このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
ポリの合成(
要約
We present a protocol to synthesize Janus microhydrogels composed entirely of the same base material, poly(N-isopropylacrylamide) (PNIPAAm), with a clearly compartmentalized structure base on the phase separation of a supersaturated NIPAAm monomer solution. The synthesized Janus microhydrogels show unique properties such as anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability.
要約
Janus microparticles are compartmentalized particles with differing molecular structures and/or functionality on each of their two sides. Because of this unique property, Janus microparticles have been recognized as a new class of materials, thereby attracting a great deal of attention from various research fields. The versatility of these microparticles has been exemplified through their uses as building blocks for self-assembly, electrically responsive actuators, emulsifiers for painting and cosmetics, and carriers for drug delivery. This study introduces a detailed protocol that explicitly describes a synthetic method for designing novel Janus microhydrogels composed of a single base material, poly(N-isopropylacrylamide) (PNIPAAm). Janus microdroplets are firstly generated via a hydrodynamic focusing microfluidic device (HFMD) based on the separation of a supersaturated aqueous NIPAAm monomer solution and subsequently polymerized through exposure to UV irradiation. The resulting Janus microhydrogels were found to be entirely composed of the same base material, featured an easily identifiable compartmentalized morphology, and exhibited anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability. We believe that the proposed method introduces a novel hydrogel platform with the potential for advanced synthesis of multi-functional Janus microhydrogels.
概要
Hydrogels are a network of hydrophilic polymer chains.1 An increasing amount of research in the field of hydrogels has promoted significant advances and revealed their similarity to biological tissues; the properties of hydrogels allow the uptake of large amounts of water while maintaining their structure. Environmentally responsive hydrogels have also been studied extensively because of their ability to swell or shrink reversibly in response to external stimuli.2 Several triggers, including temperature,3-5 pH,6,7 light,8,9 electric fields,10,11 and specific molecules, such as glucose,12,13 have been suggested to control the geometric shape of hydrogels. Among the many environmentally responsive hydrogels currently available, poly(N-isopropylacrylamide) (PNIPAAm), a well-known thermo-responsive hydrogel, exhibits volume shrinkage above a low critical solution temperature (LCST) of 32 °C.14 A recent study by Sasaki et al.15 reported the intriguing liquid-liquid phase separation of supersaturated NIPAAm, which is the monomer of PNIPAAm. According to this report, supersaturated NIPAAm was dissolved with a 10-fold molar excess of H2O, and soon after, the solution separated into two liquid phases when allows to stand at a temperature above 25 °C; by contrast, dilute NIPAAm was dissolved homogeneously under the same conditions.
Microparticles made of environmentally responsive hydrogels are fascinating candidates for application in drug delivery,16,17 catalysis,18 sensing,19,20 and photonics.21 Traditional synthetic methods including emulsion polymerization, are used to produce hydrogel microparticles with polydispersity.22,23 However, certain applications require microparticles with a narrow size distribution, for example, to stabilize the pharmacokinetics of drug delivery.24 Irregularly shaped or polydisperse embolic microparticles aggregate proximally into clusters, leading to chronic inflammatory responses in embolic particles for cancer therapeutic treatment.25,26
The microfluidic approach is at the forefront of research as a means of fabricating micro-sized particles with narrow size distributions and complex shapes.27-31 The advantages of fabricating microparticles in the microfluidic device are predicated by the small characteristic length of the microfluidic device, which results in a low Reynolds number. In contrast to traditional bulk emulsification where drops are formed in parallel, microdroplets produced in microfluidic devices are generated in series and subsequently polymerized into microparticles upon exposure to UV irradiation. The fundamental principle of droplet formation using a microfluidic device is balance between the interfacial tension and the shear force of the sheath fluid acting on the core fluid.
