生组织的长度。
- 来自静脉的另一侧,切断从基部A 11厘米的段( 图1ⅴ),并将其放置在一个15毫升管在4℃下填充有无水乙醇为至少6小时,以除去色素( 图1ⅵ)。
注:后来,仅使用前10厘米测定细胞长度的个人资料(见讨论)。 - 续约另一轮在4℃清洗至少24小时( 图1vi)的无水乙醇。
- 最后,代替无水乙醇与纯净乳酸(小心:戴手套)用于清洁和存储在4℃下24小时或直至进一步使用( 图1vi)。
3.茎尖长度测量
- 制备含有50mM氯化钠(NaCl),5mM的乙二胺四乙酸漂洗缓冲液(EDTA;注意:戴手套)和10mM的Tris(羟甲基)氨基甲烷 - 盐酸(TRIS盐酸; pH7)中。
- 从第2.4节等以3厘米的段阿克它在缓冲器20分钟( 图1vii)。
- 在等待中,使用漂洗缓冲液,制备的1μg/ ml的4',6-二脒基-2-苯基吲哚(DAPI)染色溶液,保持在冰上,并在黑暗中。
- 通过将分生组织段在DAPI染色溶液2-5分钟染色细胞核。在冰上并在黑暗中( 图1vii)工作。
- 通过快速安装在显微镜的玻璃段,并用玻璃盖覆盖它检查荧光信号。表皮细胞应该表现出荧光,而底层的细胞层不应该。
- 如果染色不充分,把段回来为一些额外分钟DAPI染色溶液。
- 要停止染色,在一个显微镜载玻片和盖有盖玻璃清洗缓冲一滴安装段。
- 在放大20倍使用,配有紫外荧光显微镜,允许大约1000表皮的可视化人细胞一次。滚动整个段和寻找增殖核分裂(中期,后期,末期,和胞质分裂),但要避免发展中的气孔( 图1viii)的形成细胞分裂9。定义哪里最远端的有丝分裂的数字的位置。
- 通过测量叶的基部和最远端表皮有丝分裂图之间的距离确定的分生组织的长度。使用图像分析软件( 例如,ImageJ的)来测量所述图像帧的全部长度。
- 算覆盖完整的分生组织的长度(从叶基部到最远端有丝分裂图)的帧的数量和一帧的长度乘以这个号码来得到完整的分生组织的长度( 图1ix)。
4.池长简介
- 以存储在乳酸(步骤2.5)段和小心将它放置在板凳上。切段机智哈手术刀在1厘米每个( 图1X)10段。
- 安装在一个显微镜载玻片连续叶段乳酸一小滴。确保始终如一地面向近轴或远轴的一面朝上。原则上,对于一个特定侧没有偏好。
- 使用带有微分干涉对比(DIC)的光学显微镜分析段,从叶基部开始。用图像分析软件测量以一致地选择相同的细胞类型的至少20复制表皮细胞中直接相邻的气孔文件文件的长度。
- 为此沿各段(每段打住4个位置)的等距位置,并一定要记下每次测量整个叶( 图1xi)的相应位置。
- 通过使用本地多项式平滑p确定在沿叶轴每毫米的平均泡孔长度rocedure,在R-脚本(;补充文件1 图1xii)实现的。
注:R-脚本提供了一系列增加平滑的数据。平滑所需的量是任意的,最好应该只是删除本地噪音,但并不影响整体的曲线。确保使用一个实验内平滑对于所有样品的相同量。 - 平均在植物之间的各位置的细胞的长度,并计算标准误差来创建沿着叶片轴线的细胞长度轮廓。
5.运动参数计算(见补充文件2)
- 通过取叶片长度的变化的两个连续时间点之间( 例如,24小时,如在步骤1.3)和由时间间隔除以计算LER。
- 计算对应于从基部远端的位置,其中的细胞达到成熟CE的95%的增长区(L GZ)的长度在平滑细胞长度轮廓LL长度。
