Analisi del ciclo cellulare: valutazione della proliferazione delle cellule T CD8 e CD4 in seguito a stimolazione tramite colorazione CFSE e citometria a flusso
Panoramica
Fonte: Perchet Thibaut1,2,3, Meunier Sylvain1,2,3, Sophie Novault4, Rachel Golub1,2,3
1 Unità di Linfopoiesi, Dipartimento di Immunologia, Istituto Pasteur, Parigi, Francia
2 INSERM U1223, Parigi, Francia
3 Université Paris Diderot, Sorbonne Paris Cité, Cellule Pasteur, Parigi, Francia
4 Platfrom, Citometria a flusso e biomarcatori UtechS, Center for Translational Science, Pasteur Institute, Parigi, Francia
Il ciclo cellulare è un processo universale di vita. Durante il ciclo cellulare, una cellula subisce diverse modifiche per dividersi in due cellule figlie. Questo meccanismo si verifica per tutta la vita di un organismo in risposta ai suoi bisogni. Le divisioni cellulari e lo sviluppo embrionale producono un organismo completo da uno zigote unicellulare. Durante l'età adulta, il ciclo cellulare è centrale in molti processi biologici critici, come le riparazioni dei tessuti.
I meccanismi di divisione cellulare sono eventi strettamente controllati in cui la cellula subisce modifiche graduali prima della divisione finale. Le cellule che non sono ancora nel ciclo sono descritte come nella fase Gap 0 (G0). Durante questa fase la cellula è considerata quiescente. Quando la cellula inizia a pedalare, vengono riconosciute quattro fasi distinte: Gap 1 (G1),Sintesi (S), Gap 2 (G2) e Mitosi (M). La fase G1 è un punto di controllo per le risorse necessarie alla cellula per la sintesi del DNA. Quindi, si verifica la fase S e inizia la replicazione del DNA, seguita dall'interfase G2, un altro checkpoint che controlla tutti gli elementi necessari per la divisione della cellula. Infine, la cellula entra nella mitosi e si divide in due cellule figlie.
La divisione cellulare è un parametro altamente informativo in molti sistemi biologici diversi. Nel campo dell'immunologia, l'analisi della proliferazione leucocitaria può indicare il meccanismo della risposta immunitaria. Altri domini di indagine si basano anche sull'analisi del ciclo cellulare. Ad esempio, l'analisi del ciclo cellulare durante lo sviluppo del tumore ha migliorato la nostra comprensione del cancro.
Molti coloranti fluorescenti sono ora disponibili per il monitoraggio della proliferazione cellulare. Questi coloranti differiscono nelle loro proprietà chimiche e spettrali. Esistono due diverse classi di coloranti: i coloranti proteici si combinano permanentemente con le proteine formando un legame covalente e i coloranti di membrana si intercalano stabilmente all'interno delle membrane cellulari attraverso forti associazioni idrofobiche. Studi in vitro e in vivo sulla proliferazione delle cellule immunitarie mediante citometria a flusso sono tra le applicazioni più comuni di entrambe le classi di coloranti a tracciamento cellulare (1, 2).
CFSE (Carboxyfluorescein succinimidyl ester) è un colorante fluorescente che segna le cellule in divisione. Inizialmente, tutte le cellule ricevono la stessa quantità di colorante; le cellule che si dividono uniformemente dividono il colorante che hanno ricevuto tra le loro due cellule figlie. Di conseguenza, il ciclo cellulare può essere seguito dalla progressiva diminuzione dell'intensità del colorante nelle cellule. La colorazione CFSE è seguita dalla citometria a flusso multiparametrica convenzionale, una tecnologia ad alto rendimento basata sulla fluorescenza che consente la caratterizzazione fenotipica e funzionale delle cellule in base al loro grado di colorazione CFSE (3).
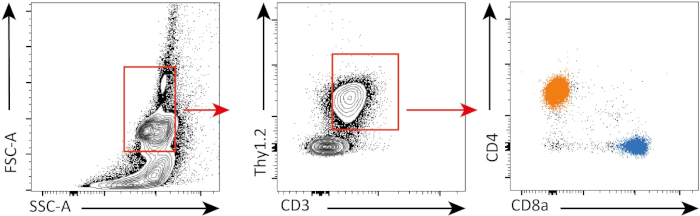
Nel seguente esperimento, valutiamo la proliferazione delle cellule T CD4+ e CD8+ in vitro, dopo stimolazione CD3, utilizzando la colorazione CFSE e la citometria a flusso.
Procedura
1. Preparazione
- Prima di iniziare, indossare guanti da laboratorio e gli indumenti protettivi appropriati.
- Sterilizzare tutti gli strumenti di dissezione, prima con un detergente e poi con etanolo al 70% e poi asciugarli accuratamente.
- Preparare 50 ml di soluzione salina bilanciata di Hank (HBSS) contenente il 2% di siero fetale per vitelli (FCS).
2. Dissezione
- Utilizzando un sistema di rilascio di anidride carboni
Risultati
In questo esperimento, abbiamo seguito la proliferazione delle cellule T spleniche CD4+ e CD8+ in coltura in vitro. Dopo 3 giorni, non abbiamo visto una forte proliferazione in entrambe le cellule T CD4+ e CD8+ con o senza stimolazione. Questo può essere visto nel pannello superiore della Figura 2 dove i picchi di CSFE non stanno diminuendo. Tuttavia, dopo 5 giorni, abbiamo iniziato a vedere la proliferazi.
Applicazione e Riepilogo
I saggi di proliferazione sono spesso utilizzati in diversi campi come l'immunologia per determinare il grado di attivazione delle cellule. Viene anche eseguito nella diagnostica oncologica per determinare l'aggressività del tumore nei pazienti. La colorazione CFSE è una tecnica utile per seguire la proliferazione delle popolazioni di cellule immunitarie nel tempo. Altri metodi consentono la caratterizzazione del ciclo cellulare. BrdU, un equivalente di CFSE è incorporato solo nelle cellule in divisione. Il recente mo...
Riferimenti
- Lyons, A. B. and Parish, C. R. Determination of lymphocyte division by flow cytometry. Journal of Immunological Methods. 171 (1): 131-37, (1994).
- Lyons, A. B. Analyzing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. Journal of Immunological Methods. 243 (1-2), 147-154, (2000).
- Quah, B. J., Warren H. S., and Parish, C. R. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nature Protocols. 2 (9): 2049-56, (2007).
Vai a...
Video da questa raccolta:

Now Playing
Analisi del ciclo cellulare: valutazione della proliferazione delle cellule T CD8 e CD4 in seguito a stimolazione tramite colorazione CFSE e citometria a flusso
Immunology
24.2K Visualizzazioni

Citometria a flusso e selezione cellulare attivata dalla fluorescenza (FACS): isolamento dei linfociti B della milza
Immunology
92.9K Visualizzazioni

Magnetic Activated Cell Sorting (MACS): isolamento dei linfociti T timici
Immunology
22.9K Visualizzazioni

Saggi ELISA: indiretti, sandwich e competitivi
Immunology
238.1K Visualizzazioni

EliSPOT Assay: Rilevamento di splenociti secernenti IFN-γ
Immunology
28.4K Visualizzazioni

Immunoistochimica e immunocitochimica: imaging dei tessuti tramite microscopia ottica
Immunology
78.8K Visualizzazioni

Generazione di anticorpi: produzione di anticorpi monoclonali attraverso l'utilizzo di ibridomi
Immunology
43.5K Visualizzazioni

Microscopia a immunofluorescenza: colorazione a immunofluorescenza di sezioni di tessuto incorporato in paraffina
Immunology
53.8K Visualizzazioni

Microscopia a fluorescenza confocale: una tecnica per determinare la localizzazione delle proteine nei fibroblasti di topo
Immunology
43.1K Visualizzazioni

Tecniche basate sull'immuno-precipitazione: purificazione di proteine endogene con l'impiego di microsfere di agarosio
Immunology
87.6K Visualizzazioni

Trasferimento di cellule adottive: introduzione degli splenociti di topo donatore a un topo ospite e valutazione del successo tramite FACS
Immunology
22.3K Visualizzazioni

Saggio per la morte cellulare: saggio di rilascio di cromo della capacità citotossica
Immunology
151.4K Visualizzazioni