Despite the obvious advantages of microfluidic fabrication of droplets/particles, Janus droplets/particles consisting of the same base material are rarely reported because the internal morphology of these droplets/particles is generally disturbed by the diffusion and perturbation of the core fluids. To circumvent this intrinsic limitation, two groups recently reported the preparation of the Janus microparticles by employing heat-induced phase separation of colloidal nanoparticles and UV-directed phase separation.32,33
To this end, we report a microfluidic approach to synthesize Janus microhydrogels entirely composed of a single base material and obtain a product with clearly compartmentalized morphology. Our approach is based on the primary concept of liquid-liquid phase separation of supersaturated NIPAAm monomer. The resulting Janus microhydrogels were found to possess unique properties including anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability.
プロトコル
フォトリソグラフィにより流体力学的フォーカシングマイクロ流体デバイス(HFMD)のためのマスターモールドの1の作製
- 製造業者のプロトコルに従って、コンピュータ支援設計(CAD)ソフトウェアを使用して、手足口病( 図1a)用のフォトマスクを設計します。
- ウェハから有機及び無機の塵埃を除去するためにアセトン、イソプロピルアルコール(IPA)、および脱イオン(DI)水を用いて4 'シリコンウエハをすすぎます。
- ウエハとSU-8との接合強度を高めるために5分間の電力100 Wで、O 2プラズマでシリコンウエハを清掃してください。
- スピンコートネガ型フォトレジスト4mlを、SU-8 2150ウェハ上に30秒間3,000 rpmで150ミクロン( 図1BにB1)の厚さを達成します。
- 65℃で5分間ホットプレート上でSU-8被覆されたウェハを置き、95℃に温度を設定し、ソフトベークに30分間ホットプレート上のウエハを残します。
- 置きウェハ上にフォトマスクを設計し、マスクアライナ( 図1bにおけるB2)で、UV光(260ミリジュールのcm -2で、10ミリワットのCMの26秒-2)に公開します。
- ホットプレート(5分間65℃で、次に95℃で12分間)で露光後ベークを行います。
- 10分間のSU-8現像液浴に浸漬することにより、ウェハを開発し、その後、清浄な表面を得るために、5秒間新鮮な現像液にそれを転送します。
- DI水で20秒間ウェハを洗浄し、N 2ガ ス( 図1bにおけるB3)で10秒間、それを乾燥させます。第2節でポリジメチルシロキサン(PDMS)鋳造用マスターモールドとして作製ウェーハを使用してください。
PDMSキャスティングを通じてHFMDの2製作
- PDMS鋳造用マスターモールドとして、第1節で得られたパターン化されたウェハを使用してください。
- 10の重量比で均一にPDMSプレポリマーと硬化剤を混合する:1。例えば、PDMSプレpolym 10gのための硬化剤1gを使用えー。
- マスターモールドにPDMSプレポリマーを注ぎ、真空チャンバー( 図1bにおけるB4)で1時間のためにそれを脱気。
- 3時間65℃のオーブンにPDMSプレポリマーとマスター型を置きます。
- 鋭いメスを用いて、単一のチップの大きさに硬化PDMSをカット。慎重に手でマスターモールドから硬化PDMSのレプリカをはがし。
- 繰り返しは、同一のPDMSレプリカを得るために、2.2〜2.5の手順。
- 入口パンチと接続するチューブの外径よりもわずかに小さい直径を有する穴パンチャーを使用して、レプリカのいずれかに穴出口。
- コロナ処理機を使用して、各レプリカの接合領域に空気プラズマ処理を施す。34