- 取为平均以下该位置( 图2)中的所有细胞的长度的平滑信元长度信息95%的各位置。
- 比较平滑的细胞长度(步骤4.4)在各位置计算出的95%的细胞长度。 (;见补充数据2 图2)从叶子的基部开始,生长区在其中电池实际长度等于以下细胞长度的95%的位置结束。
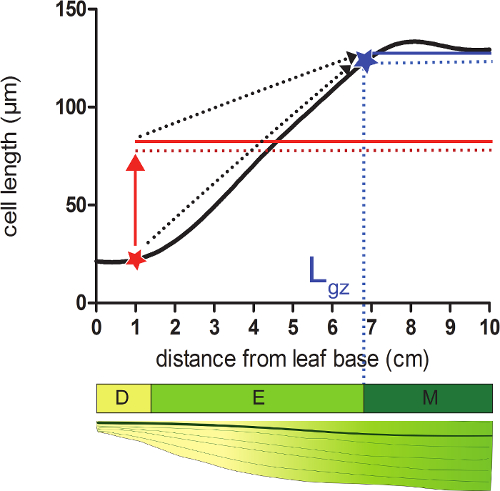
图2:确定生长区的端分生组织:在用红色星所指示的位置时,电池实际尺寸小于95%以下的该位置的所有小区的平均泡孔大小的(红色虚线)(红色固体线)。在生长区(L GZ的结束;具有AB表示泰伦星)位于其中,95%以下这个位置(蓝色实线的所有单元的平均单元尺寸(蓝色虚线))等于实际单元格大小。除法区(D)中,伸长区(E),和成熟区(M)。虚线箭头表示当地的规模和平均规模上,从基础岗位叶的尖端移动时叶的远端部分的95%之间的衔接。 请点击此处查看该图的放大版本。
- 计算伸长区(L EL)作为生长区(L GZ)和分生组织大小的长度之间的差的长度(L 聚体 ;在第3步确定)。
- 计算出的成熟细胞的长度(L 垫 )作为成熟区中的平均细胞长度。
- 用L 垫除以LER获得电池生产速率(P)。
- 计算伸长区(N EL)为N 的gz和N 聚体之间的差异细胞数。在分生组织(N- 聚体 )的细胞的数目等于设在对应于所述分生组织的间隔单元的累计数。在生长区(N GZ)的细胞的数目等于位于对应于增长区中的间隔单元的累计数。
- 计算平均细胞分裂率(D)为P / N 聚体 。细胞周期的持续时间(T C)等于LN(2)/ D。
- 除以Ñ 埃尔用P计算伸长区(T EL)的时间。在划分时区的时间等于日志2(N MER)* T C。细胞离开分生组织的长度(L 格 )等于从平滑单元长度信息在分生组织的端部的细胞长度。
- 计算使用下列公式单元格平均扩张速度(R EL):LN(L 垫 )-ln(L DIV)] / T EL。
Access restricted. Please log in or start a trial to view this content.
结果
在这里,我们将展示他们的叶子增长方面遭受干旱胁迫条件(干旱,34%SWC)精心浇灌植物(控制,54%的土壤含水量(SWC))和植物之间的比较。所有的植物在生长室生长控制的条件下(16小时白天/ 8小时,夜幕下,25℃/ 18℃,昼/夜,300-400μEm-2秒-1光合有效辐射(PAR)的干旱情况,直至正确的SWC达到再进一步保持代扣水建立的。在初步研究中,它被定义?...
Access restricted. Please log in or start a trial to view this content.
讨论
在玉米叶片完整运动学分析,使叶片生长的细胞基础的决心,并允许有效采样战略的设计。虽然该协议是相对简单的,一些谨慎以下述关键步骤,建议:(1)它不破坏分生组织分离年轻的,封闭的叶(步骤2.3)中,由于分生组织长度判定(步骤3)是重要的,需要的完整分生组织到场。事先可能需要一些练习。 (2)分生长度确定是基于的核分裂解释。因此,我们建议,一个实验中,同一个人,由?...
