내부 표준
Overview
출처: 박사의 실험실.B 질 벤턴 - 버지니아 대학
많은 화학 분석의 목표는 정량 분석이며, 시료의 물질의 양이 결정됩니다. 시료로부터 알 수 없는 농도를 정확하게 계산하기 위해서는 주의 깊은 시료 준비가 핵심이다. 샘플을 처리하거나 전송할 때마다 일부 샘플을 손실할 수 있습니다. 그러나 샘플 손실을 최소화하기 위한 전략이 있습니다. 또한 시료 손실에 대처하고 농도를 정확하게 측정하기 위한 전략도 있습니다.
샘플 손실을 최소화하기 위해 시료 처리 및 전송 단계 수를 최소화하는 것이 이상적입니다. 예를 들어, 솔리드 샘플을 플라스크에 직접 매스하여 용액이 전달 단계를 감소시키는 데 필요한 경우 한 플라스크에서 다른 플라스크로 전송해야 하고 희석이 이루어지고 있는 경우 유리 제품을 헹구면 모든 샘플이 전송되도록 합니다. 다른 전략은 샘플에 더 구체적입니다. 예를 들어, 단백질과 같은 유리에 흡착하는 샘플은 폴리프로필렌 일회용 튜브에서 더 잘 처리될 수 있습니다. 튜브는 친수성이 아니기 때문에 소량의 시료가 물에 배관될 경우 이미 튜브에 물을 첨가하여 샘플을 용매로 직접 배관할 수 있습니다. 수화 후 용성으로 인한 손실로 인해 샘플을 완전히 건조시키기보다는 농축하는 것이 더 좋을 수 있습니다.
샘플 손실의 또 다른 원인은 불완전한 샘플 조작을 통해서입니다. 예를 들어, 파생 프로시저가 사용되고 파생이 불완전한 경우 전체 양의 샘플이 관찰되지 않습니다. 이와 같은 오류는 체계적인 오류이며, 파생 절차 변경과 같은 문제를 수정하여 해결할 수 있다. 측정에서 체계적인 오류의 또 다른 원인은 매트릭스 효과입니다. 이들은 특정 물질의 측정을 방해하고 샘플이 효과를 줄일 수있는 샘플과 동일한 매트릭스에서 교정을 수행 할 수 있습니다.
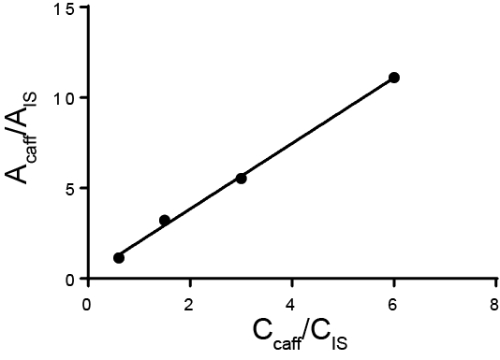
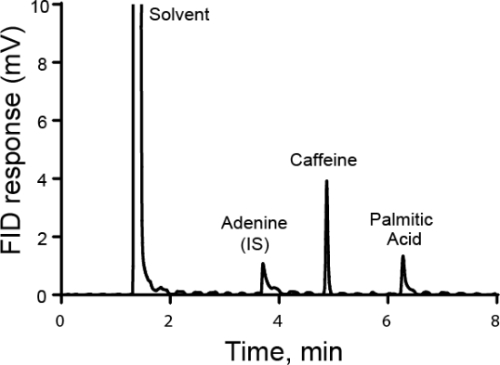
정량 분석은 일반적으로 외부 또는 내부 표준을 사용하여 수행됩니다. 외부 표준의 경우, 교정 곡선은 관심 있는 별분의 상이한 공지된 농도를 측정하여 이루어집니다. 그런 다음 샘플은 표준과 별도로 실행됩니다. 내부 표준의 경우 표준은 관심 있는 단언과 동일한 샘플에 있으므로 동시에 측정을 수행할 수 있습니다. 전형적으로, 다른 종은 내부 표준이라고 불리며 그 내부 표준및 분석물의 반응 비율이 계산된다. 응답 계수라고 하는 응답 비율이 농도에 비례한다는 것이 아이디어입니다. 방법은 관심 분석기와 내부 표준을 구별할 수 있어야 하지만 내부 표준이 추가된 후에 발생하는 모든 샘플 손실은 두 물질 모두에 대해 유사해야 하므로 응답 비율은 동일하게 유지됩니다. 내부 표준을 사용하는 특별한 경우는 표준 추가 방법이며, 여기서 는 Aalyte의 양이 증가함에 따라 솔루션에 추가되고 원래의 양의 딜리바이트가 다시 계산됩니다. 내부 표준은 크로마토그래피, 전기화학 및 분광법에 사용될 수 있습니다.
Procedure
1. 적절한 샘플 처리: 솔루션 만들기