注意:オゾンの蓄積を避けるために、通気性のいいエリアにコロナ処理機を使ってください。 - 空気プラズマ処理された領域上にメタノール5μLをドロップします。細かく手manipuによってHFMDを作製するために、2つの同一のPDMSレプリカを揃えますレーション、および顕微鏡( 図1bにおけるB5)を介して位置合わせを確認してください。
注意:空気プラズマ処理したPDMSレプリカはかなり粘着性および操作することが困難です。このように、メタノールの5μlを、潤滑剤として機能する空気プラズマ処理した表面に添加されます。 - 2 PDMSレプリカ( 図1bにおけるB6)との間の結合を強化するために65℃で一晩に設定したオーブンでHFMDを置きます。 HFMDのマイクロチャネルの高さを高くし、動作中のマイクロ流体チャネル内に微小液滴の目詰まりを避けるために、ボンド二つの同一のPDMSのレプリカ。
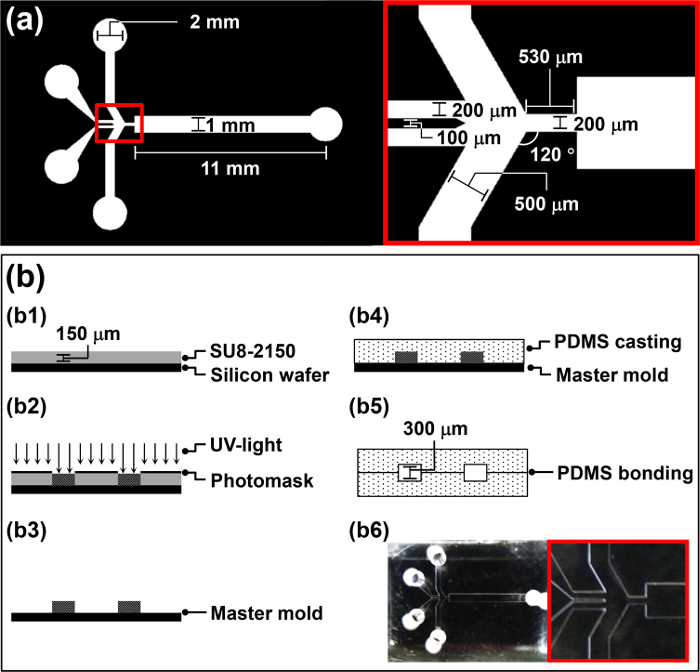
図1:手足口病の製造手順の概要 HFMDのためのフォトマスクの(a)の設計パラメータ。 HFMDの製造手順の(b)のイラスト。ftp_upload / 52813 / 52813fig1large.jpg "ターゲット=" _空白 ">この図の拡大版をご覧になるにはこちらをクリックしてください。
過飽和NIPAAMの相分離により、リッチNIPAAM-(N-リッチ)とNIPAAMに乏しい(N-貧困層)相の調製
- ボルテックスミキサーを用いて、1; 1のAW / wの割合でDI水にNIPAAMモノマーを溶解例えば、DI水( 図2aの第一の画像)10ml中NIPAAM 10gを溶解します。
注:NIPAAMモノマーが完全に室温で溶解されると、溶液は濁った( 図2aの第二の画像)が表示されます。この現象は、過飽和NIPAAMモノマーの溶解度誘起相分離が正常に発生していると最初のキューです。 - モノマー溶液は、少なくとも15分間室温で垂直位置で休むことを可能にします。上相は、Nリッチ相であり、そしてより高密度下相はN-乏しい相( 図2aの第三の画像)です。目の密度電子N-豊富で、N-乏しい相は、それぞれ0.93±0.01および0.99±0.01グラムcm -3である。15
- 2相を分離インタフェースが明確になると、慎重にピペットを使用して、このインターフェイスを乱すことなくN-豊富で、N-貧しい段階からモノマー溶液の2ミリリットルを抽出します。
- 架橋剤としてN 4mgの、N '-methylenebisacrylamide(MBAAm)を追加し、4- 4mgの(2-ヒドロキシエトキシ)フェニル(2-ヒドロキシ-2-プロピル)ケトン抽出リッチN末端 およびNの光開始剤として低い架橋剤濃度(2ミリグラムmlの-1)( 図2bにおけるB1とB2)のサンプルについて、コア流体1及び2を調製するための乏しいモノマー溶液。
- 前のステップ3.3を繰り返し、コア流体を調製するために抽出されたNリッチとN-乏しいモノマー溶液のそれぞれにMBAAm 80mgの4-(2-ヒドロキシエトキシ)フェニル(2-ヒドロキシ-2-プロピル)ケトンの4 mgの追加高架橋剤の濃度図中(40 mgをミリリットル-1)サンプル(B3およびB4のための1および2URE 2B)。
- シース流体( 図2bにおけるB5)を作製するためにミネラルオイルに、オイル、界面活性剤の10重量%を溶解させます。
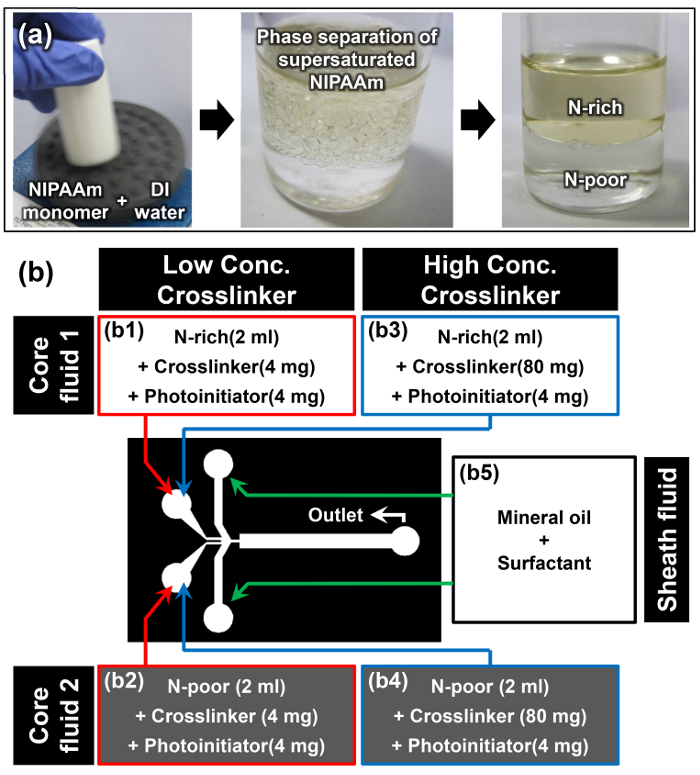
図2:ヤヌスMicrohydrogel合成のための材料の準備 (a)は、過飽和NIPAAMの相分離を介して、N-豊富で、N-乏しいモノマー溶液の調製。 (b)の材料とプロトコルで使用される実験の詳細。 この図の拡大版をご覧になるにはこちらをクリックしてください。
ヤヌスMicrohydrogelsの4合成HFMDを使用しました
- 負荷コア流体2mlの1及び2(B1、B2又はB3、B4でFiのグレ2b)は 、3つの別々の3 mlの注射器へとシース流体( 図2BのB5)。
- シリンジポンプにシリンジを取り付け、チューブ( 図の(b)を用いて、HFMDの適切な流体入口にそれぞれ注射器を接続してください。収集容器にHFMDの流体出口を接続するためのチューブを使用してください。
- それぞれ、シリンジポンプを設定し、2,2、および10μL分-1の流速でコア流体1及び2と、シース流体を注入します。
- ヤヌスの微小液滴の各側の相対的な量比を調整する(オプション)チューンコア流体1及び2の流量。
- 垂直に約1cm離れた収集容器からのUV光源を配置します。 UV光源に切り替えて、視覚的にヤヌスmicrohydrogelsの連続生産を監視します。