Access restricted. Please log in or start a trial to view this content.
披露声明
作者宣称,他们没有竞争的经济利益。
致谢
这项工作是由来自安特卫普大学VA一个博士研究生奖学金的支持;从法兰德斯科学基金会(FWO,11ZI916N),以KS博士奖学金;从FWO(G0D0514N)项目赠款;协调一致的研究活动(GOA)研究经费,从安特卫普大学的研究委员会"叶形态的系统生物学方法";和校际景点波兰人(IUAP VII / 29,MARS),"玉米和拟南芥幼苗和根生长",从比利时联邦科学政策办公室(BELSPO),以GTSB韩Asard,Bulelani L. Sizani和滨田AbdElgawad都促使视频。
Access restricted. Please log in or start a trial to view this content.
材料
| Name | Company | Catalog Number | Comments |
| Pots | Any | Any | We use pots with the following measures, but can be different depending on the treatment/study: bottom diameter: 11 cm, opening diameter: 15 cm, height: 12 cm. We grow one maize plant per pot. |
| Planting substrate | Any | Any | We use potting medium (Jiffy, The Netherlands), but other substrates can be used, depending on treatment/study. |
| Ruler | Any | Any | An extension ruler that covers at least 1.5 meters is needed to measure the final leaf length of the plants. |
| Seeds | Any | NA | Seeds can be ordered from a breeder. |
| Scalpel | Any | Any | The scalpel is used during leaf harvesting to detach the leaf of interest from its surrounding leaves and right after harvesting to cut a proper sample for cell length and meristem length measurements. |
| 15 mL falcon tubes | Any | Any | The 15 mL falcon tubes are used for storing samples used for cell length measurements during sample clearing with absolute ethanol and lactic acid. |
| Eppendorf tubes | Any | Any | The eppendorf tubes are used for storing samples used for meristem length measurements in ethanol:acetic acid 3:1 (v:v) solution. |
| Gloves | Any | Any | Latex gloves, which protect against corrosive reagents. |
| Acetic acid | Any | Any | CAUTION: Corrosive to metals, category 1 Skin corrosion, categories 1A,1B,1C Serious eye damage, category 1; Flammable liquids, categories 1,2,3 |
| Absolute ethanol | Any | Any | CAUTION: Hazardous in case of skin contact (irritant), of eye contact (irritant), of inhalation. Slightly hazardous in case of skin contact (permeator), of ingestion |
| Lactic acid >98% | Any | Any | CAUTION: Corrosive to metals, category 1 Skin corrosion, categories 1A,1B,1C Serious eye damage, category 1 |
| Sodium chloride (NaCl) | Any | Any | |
| Ethylenediaminetetraacetic acid (EDTA) | Any | Any | CAUTION: Acute toxicity (oral, dermal, inhalation), category 4 Skin irritation, category 2 Eye irritation, category 2 Skin sensitisation, category 1 Specific Target Organ Toxicity – Single exposure, category 3 |
| Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) | Any | Any | This material can be an irritant, contact with eyes and skin should be avoided. Inhalation of dust may be irritating to the respiratory tract. |
| 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) | Any | Any | Cell permeable fluorescent minor groove-binding probe for DNA. Causes skin irritation. May cause an allergic skin reaction. May cause respiratory irritation. |
| Ice | Any | NA | The DAPI solution has to be kept on ice. |
| Fluorescent microscope | AxioScope A1, Axiocam ICm1 from Zeiss or other | Any fluorescent microscope can be used for determining meristem length. | |
| Microscopic slide | Any | Any | |
| Cover glass | Any | Any | |
| Tweezers | Any | Any | Tweezers are needed for unfolding the rolled maize leaf right after harvesting in order to cut a proper sample for cell length and meristem length measurements. |
| Image-analysis software | Axiovision (Release 4.8) from Zeiss | NA | The software can be downloaded at: http://www.zeiss.com/microscopy/en_de/downloads/axiovision.html. Other softwares such as ImageJ (https://imagej.nih.gov/ij/) could be used as well. |
| Microscope equipped with DIC | AxioScope A1, Axiocam ICm1 from Zeiss or other | Any microscope, equipped with differential interference contrast (DIC) can be used to measure cell lengths. | |
| R statistical analysis software | R Foundation for Statistical Computing | NA | Open source; Could be downloaded at https://www.r-project.org/ |
| R script | NA | NA | We use the kernel smoothing function locpoly of the Kern Smooth package (Wand MP, Jones MC. Kernel Smoothing: Chapman & Hall/CRC (1995)). The script is available for Mac and Windows upon inquiry with the corresponding author. |
参考文献
- Fiorani, F., Beemster, G. T. S. Quantitative analyses of cell division in plants. Plant Mol. Biol. 60, 963-979 (2006).
