Spettroscopia a risonanza magnetica nucleare (NMR)
Panoramica
Fonte: Laboratorio del Dr. Henrik Sundén – Chalmers University of Technology
La spettroscopia di risonanza magnetica nucleare (NMR) è una tecnica di analisi vitale per i chimici organici. Con l'aiuto di NMR, il lavoro nel laboratorio organico è stato facilitato enormemente. Non solo può fornire informazioni sulla struttura di una molecola, ma anche determinare il contenuto e la purezza di un campione. Rispetto ad altre tecniche comunemente incontrate per i chimici organici – come l'analisi termica e la spettrometria di massa (MS) – la NMR è un metodo non distruttivo che è prezioso quando il recupero del campione è importante.
Una delle tecniche NMR più frequentemente utilizzate per un chimico organico è la NMR protonica(1H). I protoni presenti in una molecola si comporteranno in modo diverso a seconda dell'ambiente chimico circostante, rendendo possibile chiarire la sua struttura. Inoltre, è possibile monitorare il completamento di una reazione confrontando gli spettri NMR del materiale di partenza con quelli del prodotto finale.
Questo video esemplifica come la spettroscopia NMR può essere utilizzata nel lavoro quotidiano di un chimico organico. Verrà mostrato quanto segue: i) preparazione di un campione NMR. ii) Utilizzo di 1H NMR per monitorare una reazione. iii) Identificazione del prodotto ottenuto da una reazione con 1H NMR. La reazione che verrà mostrata è la sintesi di un E-calcone (3) da un'aldeide (1) e un chetone (2) (Schema 1). 1
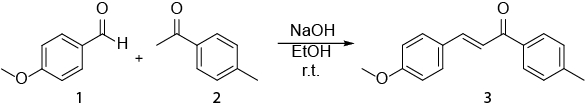
Schema 1. Sintesi di (2E)-3-(4-metossifenil)-1-(4-metilfenil)-2-propen-1-one.
Procedura
1. Preparazione del materiale di partenza NMR
- Aggiungere ~ 10 mg di materiale di partenza a un tubo NMR pulito.
- Sciogliere il materiale di partenza in ~0,7 mL di solvente deuterato (esempio dato CDCl3). Un'altezza adatta del solvente per un buon spettro è di 4,5-5 cm.
- Tappare attentamente il tubo NMR e scrivere il nome del campione sul cappuccio.
- Agitare delicatamente il campione per assicurarsi che tutto il materiale si sia sciolto. Fare attenzione ad evitare il contatt
Risultati
Confrontando gli spettri delle materie prime (Figure 1 e 2) con quelli del prodotto finale (Figura 5) si può osservare una chiara differenza tra gli spettri, che indica la formazione del calcone. L'endpoint della reazione può essere determinato prelevando campioni NMR a diversi intervalli di tempo; ad esempio, il picco del protone aldeide (C(=O)H) (1) può essere visto in Figura 3 ma non in Figu...
Applicazione e Riepilogo
La NMR può, ad esempio, essere utilizzata per rilevare intermedi di reazione, facilitando il lavoro di chiarimento di un meccanismo di reazione. Con l'aiuto della NMR è anche possibile osservare movimenti molecolari e interazioni importanti per lo sviluppo di farmaci. Inoltre, NMR può fornire informazioni strutturali sui materiali solidi; ad esempio per fornire una motivazione per le proprietà osservate dei materiali. Altre applicazioni della NMR possono essere trovate nel campo della medicina, dove la risonanza magnetica (MRI) viene spe...
Riferimenti
- Ta, L., Axelsson, A., Bijl, J., Haukka, M., Sundén, H., Ionic Liquids as Precatalysts in the Highly Stereoselective Conjugate Addition of α,β-Unsaturated Aldehydes to Chalcones. Chem. Eur. J. 20 (43), 13889-13893 (2014).
- Table adapted from Graham Solomons, T. W. Fryhle, C. B., Organic Chemistry, 10th edition, Wiley, p. 387, 418 (2011).
- Clayden, J., Greeves, N., Warren, S., Wothers, P. Proton nuclear magnetic resonance. Organic Chemistry, Chapter 11, Oxford University Press, 269 (2001).
- Wu, X.-F., Neumann, H., Spannenberg, A., Schulz, T., Jiao, H., Beller, M.,Development of a General Palladium-Catalyzed Carbonylative Heck Reaction of Aryl Halides. J. Am. Chem. Soc. 132 (41), 14596-14602 (2010).
Tags
Vai a...
Video da questa raccolta:

Now Playing
Spettroscopia a risonanza magnetica nucleare (NMR)
Organic Chemistry
247.8K Visualizzazioni

Introduzione alla catalisi
Organic Chemistry
34.5K Visualizzazioni

Assemblaggio di un sistema a riflusso per reazioni chimiche riscaldate
Organic Chemistry
167.5K Visualizzazioni

Esecuzione di reazioni al di sotto della temperatura ambiente
Organic Chemistry
70.6K Visualizzazioni

Trasferimento di solventi tramite linea Schlenk
Organic Chemistry
41.6K Visualizzazioni

Degasaggio di liquidi con ciclo freeze-pump-thaw
Organic Chemistry
56.1K Visualizzazioni

Preparazione di reagenti anidri e relativa strumentazione
Organic Chemistry
79.4K Visualizzazioni

Purificazione di composti tramite ricristallizzazione
Organic Chemistry
708.6K Visualizzazioni

Separazione di miscele tramite precipitazione
Organic Chemistry
157.8K Visualizzazioni

Estrazione solido-liquido (lisciviazione)
Organic Chemistry
237.8K Visualizzazioni

Rimozione dei solventi con evaporatore rotante
Organic Chemistry
212.9K Visualizzazioni

Distillazione frazionata
Organic Chemistry
334.4K Visualizzazioni

Preparazione di cristalli per l'analisi mediante diffrazione dei raggi X
Organic Chemistry
32.4K Visualizzazioni

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.7K Visualizzazioni

Cromatografia su colonna
Organic Chemistry
360.1K Visualizzazioni