注意:使用UV保護-ゴーグルmicrohydrogelの生産を監視します。 - コニカルチューブに加工ヤヌスmicrohydrogelsを収集し、IPAを使用してそれらを洗います。その後、決済するためにコニカルチューブ(5分間、780グラム)を遠心分離microhydrogels。
- 完全ヤヌスmicrohydrogelsを取り巻く鉱物油を除去するために繰り返して、ステップ4.6に数回。
- 繰り返し、ステップ4.6が、ヤヌスmicrohydrogelsの周りに残ったIPAを除去するために、代わりにIPAの(v / v)の0.005%の水の界面活性剤でDI水を使用します。
- DI水を含む10ミリリットルバイアルに完全に洗浄しヤヌスmicrohydrogelsを保管してください。
ヤヌスMicrohydrogelsの異方性熱応答性の5.分析
- 24ウェルプレートに4節から合成ヤヌスmicrohydrogelsを配置するために、ピペットを使用してください。単層がウェルの底面に形成されるまでmicrohydrogelsは15秒のために解決することができます。
- 5X対物レンズと直立光学顕微鏡を用いて24℃でヤヌスmicrohydrogelの画像を取得します。
- ウェルプレートの下に熱電モジュールを設定し、ヤヌスマイクロを含む溶液の温度を上昇させるために、このモジュールの電圧を制御32℃にヒドロゲル。
- 5X対物レンズと直立光学顕微鏡を用いて、もう一度32℃でヤヌスmicrohydrogelの画像を取得します。
- 繰り返しは、統計分析のために異なるヤヌスmicrohydrogelを選ぶように注意しながら、5.2から5.4の24倍をステップ実行します。
- 24と32℃で異なるヤヌスmicrohydrogelsの25画像からは、製造元の指示に従って画像解析ソフトウェアを使用して、ヤヌスmicrohydrogelsのPN-豊富で、PN-貧しい部品の半径を測定します。
結果
図3aは、HFMD 経由ヤヌスmicrohydrogelsを合成するために使用される実験のセットアップの概略図を示します。 N-金持ちとN-乏しい相が正確に芯液1及び2のように手足口病に注入し、その後合併し、理由レイリーキャピラリー不安定性の鉱物油のシース流体によるオリフィスでヤヌス微小液滴に分かれていました。 図3bに示すようにその結果?...
ディスカッション
二つの不混和性の基材は、一般に、ヤヌスmicrohydrogelsを合成するために使用されます。このプロトコルでは最近まで、同一の基材からなるヤヌスmicrohydrogelsはほとんど報告されなかったと報告されたヤヌスmicrohydrogelsが原因で構成材料の相溶性による外乱に明確な内部形態を持っていませんでした。35、36、我々は法を実証します明らかに区画構成で、完全に単一の基材のPNIPAAmからなる...
開示事項
The authors declare that they have no competing financial interests.
謝辞
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP (Nos. 2014R1A2A1A01006527 and 2011-0030075).
資料
| Name | Company | Catalog Number | Comments |
| Silicon wafer | LG Siltron | 4", Test grade | Wafer for master mold fabrication |
| Acetone | Samchun Pure Chemical | A0097 | Cleaning silicon wafer |
| Isopropyl alcohol (IPA) | Daejung Chemicals & Metals | 5035-4404 | Cleaning silicon wafer |
| Water purification system | Merck Millipore | EMD Millipore RIOs Essential 5 | Prepering deionized water |
| O2 plasma machine | Femto Science | VITA-A | Cleaning silicon wafer |
| SU-8 2150 negative photoresist | MicroChem | Y111077 0500L1GL | Photoresist for master mold fabrication |
| Hot plate | Misung Scientific | HP330D, HP150D | Baking SU-8 |
| SU-8 developer | Microchem | Y020100 4000L1PE | Developing SU-8 |
| Mask aligner system for photolithograpy | Shinu Mst Co. | CA-6M | Photolithography |