- Silk, W. K., Erickson, R. O. Kinematics of Plant-Growth. J. Theor. Biol. 76, 481-501 (1979).
- Rymen, B., Coppens, F., Dhondt, S., Fiorani, F., Beemster, G. T. S. Kinematic Analysis of Cell Division and Expansion. Plant Developmental Biology. Hennig, L., Köhler, C. , Chapter 14 (2010).
- Avramova, V., Sprangers, K., Beemster, G. T. S. The Maize Leaf: Another Perspective on Growth Regulation. Trends Plant Sci. 20, 787-797 (2015).
- Rymen, B., et al. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol. 143, 1429-1438 (2007).
- Muller, B., Reymond, M., Tardieu, F. The elongation rate at the base of a maize leaf shows an invariant pattern during both the steady-state elongation and the establishment of the elongation zone. J. Exp. Bot. 52, 1259-1268 (2001).
- Beemster, G. T. S., Masle, J., Williamson, R. E., Farquhar, G. D. Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L): Kinematic analysis of leaf elongation. J. Exp. Bot. 47, 1663-1678 (1996).
- Bernstein, N., Silk, W. K., Lauchli, A. Growth and Development of Sorghum Leaves under Conditions of Nacl Stress - Spatial and Temporal Aspects of Leaf Growth-Inhibition. Planta. 191, 433-439 (1993).
- Sylvester, A. W., Smith, L. G. Cell Biology of Maize Leaf Development. Handbook of maize: It's Biology. Bennetzen, J. L., Hake, S. C. , Springer. NY. (2009).
- Nelissen, H., et al. A Local Maximum in Gibberellin Levels Regulates Maize Leaf Growth by Spatial Control of Cell Division. Curr. Biol. 22, 1183-1187 (2012).
- Avramova, V., et al. Drought Induces Distinct Growth Response, Protection, and Recovery Mechanisms in the Maize Leaf Growth Zone. Plant Physiol. 169, 1382-1396 (2015).
- Picaud, J. C., et al. Total malondialdehyde (MDA) concentrations as a marker of lipid peroxidation in all-in-one parenteral nutrition admixtures (APA) used in newborn infants. Pediatr. Res. 53, 406(2003).
- Basu, P., Pal, A., Lynch, J. P., Brown, K. M. A novel image-analysis technique for kinematic study of growth and curvature. Plant Physiol. 145, 305-316 (2007).
- Vander Weele, C. M., et al. A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiol. 132, 1138-1148 (2003).
- Nelissen, H., Rymen, B., Coppens, F., Dhondt, S., Fiorani, F., Beemster, G. T. S. Plant Organogenesis. DeSmet, I. , Chapter 17 (2013).
- Ben-Haj-Salah, H., Tardieu, F. Temperature Affects Expansion Rate of Maize Leaves without Change in Spatial-Distribution of Cell Length - Analysis of the Coordination between Cell-Division and Cell Expansion. Plant Physiol. 109, 861-870 (1995).
- Fiorani, F., Beemster, G. T. S., Bultynck, L., Lambers, H. Can meristematic activity determine variation in leaf size and elongation rate among four Poa species? A kinematic study. Plant Physiol. 124, 845-855 (2000).
