Standard interni
Panoramica
Fonte: Laboratorio del Dr.B. Jill Venton - Università della Virginia
L'obiettivo di molte analisi chimiche è un'analisi quantitativa, in cui viene determinata la quantità di una sostanza in un campione. Per calcolare con precisione la concentrazione di uno sconosciuto da un campione, è fondamentale un'attenta preparazione del campione. Ogni volta che un campione viene maneggiato o trasferito, parte del campione può andare perso. Esistono tuttavia strategie per ridurre al minimo la perdita di campioni. Esistono anche strategie per far fronte alla perdita del campione e fare ancora misurazioni accurate della concentrazione.
Per ridurre al minimo la perdita di campioni, l'ideale è ridurre al minimo il numero di passaggi di gestione e trasferimento del campione. Ad esempio, la massa di un campione solido direttamente in un pallone in cui verrà realizzata una soluzione riduce una fase di trasferimento. Se è necessario trasferire da un pallone all'altro e si sta facendo una diluizione, il triplo risciacquo della vetreria aiuta a garantire che tutto il campione venga trasferito. Altre strategie sono più specifiche per il campione. Ad esempio, i campioni che assorbono al vetro, come le proteine, potrebbero essere gestiti meglio in tubi monouso in polipropilene. I tubi non sono idrofili, quindi se una piccola quantità di campione deve essere pipettata in acqua, è meglio aver già aggiunto l'acqua al tubo, in modo che il campione possa essere pipettato direttamente nel solvente. Potrebbe essere meglio concentrarsi, piuttosto che asciugare completamente un campione, a causa delle perdite da insolubilità dopo la reidratazione.
Un'altra fonte di perdita del campione è attraverso manipolazioni incomplete del campione. Ad esempio, se viene utilizzata una procedura di derivatizzazione e la derivatizzazione è incompleta, l'intera quantità di campione non viene osservata. Errori come questo sono errori sistematici e possono essere risolti correggendo il problema, ad esempio modificando la procedura di derivatizzazione. Un'altra causa di errore sistematico nelle misurazioni sono gli effetti della matrice. Questi possono interferire con la misurazione di determinate sostanze e l'esecuzione di calibrazioni nella stessa matrice del campione può ridurre questo effetto.
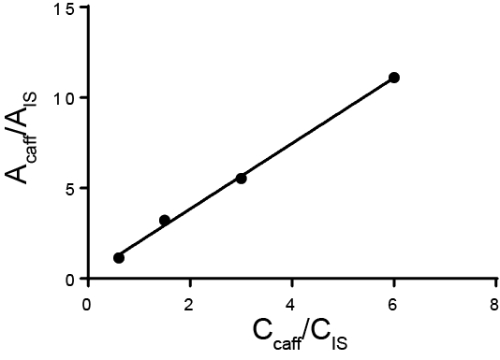
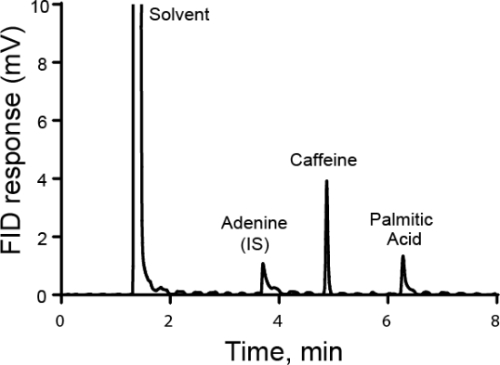
L'analisi quantitativa viene in genere eseguita utilizzando standard esterni o interni. Per gli standard esterni, viene effettuata una curva di calibrazione misurando diverse concentrazioni note dell'analita di interesse. Quindi, l'esempio viene eseguito separatamente dallo standard. Per gli standard interni, lo standard si trova nello stesso campione dell'analita di interesse, consentendo di eseguire la misurazione contemporaneamente. Tipicamente, viene aggiunta una specie diversa chiamata standard interno e viene calcolato il rapporto tra la risposta per quello standard interno e l'analita. L'idea è che il rapporto della risposta, chiamato fattore di risposta, sia proporzionale alla loro concentrazione. Mentre il metodo deve essere in grado di distinguere tra l'analita di interesse e lo standard interno, eventuali perdite di campione che si verificano dopo l'aggiunta dello standard interno dovrebbero essere simili per entrambe le sostanze e quindi il rapporto della risposta rimane lo stesso. Un caso speciale di utilizzo di standard interni è il metodo delle aggiunte standard, in cui quantità crescenti di analita vengono aggiunte alla soluzione e la quantità originale di analita viene retro-calcolata. Gli standard interni possono essere utilizzati in cromatografia, elettrochimica e spettroscopia.
Procedura
1. Corretta gestione del campione: creazione di una soluzione
- Prendi un becher pulito e ammassa la quantità corretta di campione in esso. Registrare la massa effettiva utilizzata. In questo esempio, una soluzione di adenina viene realizzata in un pallone volumetrico per l'uso come standard interno per l'analisi successiva. La massa di adenina è di 100 mg. Non ammassare direttamente in un matraccio volumetrico perché ha un collo lungo e l'adenina non può essere facilmente aggiunta o rimossa.
- Aggiungere
Applicazione e Riepilogo
Gli standard interni sono utilizzati in molti campi, tra cui la spettroscopia e la cromatografia. In spettroscopia, gli standard interni possono aiutare a correggere errori casuali dovuti a cambiamenti nell'intensità della sorgente luminosa. Se una lampada o un'altra sorgente luminosa ha una potenza variabile, influenzerà l'assorbimento e, di conseguenza, l'emissione di un campione. Tuttavia, il rapporto tra uno standard interno e l'analita rimarrà costante, anche se la sorgente luminosa non lo fa. Un esempio di quest...
Tags
Vai a...
Video da questa raccolta:

Now Playing
Standard interni
Analytical Chemistry
204.3K Visualizzazioni

Preparazione del campione per la caratterizzazione analitica
Analytical Chemistry
84.2K Visualizzazioni

Metodo delle aggiunte standard
Analytical Chemistry
319.3K Visualizzazioni

Curve di calibrazione
Analytical Chemistry
794.6K Visualizzazioni

Spettroscopia ultravioletta/visibile (UV-VIs)
Analytical Chemistry
621.7K Visualizzazioni

Spettroscopia Raman per analisi chimiche
Analytical Chemistry
51.0K Visualizzazioni

Fluorescenza a raggi X (XRF)
Analytical Chemistry
25.3K Visualizzazioni

Gascromatografia con rivelatore a ionizzazione di fiamma
Analytical Chemistry
281.1K Visualizzazioni

Cromatografia liquida ad alta prestazione (HPLC)
Analytical Chemistry
383.0K Visualizzazioni

Cromatografia a scambio ionico
Analytical Chemistry
263.9K Visualizzazioni

Elettroforesi capillare
Analytical Chemistry
93.3K Visualizzazioni

Introduzione alla spettrometria di massa
Analytical Chemistry
111.9K Visualizzazioni

Microscopia elettronica a scansione (SEM)
Analytical Chemistry
86.8K Visualizzazioni

Misurazioni elettrochimiche di catalizzatori supportati mediante l'utilizzo di un potenziometro/galvanometro
Analytical Chemistry
51.3K Visualizzazioni

Voltammetria ciclica
Analytical Chemistry
124.2K Visualizzazioni