| Sylgard 184 silicone elastomer kit | Dow Corning | 1064891 | PDMS casting |
| Laboratory Corona Treater | Electro-technic Products Inc. | Model BD-20AC | PDMS air plasma treatment |
| N-isopropylacrylamide (NIPAAm) | Sigma-Aldrich | 415324-50G | Monomer |
| N,N'-methylenebisacrylamide (MBAAm) | Sigma-Aldrich | 146072-100G | Crosslinker of NIPAAm |
| 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-propyl)ketone, Irgacure 2959 | BASF | 55047962 | Photoinitiator of NIPAAm |
| ABIL EM 90 | Evonik Industries | 201109 | Sufactant for oil |
| Vortex mixer | Scientific Industries Inc. | Vortex-Genie 2 | Mixing |
| Tygon tubing | Saint-Gobain | I.D. 1/32", O.D. 3/32", Wall 1/32" | Connecting tube between syringes and HFMD |
| UV light source | Hamamatsu | Spot light source LC8 | Polymerization from NIPAAm to PNIPAAm |
| Syringes, NORM-JECT (3ml) | Henke-Sass Wolf GmbH | 22767 | Loading of materials |
| Syringe pump | KD Scientific | KDS model 200 | Perfusion of materials |
| Tegitol Type NP-10 | Sigma-Aldrich | NP10-500ML | Surfactant for water |
| Oil red O | Sigma-Aldrich | O0625-25G | Dye for N-rich phase |
| Oil Blue N | Sigma-Aldrich | 391557-5G | Dye for N-rich phase |
| Yellow food dye | Edentown F&B | NA | Dye for N-poor phase |
| Green food dye | Edentown F&B | NA | Dye for N-poor phase |
| Power supply | Agilent | E3649A | Power soruce for thermoelectric moduel |
| Thermoelectric module | Peltier | FALC1-12710T125 | Temparature control |
| Centrifuge machine | Labogene | 1248R | Settling down microhydrogels |
| 24-well plate | SPL Life Sciences | 32024 | Reservoir for observation |
| Optical microscope | Nikon | ECLIPSE 80i | Optical observation |
| Image analysis software | IMT i-Solution Inc. | iSolutions DT | Measurement of radius |
参考文献
- Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Delivery Rev. 54 (1), 3-12 (2002).
- Qiu, Y., Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev. 53 (3), 321-339 (2001).
- Hirokawa, Y., Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 81 (12), 6379-6380 (1984).