- Pettko-Szandtner, A., et al. Core cell cycle regulatory genes in rice and their expression profiles across the growth zone of the leaf. J. Plant Res. 128, 953-974 (2015).
- Poorter, H., Remkes, C. Leaf-Area Ratio and Net Assimilation Rate of 24 Wild-Species Differing in Relative Growth-Rate. Oecologia. 83, 553-559 (1990).
- Macadam, J. W., Volenec, J. J., Nelson, C. J. Effects of Nitrogen on Mesophyll Cell-Division and Epidermal-Cell Elongation in Tall Fescue Leaf Blades. Plant Physiol. 89, 549-556 (1989).
- Tardieu, F., Granier, C. Quantitative analysis of cell division in leaves: methods, developmental patterns and effects of environmental conditions. Plant Mol. Biol. 43, 555-567 (2000).
- Bernstein, N., Silk, W. K., Lauchli, A. Growth and Development of Sorghum Leaves under Conditions of Nacl Stress - Possible Role of Some Mineral Elements in Growth-Inhibition. Planta. 196, 699-705 (1995).
- Erickson, R. O., Sax, K. B. Rates of Cell-Division and Cell Elongation in the Growth of the Primary Root of Zea-Mays. P. Am. Philos. Soc. 100, 499-514 (1956).
- Beemster, G. T. S., Baskin, T. I. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 116, 1515-1526 (1998).
- Goodwin, R. H., Stepka, W. Growth and differentiation in the root tip of Phleum pratense. Am. J. Bot. 32, 36-46 (1945).
- Hejnowicz, Z. Growth and Cell Division in the Apical Meristem of Wheat Roots. Physiologia Plantarum. 12, 124-138 (1959).
- Gandar, P. W. Growth in Root Apices .1. The Kinematic Description of Growth. Bot. Gaz. 144, 1-10 (1983).
- Baskin, T. I., Cork, A., Williamson, R. E., Gorst, J. R. Stunted-Plant-1, a Gene Required for Expansion in Rapidly Elongating but Not in Dividing Cells and Mediating Root-Growth Responses to Applied Cytokinin. Plant Physiol. 107, 233-243 (1995).
- Sacks, M. M., Silk, W. K., Burman, P. Effect of water stress on cortical cell division rates within the apical meristem of primary roots of maize. Plant Physiol. 114, 519-527 (1997).
- Granier, C., Tardieu, F. Spatial and temporal analyses of expansion and cell cycle in sunflower leaves - A common pattern of development for all zones of a leaf and different leaves of a plant. Plant Physiol. 116, 991-1001 (1998).
- De Veylder, L., et al. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell. 13, 1653-1667 (2001).
- Kwiatkowska, D. Surface growth at the reproductive shoot apex of Arabidopsis thaliana pin-formed 1 and wild type. J. Exp. Bot. 55, 1021-1032 (2004).
- Kutschmar, A., et al. PSK-alpha promotes root growth in Arabidopsis. New Phytol. 181, 820-831 (2009).
- Vanneste, S., et al. Plant CYCA2s are G2/M regulators that are transcriptionally repressed during differentiation. Embo J. 30, 3430-3441 (2011).
- Eloy, N. B., et al. Functional Analysis of the anaphase-Promoting Complex Subunit 10. Plant J. 68, 553-563 (2011).
- Eloy, N. B., et al. SAMBA, a plant-specific anaphase-promoting complex/cyclosome regulator is involved in early development and A-type cyclin stabilization. P. Natl. Acad. Sci. USA. 109, 13853-13858 (2012).
- Dhondt, S., et al. SHORT-ROOT and SCARECROW Regulate Leaf Growth in Arabidopsis by Stimulating S-Phase Progression of the Cell Cycle. Plant Physiol. 154, 1183-1195 (2010).
- Baute, J., et al. Correlation analysis of the transcriptome of growing leaves with mature leaf parameters in a maize RIL population. Genome Biol. 16, (2015).