- 깨끗한 비커를 가지고 정확한 양의 샘플을 대량으로 넣습니다. 사용된 실제 질량을 기록합니다. 이 예에서 아데닌의 용액은 다음 분석을 위한 내부 표준으로 사용하기 위해 볼륨 플라스크에서 만들어집니다. 아데닌의 질량은 100 mg. 긴 목을 가지고 있고 아데닌을 쉽게 추가하거나 제거할 수 없기 때문에 체적 플라스크에 직접 질량을 두지 마십시오.
- 약 25mL의 용매(이 경우 디메틸 설산화물(DMSO)를 비커에 넣고 저어서 용해하도록 한다. 이 예에서 최종 용액은 50mL 체피 플라스크로 만들어지므로 약 25mL만 추가하여 비커를 헹구고 최종 볼륨으로 구성된 솔루션이 가능합니다.
- 고체가 용해되면 용액을 체적 플라스크에 붓습니다.
- 비커와 교반바를 소량의 용매로 헹구고 약 10mL로 헹구고 헹구는 플라스크에 붓습니다. 두 번 더 반복합니다. 이렇게 하면 적절한 솔루션 전송을 보장할 수 있습니다.
Application and Summary
내부 표준은 분광및 크로마토그래피를 포함하여 많은 분야에서 사용됩니다. 분광법에서 내부 표준은 광원 강도의 변화로 인해 임의 오류를 수정하는 데 도움이 될 수 있습니다. 램프 또는 다른 광원이 가변 전력을 가지고 있는 경우, 이는 시료의 흡수에 영향을 미치고 결과적으로 시료의 방출에 영향을 미칩니다. 그러나 광원이 없는 경우에도 내부 표준에서 분석기의 비율은 일정하게 유지됩니다...
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
내부 표준
Analytical Chemistry
205.4K Views

분석 특성화를 위한 샘플 준비
Analytical Chemistry
85.3K Views

표준 추가 방법
Analytical Chemistry
320.9K Views

교정 곡선
Analytical Chemistry
798.8K Views

자외선 눈에 보이는 (UV-Vis) 분광법
Analytical Chemistry
625.4K Views

화학 분석을 위한 라만 분광법
Analytical Chemistry
51.4K Views

엑스레이 형광 (XRF)
Analytical Chemistry
25.9K Views

화염 이온화 감지를 갖춘 가스 크로마토그래피(GC)
Analytical Chemistry
283.1K Views

고성능 액체 크로마토그래피 (HPLC)
Analytical Chemistry
386.1K Views

이온 교환 크로마토그래피
Analytical Chemistry
265.2K Views

모세관 전기 포레시스 (CE)
Analytical Chemistry
94.6K Views

질량 분광법 소개
Analytical Chemistry
112.9K Views

스캐닝 전자 현미경 검사법 (SEM)
Analytical Chemistry
87.7K Views

전위요스타트/갈바노스타트를 사용하여 지원되는 촉매의 전기화학적 측정
Analytical Chemistry
51.8K Views

순환 볼탐량 (CV)
Analytical Chemistry
126.0K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유