- Bae, Y. H., Okano, T., Hsu, R., Kim, S. W. Thermo-sensitive polymers as on-off switches for drug release. Macromol. Rapid Commun. 8 (10), 481-485 (1987).
- Yoshida, R., et al. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 374 (6519), 240-242 (1995).
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 40 (12), 820-823 (1978).
- Tanaka, T., et al. Phase transitions in ionic gels. Phys. Rev. Lett. 45 (20), 1636-1639 (1980).
- Zhao, Y. L., Stoddart, J. F. Azobenzene-based light-responsive hydrogel system. Langmuir. 25 (15), 8442-8446 (2009).
- Alvarez-Lorenzo, C., Bromberg, L., Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 85 (4), 848-860 (2009).
- Tanaka, T., Nishio, I., Sun, S. T., Ueno-Nishio, S. Collapse of gels in an electric field. Science. 218 (4571), 467-469 (1982).
- Kwon, I. C., Bae, Y. H., Kim, S. W. Electrically credible polymer gel for controlled release of drugs. Nature. 354 (6351), 291-293 (1991).
- Obaidat, A. A., Park, K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials. 18 (11), 801-806 (1997).
- Kataoka, K., Miyazaki, H., Bunya, M., Okano, T., Sakurai, Y. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off regulation of insulin release. J. Am. Chem. Soc. 120 (48), 12694-12695 (1998).
- Heskins, M., Guillet, J. E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Part A Pure Appl. Chem. 2 (8), 1441-1455 (1968).
- Sasaki, S., Okabe, S., Miyahara, Y. Thermodynamic properties of N-isopropylacrylamide in water: solubility transition, phase separation of supersaturated solution, and glass formation. J. Phys. Chem. B. 114 (46), 14995-15002 (2010).
- Bromberg, L., Alakhov, V. Effects of polyether-modified poly(acrylic acid) microgels on doxorubicin transport in human intestinal epithelial Caco-2 cell layers. J. Controlled Release. 88 (1), 11-22 (2003).
- Coughlan, D. C., Quilty, F. P., Corrigan, O. I. Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopropylacrylamide) hydrogels. J. Controll. Release. 98 (1), 97-114 (2004).
- Bergbreiter, D. E., Case, B. L., Liu, Y. S., Caraway, J. W. Poly(N-isopropylacrylamide) soluble polymer supports in catalysis and synthesis. Macromolecules. 31 (18), 6053-6062 (1998).