- Andriankaja, M., et al. Exit from Proliferation during Leaf Development in Arabidopsis thaliana: A Not-So-Gradual Process. Dev. Cell. 22, 64-78 (2012).
- Beemster, G. T. S., et al. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol. 138, 734-743 (2005).
- Spollen, W. G., et al. Spatial distribution of transcript changes in the maize primary root elongation zone at low water potential. Bmc Plant Biol. 8, (2008).
- Candaele, J., et al. Differential Methylation during Maize Leaf Growth Targets Developmentally Regulated Genes. Plant Physiol. 164, 1350-1364 (2014).
- West, G., Inze, D., Beemster, G. T. S. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 135, 1050-1058 (2004).
- Zhang, Z., Voothuluru, P., Yamaguchi, M., Sharp, R. E., Peck, S. C. Developmental distribution of the plasma membrane-enriched proteome in the maize primary root growth zone. Front. Plant Sci. 4, (2013).
- Bonhomme, L., Valot, B., Tardieu, F., Zivy, M. Phosphoproteome Dynamics Upon Changes in Plant Water Status Reveal Early Events Associated With Rapid Growth Adjustment in Maize Leaves. Mol. Cell Proteomics. 11, 957-972 (2012).
- Schnyder, H., Nelson, C. J. Growth-Rates and Assimilate Partitioning in the Elongation Zone of Tall Fescue Leaf Blades at High and Low Irradiance. Plant Physiol. 90, 1201-1206 (1989).
- Schnyder, H., Nelson, C. J., Spollen, W. G. Diurnal Growth of Tall Fescue Leaf Blades .2. Dry-Matter Partitioning and Carbohydrate-Metabolism in the Elongation Zone and Adjacent Expanded Tissue. Plant Physiol. 86, 1077-1083 (1988).
- Schnyder, H., Nelson, C. J. Growth-Rates and Carbohydrate Fluxes within the Elongation Zone of Tall Fescue Leaf Blades. Plant Physiol. 85, 548-553 (1987).
- Vassey, T. L., Shnyder, H. S., Spollen, W. G., Nelson, C. J. Cellular Characterisation and Fructan Profiles in Expanding Tall Fescue. Curr. T. Pl. B. 4, 227-229 (1985).
- Allard, G., Nelson, C. J. Photosynthate Partitioning in Basal Zones of Tall Fescue Leaf Blades. Plant Physiol. 95, 663-668 (1991).
- Spollen, W. G., Nelson, C. J. Response of Fructan to Water-Deficit in Growing Leaves of Tall Fescue. Plant Physiol. 106, 329-336 (1994).
- Volenec, J. J., Nelson, C. J. Carbohydrate-Metabolism in Leaf Meristems of Tall Fescue .1. Relationship to Genetically Altered Leaf Elongation Rates. Plant Physiol. 74, 590-594 (1984).
- Volenec, J. J., Nelson, C. J. Carbohydrate-Metabolism in Leaf Meristems of Tall Fescue .2. Relationship to Leaf Elongation Rates Modified by Nitrogen-Fertilization. Plant Physiol. 74, 595-600 (1984).
- Silk, W. K., Walker, R. C., Labavitch, J. Uronide Deposition Rates in the Primary Root of Zea-Mays. Plant Physiol. 74, 721-726 (1984).
- Granier, C., Inze, D., Tardieu, F. Spatial distribution of cell division rate can be deduced from that of p34(cdc2) kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol. 124, 1393-1402 (2000).
- Voothuluru, P., Sharp, R. E. Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress.1. Increased levels are specific to the apical region of growth maintenance. J. Exp. Bot. 64, 1223-1233 (2012).
- Wu, Y. J., Jeong, B. R., Fry, S. C., Boyer, J. S. Change in XET activities, cell wall extensibility and hypocotyl elongation of soybean seedlings at low water potential. Planta. 220, 593-601 (2005).
- Macadam, J. W., Nelson, C. J., Sharp, R. E. Peroxidase-Activity in the Leaf Elongation Zone of Tall Fescue .1. Spatial-Distribution of Ionically Bound Peroxidase-Activity in Genotypes Differing in Length of the Elongation Zone. Plant Physiol. 99, 872-878 (1992).
- Macadam, J. W., Sharp, R. E., Nelson, C. J. Peroxidase-Activity in the Leaf Elongation Zone of Tall Fescue .2. Spatial-Distribution of Apoplastic Peroxidase-Activity in Genotypes Differing in Length of the Elongation Zone. Plant Physiol. 99, 879-885 (1992).
- Beemster, G. T. S., De Vusser, K., De Tavernier, E., De Bock, K., Inze, D. Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol. 129, 854-864 (2002).
- Kavanova, M., Lattanzi, F. A., Schnyder, H. Nitrogen deficiency inhibits leaf blade growth in Lolium perenne by increasing cell cycle duration and decreasing mitotic and post-mitotic growth rates. Plant Cell Environ. 31, 727-737 (2008).
- Macadam, J. W., Nelson, C. J. Secondary cell wall deposition causes radial growth of fibre cells in the maturation zone of elongating tall fescue leaf blades. Ann. Bot-London. 89, 89-96 (2002).
- Schnyder, H., Nelson, C. J. Diurnal Growth of Tall Fescue Leaf Blades .1. Spatial-Distribution of Growth, Deposition of Water, and Assimilate Import in the Elongation Zone. Plant Physiol. 86, 1070-1076 (1988).
- Gastal, F., Nelson, C. J. Nitrogen Use within the Growing Leaf Blade of Tall Fescue. Plant Physiol. 105, 191-197 (1994).
- Vanvolkenburgh, E., Boyer, J. S. Inhibitory Effects of Water Deficit on Maize Leaf Elongation. Plant Physiol. 77, 190-194 (1985).
- Silk, W. K., Hsiao, T. C., Diedenhofen, U., Matson, C. Spatial Distributions of Potassium, Solutes, and Their Deposition Rates in the Growth Zone of the Primary Corn Root. Plant Physiol. 82, 853-858 (1986).
- Meiri, A., Silk, W. K., Lauchli, A. Growth and Deposition of Inorganic Nutrient Elements in Developing Leaves of Zea-Mays L. Plant Physiol. 99, 972-978 (1992).
- Neves-Piestun, B. G., Bernstein, N. Salinity-induced inhibition of leaf elongation in maize is not mediated by changes in cell wall acidification capacity. Plant Physiol. 125, 1419-1428 (2001).
- Bouchabke, O., Tardieu, F., Simonneau, T. Leaf growth and turgor in growing cells of maize (Zea mays L.) respond to evaporative demand under moderate irrigation but not in water-saturated soil. Plant Cell Environ. 29, 1138-1148 (2006).
- Westgate, M. E., Boyer, J. S. Transpiration-Induced and Growth-Induced Water Potentials in Maize. Plant Physiol. 74, 882-889 (1984).
- Horiguchi, G., Gonzalez, N., Beemster, G. T. S., Inze, D., Tsukaya, H. Impact of segmental chromosomal duplications on leaf size in the grandifolia-D mutants of Arabidopsis thaliana. Plant J. 60, 122-133 (2009).
- Fleury, D., et al. The Arabidopsis thaliana homolog of yeast BRE1 has a function in cell cycle regulation during early leaf and root growth. Plant Cell. 19, 417-432 (2007).
- Vlieghe, K., et al. The DP-E2F-like gene DEL1 controls the endocycle in Arabidopsis thaliana. Curr. Biol. 15, 59-63 (2005).
- Boudolf, V., et al. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell. 16, 2683-2692 (2004).
- Baskin, T. I., Beemster, G. T. S., Judy-March, J. E., Marga, F. Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis. Plant Physiol. 135, 2279-2290 (2004).
Access restricted. Please log in or start a trial to view this content.
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。