- Lapeyre, V., Gosse, I., Chevreux, S., Ravaine, V. Monodispersed glucose-responsive microgels operating at physiological salinity. Biomacromolecules. 7 (12), 3356-3363 (2006).
- Hoare, T., Pelton, R. Engineering glucose swelling responses in poly(N-isopropylacrylamide)-based microgels. Macromolecules. 40 (3), 670-678 (2007).
- Xu, S., Zhang, J., Paquet, C., Lin, Y., Kumacheva, E. From hybrid microgels to photonic crystals. Adv. Funct. Mater. 13 (6), 468-472 (2003).
- Clarke, J., Vincent, B. Stability of non-aqueous microgel dispersions in the presence of free polymer. J. Chem. Soc., Faraday Trans. 1. 77 (8), 1831-1843 (1981).
- Mears, S. J., Deng, Y., Cosgrove, T., Pelton, R. Structure of sodium dodecyl sulfate bound to a poly (NIPAM) microgel particle. Langmuir. 13 (7), 1901-1906 (1997).
- Shah, R. K., Kim, J. W., Agresti, J. J., Weitz, D. A., Chu, L. Y. Fabrication of monodisperse thermosensitive microgels and gel capsules in microfluidic devices. Soft Matter. 4 (12), 2303-2309 (2008).
- Jack, C. R., Forbes, G., Dewanjee, M. K., Brown, M. L., Earnest, F. Polyvinyl alcohol sponge for embolotherapy: particle size and morphology. Am. J. Neuroradiol. 6 (4), 595-597 (1985).
- Derdeyn, C. P., Moran, C. J., Cross, D. T., Dietrich, H. H., Dacey, R. G. Polyvinyl alcohol particle size and suspension characteristics. Am. J. Neuroradiol. 16 (6), 1335-1343 (1995).
- Han, K., et al. Effect of flow rates on generation of monodisperse clay-poly(N-isopropylacrylamide) embolic microspheres using hydrodynamic focusing microfluidic device. Jpn. J. Appl. Phys. 50 (6), 06-12 (2011).
- Seo, K. D., Doh, J., Kim, D. S. One-step microfluidic synthesis of Janus microhydrogels with anisotropic thermo-responsive behavior and organophilic/hydrophilic loading capability. Langmuir. 29 (49), 15137-15141 (2013).
- Seo, K. D., Kim, D. S. Microfluidic synthesis of thermo-responsive poly(N-isopropylacrylamide)-poly(ethylene glycol) diacrylate microhydrogels as chemo-embolic microspheres. J. Micromech. Microeng. 24 (8), 085001 (2014).
- Seo, K. D., Kwak, B. K., Kim, D. S., Sánchez, S. Microfluidic-assisted fabrication of flexible and location traceable organo-motor. IEEE Trans. Nanobiosci. 14 (3), 298-304 (2015).
- Seo, K. D., Kim, D. S., Sánchez, S. Fabrication and application of complex-shaped microparticles via microfluidics. Lab Chip. , (2015).
- Shah, R. K., Kim, J. W., Weitz, D. A. Janus supraparticles by induced phase separation of nanoparticles in droplets. Adv. Mater. 21 (19), 1949-1953 (2009).
- Lone, S., et al. Microfluidic synthesis of Janus particles by UV-directed phase separation. Chem. Commun. 47 (9), 2634-2636 (2011).
- Hauber, K., Drier, T., Beebe, D. PDMS bonding by means of a portable, low-cost corona system. Lab chip. 6 (12), 1548-1549 (2006).
- Nisisako, T., Torii, T., Takahashi, T., Takizawa, Y. Synthesis of monodisperse bicolored Janus particles with electrical anisotropy using a microfluidic co-flow system. Adv. Mater. 18 (9), 1152-1156 (2006).
- Seiffert, S., Romanowsky, M. B., Weitz, D. A. Janus microgels produced from functional precursor polymers. Langmuir. 26 (18), 14842-14847 (2010).
- Peppas, N. A., Hilt, J. Z., Khademhosseini, A., Langer, R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 18 (11), 1345-1360 (2006).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved