È necessario avere un abbonamento a JoVE per visualizzare questo. Accedi o inizia la tua prova gratuita.
Method Article
Sintesi di Poly (
In questo articolo
Riepilogo
We present a protocol to synthesize Janus microhydrogels composed entirely of the same base material, poly(N-isopropylacrylamide) (PNIPAAm), with a clearly compartmentalized structure base on the phase separation of a supersaturated NIPAAm monomer solution. The synthesized Janus microhydrogels show unique properties such as anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability.
Abstract
Janus microparticles are compartmentalized particles with differing molecular structures and/or functionality on each of their two sides. Because of this unique property, Janus microparticles have been recognized as a new class of materials, thereby attracting a great deal of attention from various research fields. The versatility of these microparticles has been exemplified through their uses as building blocks for self-assembly, electrically responsive actuators, emulsifiers for painting and cosmetics, and carriers for drug delivery. This study introduces a detailed protocol that explicitly describes a synthetic method for designing novel Janus microhydrogels composed of a single base material, poly(N-isopropylacrylamide) (PNIPAAm). Janus microdroplets are firstly generated via a hydrodynamic focusing microfluidic device (HFMD) based on the separation of a supersaturated aqueous NIPAAm monomer solution and subsequently polymerized through exposure to UV irradiation. The resulting Janus microhydrogels were found to be entirely composed of the same base material, featured an easily identifiable compartmentalized morphology, and exhibited anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability. We believe that the proposed method introduces a novel hydrogel platform with the potential for advanced synthesis of multi-functional Janus microhydrogels.
Introduzione
Hydrogels are a network of hydrophilic polymer chains.1 An increasing amount of research in the field of hydrogels has promoted significant advances and revealed their similarity to biological tissues; the properties of hydrogels allow the uptake of large amounts of water while maintaining their structure. Environmentally responsive hydrogels have also been studied extensively because of their ability to swell or shrink reversibly in response to external stimuli.2 Several triggers, including temperature,3-5 pH,6,7 light,8,9 electric fields,10,11 and specific molecules, such as glucose,12,13 have been suggested to control the geometric shape of hydrogels. Among the many environmentally responsive hydrogels currently available, poly(N-isopropylacrylamide) (PNIPAAm), a well-known thermo-responsive hydrogel, exhibits volume shrinkage above a low critical solution temperature (LCST) of 32 °C.14 A recent study by Sasaki et al.15 reported the intriguing liquid-liquid phase separation of supersaturated NIPAAm, which is the monomer of PNIPAAm. According to this report, supersaturated NIPAAm was dissolved with a 10-fold molar excess of H2O, and soon after, the solution separated into two liquid phases when allows to stand at a temperature above 25 °C; by contrast, dilute NIPAAm was dissolved homogeneously under the same conditions.
Microparticles made of environmentally responsive hydrogels are fascinating candidates for application in drug delivery,16,17 catalysis,18 sensing,19,20 and photonics.21 Traditional synthetic methods including emulsion polymerization, are used to produce hydrogel microparticles with polydispersity.22,23 However, certain applications require microparticles with a narrow size distribution, for example, to stabilize the pharmacokinetics of drug delivery.24 Irregularly shaped or polydisperse embolic microparticles aggregate proximally into clusters, leading to chronic inflammatory responses in embolic particles for cancer therapeutic treatment.25,26
The microfluidic approach is at the forefront of research as a means of fabricating micro-sized particles with narrow size distributions and complex shapes.27-31 The advantages of fabricating microparticles in the microfluidic device are predicated by the small characteristic length of the microfluidic device, which results in a low Reynolds number. In contrast to traditional bulk emulsification where drops are formed in parallel, microdroplets produced in microfluidic devices are generated in series and subsequently polymerized into microparticles upon exposure to UV irradiation. The fundamental principle of droplet formation using a microfluidic device is balance between the interfacial tension and the shear force of the sheath fluid acting on the core fluid.
Despite the obvious advantages of microfluidic fabrication of droplets/particles, Janus droplets/particles consisting of the same base material are rarely reported because the internal morphology of these droplets/particles is generally disturbed by the diffusion and perturbation of the core fluids. To circumvent this intrinsic limitation, two groups recently reported the preparation of the Janus microparticles by employing heat-induced phase separation of colloidal nanoparticles and UV-directed phase separation.32,33
To this end, we report a microfluidic approach to synthesize Janus microhydrogels entirely composed of a single base material and obtain a product with clearly compartmentalized morphology. Our approach is based on the primary concept of liquid-liquid phase separation of supersaturated NIPAAm monomer. The resulting Janus microhydrogels were found to possess unique properties including anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability.
Protocollo
1. Realizzazione di uno stampo master per la focalizzazione idrodinamica dispositivo a microfluidi (HFMD) attraverso la fotolitografia
- Progettare una fotomaschera per il HFMD (Figura 1a) utilizzando il software di progettazione assistita da computer (CAD) secondo il protocollo del produttore.
- Risciacquare una fetta 4 'silicio con acetone, alcol isopropilico (IPA), e acqua deionizzata (DI) per rimuovere la polvere organica e inorganica dal wafer.
- Pulire il wafer di silicio con O 2 al plasma a 100 W di potenza per 5 minuti per aumentare la forza di legame tra il wafer e SU-8.
- Spin-coat 4 ml del fotoresist negativo, SU-8 2150, sul wafer a 3.000 rpm per 30 sec per ottenere uno spessore di 150 micron (b1 nella Figura 1b).
- Posizionare il wafer rivestito SU-8 su una piastra per 5 min a 65 ° C, impostare la temperatura a 95 ° C, quindi lasciare il wafer sulla piastra per 30 minuti per cuocere morbido.
- Posiziona ilprogettata photomask sopra il wafer ed esporre alla luce UV (260 mJ cm -2, 26 sec per 10 mW cm -2) in un allineatore maschera (b2 nella Figura 1b).
- Eseguire post-esposizione cottura su una piastra (65 ° C per 5 minuti e poi 95 ° C per 12 min).
- Sviluppare il wafer mediante immersione in un bagno di sviluppo SU-8 per 10 minuti, e poi trasferisce in sviluppatore fresco per 5 secondi per ottenere una superficie pulita.
- Lavare il wafer per 20 secondi con acqua deionizzata e asciugare per 10 sec con N 2 gas (b3 in figura 1b). Utilizzare il wafer fabbricati come uno stampo master per polidimetilsilossano (PDMS) fusione nella sezione 2.
2. Realizzazione della HFMD attraverso PDMS Casting
- Utilizzare il wafer modellato ottenuto nella sezione 1, come lo stampo PDMS master per fusione.
- Mescolare il PDMS pre-polimero e un agente indurente omogeneamente in un rapporto in peso di 10: 1; per esempio, usare 1 g di induritore per 10 g di PDMS pre-polimeri eER.
- Versare il PDMS pre-polimero nello stampo master e degassare per 1 ora in una camera a vuoto (b4 nella Figura 1b).
- Posizionare lo stampo master con il PDMS pre-polimero in un forno a 65 ° C per 3 ore.
- Tagliare il PDMS vulcanizzati nella dimensione di un singolo chip utilizzando un bisturi affilato. sbucciare con cautela la replica PDMS curato dallo stampo maestro a mano.
- Ripetere i punti da 2.2 a 2.5 per ottenere un identico PDMS replica.
- Punzone ingresso e uscita fori in una delle repliche utilizzando un foro-perforatore con un diametro leggermente inferiore al diametro esterno del tubo di collegamento.
- Applicare trattamento al plasma aria alla zona di incollaggio di ciascuna replica con un trattamento corona. 34
Attenzione: Utilizzare il trattamento corona in una zona con una buona ventilazione per evitare l'accumulo di ozono. - Goccia 5 ml di metanolo sulle zone plasma trattati aria. Finemente allineare due PDMS identici repliche per fabbricare la HFMD per mano alla manipolazione mento, e verificare l'allineamento tramite un microscopio (b5 nella figura 1b).
Nota: il plasma trattato con repliche PDMS aria sono piuttosto appiccicoso e difficile da manipolare. Così, 5 ml di metanolo vengono aggiunti alla superficie plasma aria trattata per funzionare come lubrificante. - Posizionare il HFMD in forno a 65 ° C per una notte per rafforzare il legame tra due repliche PDMS (b6 nella Figura 1b). Legame due identiche PDMS repliche per aumentare l'altezza del microcanale del HFMD ed evitare l'intasamento del microgoccioline nel canale microfluidico durante il funzionamento.
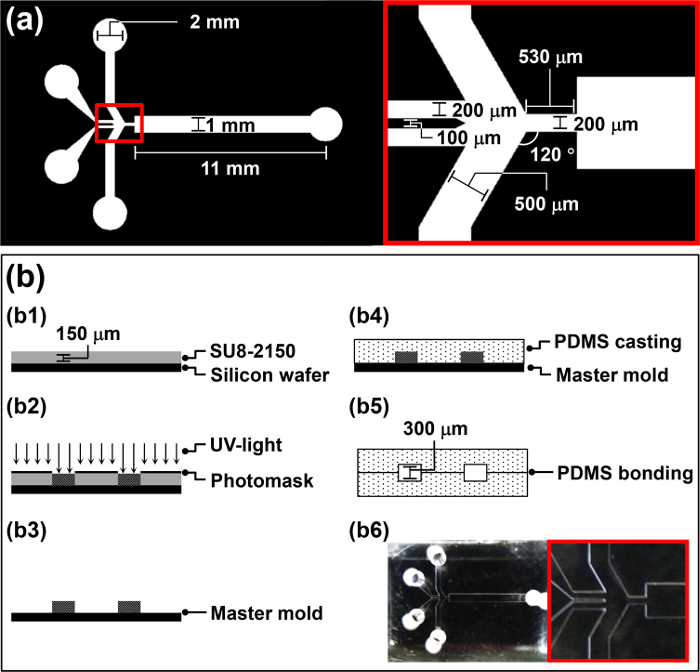
Figura 1: Panoramica sulla procedura di HFMD Fabrication (a) i parametri di progettazione del fotomaschera per la HFMD.. (B) Illustrazione del procedimento di fabbricazione per la HFMD.ftp_upload / 52813 / 52813fig1large.jpg "target =" _ blank "> Clicca qui per vedere una versione più grande di questa figura.
3. Preparazione di NIPAAm-ricchi (N-ricchi) e NIPAAm-poveri (N-poveri) Fasi di fase di separazione di Supersaturated NIPAAm
- Sciogliere NIPAAm monomero in acqua deionizzata con rapporto p / w di 1: 1 utilizzando un vortex; per esempio, sciogliere 10 g di NIPAAm in 10 ml di acqua deionizzata (prima immagine di figura 2a).
Nota: Una volta che il monomero NIPAAm è completamente sciolto a temperatura ambiente, la soluzione appare torbida (seconda immagine di figura 2a). Questo fenomeno è il primo cue è verificato con successo che solubilità indotta fase di separazione del sovrasatura monomero NIPAAm. - Lasciare la soluzione monomero di riposare in posizione verticale a temperatura ambiente per almeno 15 min. La fase superiore è la fase N-ricco, e la fase più densa inferiore è la fase di N-poveri (terza immagine di figura 2a). Le densità dei thfasi e N-ricchi e N-poveri sono 0,93 ± 0,01 e 0,99 ± 0,01 g cm -3, rispettivamente. 15
- Quando l'interfaccia che separa le due fasi diventa chiaro, estrarre con attenzione 2 ml di soluzione di monomero dalle fasi N-ricchi e N-poveri senza disturbare questa interfaccia utilizzando una pipetta.
- Aggiungere 4 mg di N, N '-methylenebisacrylamide (MBAAm) come reticolante e 4 mg di 4- (2-idrossietossi) fenil (2-idrossi-2-propil) chetone come fotoiniziatore alla estratta N-ricco e N -poor soluzioni monomero per preparare fluidi di base 1 e 2 per la bassa concentrazione di reticolante (2 mg ml -1) del campione (B1 e B2 nella Figura 2b).
- Ripetere precedente Fase 3.3 e aggiungere 80 mg di MBAAm e 4 mg di 4- (2-idrossietossi) fenil (2-idrossi-2-propil) chetone in ciascun estratto soluzione N-ricco e N-poveri monomero per preparare fluidi di base 1 e 2 per l'alta concentrazione di reticolante (40 mg ml -1) del campione (b3 e b4 in Figure 2b).
- Sciogliere 10% in peso di tensioattivo olio in olio minerale per preparare il liquido guaina (b5 nella Figura 2b).
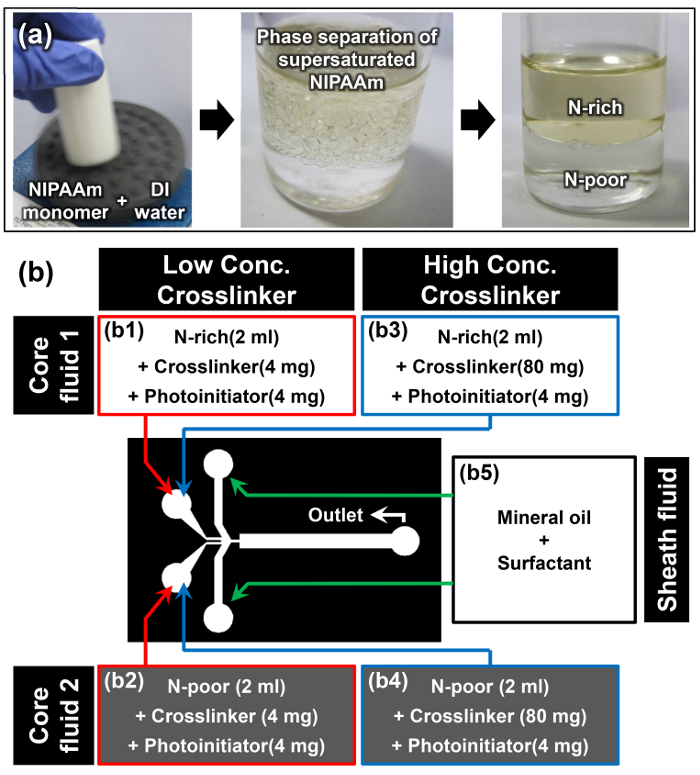
Figura 2:. Preparazione dei Materiali per Janus Microhydrogel Synthesis (a) Preparazione di soluzioni N-ricchi e N-poveri monomero mediante separazione di fase di supersaturated NIPAAm. (B) i dettagli dei materiali e setup sperimentale utilizzato nel protocollo. Clicca qui per vedere una versione più grande di questa figura.
4. Sintesi di Giano Microhydrogels Uso del HFMD
- Carico 2 ml di fluidi fondamentali 1 e 2 (b1, b2 B3 o, b4 in Fi gura 2b) e il liquido guaina (b5 nella Figura 2b) in tre separati 3 ml siringhe.
- Montare le siringhe nelle pompe a siringa e collegare ciascuna siringa all'ingresso del fluido appropriato del HFMD utilizzano tubi (figura (b). Usare tubo per collegare l'uscita del fluido della HFMD ad un serbatoio di raccolta.
- Impostare le pompe siringa e infondere fluidi di base 1 e 2 e del liquido guaina a portate di 2, 2, e 10 min -1 ml, rispettivamente.
- (Opzionale) Tune la portata di fluidi fondamentali 1 e 2 per regolare i rapporti volumetrici relative di ciascun lato del microdroplet Janus.
- Posizionare la sorgente di luce UV perpendicolarmente a circa 1 cm dal serbatoio di raccolta. Accendere la sorgente di luce UV e monitorare visivamente la produzione continua di microhydrogels Janus.
Attenzione: L'uso UV di protezione-occhiali durante il monitoraggio della produzione microhydrogel. - Raccogliere le microhydrogels Janus fabbricate in un tubo conico e lavare utilizzando IPA. Poi, centrifugare il tubo conico (780 g per 5 minuti) per risolvere lamicrohydrogels.
- Ripetere il punto 4.6 più volte per rimuovere l'olio minerale che circonda completamente il microhydrogels Janus.
- Ripetere il punto 4.6 ma usare acqua DI con un tensioattivo acqua 0,005% (v / v) invece di IPA per rimuovere il residuo IPA intorno alle microhydrogels Janus.
- Conservare microhydrogels Janus completamente lavati in un flaconcino da 10 ml di acqua contenente DI.
5. Analisi della anisotropico Thermo-reattività di Giano Microhydrogels
- Utilizzare una pipetta per posizionare microhydrogels Janus sintetizzati dalla sezione 4 in una piastra da 24 pozzetti. Lasciare le microhydrogels di stabilirsi per 15 sec fino ad un monostrato viene formato sulla superficie inferiore del pozzetto.
- Ottenere un'immagine del microhydrogel Janus a 24 ° C utilizzando un microscopio ottico verticale con una lente obiettivo 5X.
- Impostare un modulo termoelettrico sotto la piastra bene e controllare la tensione di questo modulo per aumentare la temperatura della soluzione contenente Janus microidrogel a 32 ° C.
- Ottenere un'immagine del microhydrogel Janus a 32 ° C una volta utilizzando un microscopio ottico verticale con una lente obiettivo 5X.
- Ripetere i punti 5.2-5.4 24 volte, avendo cura di scegliere un diverso microhydrogel Janus per l'analisi statistica.
- Dalle 25 immagini di diversi microhydrogels Janus a 24 e 32 ° C, misurare il raggio delle parti PN-ricchi e PN-poveri del microhydrogels Janus utilizzando il software di analisi di immagine in base alle istruzioni del produttore.
Risultati
Figura 3a presenta uno schema del setup sperimentale utilizzato per sintetizzare microhydrogels Janus tramite il HFMD. Le fasi N-ricchi e N-poveri sono stati iniettati nel proprio HFMD come fluidi fondamentali 1 e 2 e poi fuse e suddivise in microgoccioline Janus all'orifizio dal fluido di trasporto di olio minerale a causa del capillare instabilità Rayleigh. Di conseguenza, microgoccioline Janus composto di fasi N-ricchi e N-poveri sono stati generati con ...
Discussione
Due materiali di base immiscibili sono generalmente utilizzati per sintetizzare le microhydrogels Janus. Fino a poco tempo, microhydrogels Janus costituiti dello stesso materiale di base sono stati segnalati raramente e le microhydrogels Janus riportati non hanno una chiara morfologia interna a causa del disturbo causato dal miscibilità dei materiali che lo compongono. 35, 36 In questo protocollo, dimostriamo un metodo per sintetizzare microhydrogels Janus interamente composti da materiale di base singola, P...
Divulgazioni
The authors declare that they have no competing financial interests.
Riconoscimenti
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP (Nos. 2014R1A2A1A01006527 and 2011-0030075).
Materiali
| Name | Company | Catalog Number | Comments |
| Silicon wafer | LG Siltron | 4", Test grade | Wafer for master mold fabrication |
| Acetone | Samchun Pure Chemical | A0097 | Cleaning silicon wafer |
| Isopropyl alcohol (IPA) | Daejung Chemicals & Metals | 5035-4404 | Cleaning silicon wafer |
| Water purification system | Merck Millipore | EMD Millipore RIOs Essential 5 | Prepering deionized water |
| O2 plasma machine | Femto Science | VITA-A | Cleaning silicon wafer |
| SU-8 2150 negative photoresist | MicroChem | Y111077 0500L1GL | Photoresist for master mold fabrication |
| Hot plate | Misung Scientific | HP330D, HP150D | Baking SU-8 |
| SU-8 developer | Microchem | Y020100 4000L1PE | Developing SU-8 |
| Mask aligner system for photolithograpy | Shinu Mst Co. | CA-6M | Photolithography |
| Sylgard 184 silicone elastomer kit | Dow Corning | 1064891 | PDMS casting |
| Laboratory Corona Treater | Electro-technic Products Inc. | Model BD-20AC | PDMS air plasma treatment |
| N-isopropylacrylamide (NIPAAm) | Sigma-Aldrich | 415324-50G | Monomer |
| N,N'-methylenebisacrylamide (MBAAm) | Sigma-Aldrich | 146072-100G | Crosslinker of NIPAAm |
| 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-propyl)ketone, Irgacure 2959 | BASF | 55047962 | Photoinitiator of NIPAAm |
| ABIL EM 90 | Evonik Industries | 201109 | Sufactant for oil |
| Vortex mixer | Scientific Industries Inc. | Vortex-Genie 2 | Mixing |
| Tygon tubing | Saint-Gobain | I.D. 1/32", O.D. 3/32", Wall 1/32" | Connecting tube between syringes and HFMD |
| UV light source | Hamamatsu | Spot light source LC8 | Polymerization from NIPAAm to PNIPAAm |
| Syringes, NORM-JECT (3ml) | Henke-Sass Wolf GmbH | 22767 | Loading of materials |
| Syringe pump | KD Scientific | KDS model 200 | Perfusion of materials |
| Tegitol Type NP-10 | Sigma-Aldrich | NP10-500ML | Surfactant for water |
| Oil red O | Sigma-Aldrich | O0625-25G | Dye for N-rich phase |
| Oil Blue N | Sigma-Aldrich | 391557-5G | Dye for N-rich phase |
| Yellow food dye | Edentown F&B | NA | Dye for N-poor phase |
| Green food dye | Edentown F&B | NA | Dye for N-poor phase |
| Power supply | Agilent | E3649A | Power soruce for thermoelectric moduel |
| Thermoelectric module | Peltier | FALC1-12710T125 | Temparature control |
| Centrifuge machine | Labogene | 1248R | Settling down microhydrogels |
| 24-well plate | SPL Life Sciences | 32024 | Reservoir for observation |
| Optical microscope | Nikon | ECLIPSE 80i | Optical observation |
| Image analysis software | IMT i-Solution Inc. | iSolutions DT | Measurement of radius |
Riferimenti
- Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Delivery Rev. 54 (1), 3-12 (2002).
- Qiu, Y., Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev. 53 (3), 321-339 (2001).
- Hirokawa, Y., Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 81 (12), 6379-6380 (1984).
- Bae, Y. H., Okano, T., Hsu, R., Kim, S. W. Thermo-sensitive polymers as on-off switches for drug release. Macromol. Rapid Commun. 8 (10), 481-485 (1987).
- Yoshida, R., et al. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 374 (6519), 240-242 (1995).
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 40 (12), 820-823 (1978).
- Tanaka, T., et al. Phase transitions in ionic gels. Phys. Rev. Lett. 45 (20), 1636-1639 (1980).
- Zhao, Y. L., Stoddart, J. F. Azobenzene-based light-responsive hydrogel system. Langmuir. 25 (15), 8442-8446 (2009).
- Alvarez-Lorenzo, C., Bromberg, L., Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 85 (4), 848-860 (2009).
- Tanaka, T., Nishio, I., Sun, S. T., Ueno-Nishio, S. Collapse of gels in an electric field. Science. 218 (4571), 467-469 (1982).
- Kwon, I. C., Bae, Y. H., Kim, S. W. Electrically credible polymer gel for controlled release of drugs. Nature. 354 (6351), 291-293 (1991).
- Obaidat, A. A., Park, K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials. 18 (11), 801-806 (1997).
- Kataoka, K., Miyazaki, H., Bunya, M., Okano, T., Sakurai, Y. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off regulation of insulin release. J. Am. Chem. Soc. 120 (48), 12694-12695 (1998).
- Heskins, M., Guillet, J. E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Part A Pure Appl. Chem. 2 (8), 1441-1455 (1968).
- Sasaki, S., Okabe, S., Miyahara, Y. Thermodynamic properties of N-isopropylacrylamide in water: solubility transition, phase separation of supersaturated solution, and glass formation. J. Phys. Chem. B. 114 (46), 14995-15002 (2010).
- Bromberg, L., Alakhov, V. Effects of polyether-modified poly(acrylic acid) microgels on doxorubicin transport in human intestinal epithelial Caco-2 cell layers. J. Controlled Release. 88 (1), 11-22 (2003).
- Coughlan, D. C., Quilty, F. P., Corrigan, O. I. Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopropylacrylamide) hydrogels. J. Controll. Release. 98 (1), 97-114 (2004).
- Bergbreiter, D. E., Case, B. L., Liu, Y. S., Caraway, J. W. Poly(N-isopropylacrylamide) soluble polymer supports in catalysis and synthesis. Macromolecules. 31 (18), 6053-6062 (1998).
- Lapeyre, V., Gosse, I., Chevreux, S., Ravaine, V. Monodispersed glucose-responsive microgels operating at physiological salinity. Biomacromolecules. 7 (12), 3356-3363 (2006).
- Hoare, T., Pelton, R. Engineering glucose swelling responses in poly(N-isopropylacrylamide)-based microgels. Macromolecules. 40 (3), 670-678 (2007).
- Xu, S., Zhang, J., Paquet, C., Lin, Y., Kumacheva, E. From hybrid microgels to photonic crystals. Adv. Funct. Mater. 13 (6), 468-472 (2003).
- Clarke, J., Vincent, B. Stability of non-aqueous microgel dispersions in the presence of free polymer. J. Chem. Soc., Faraday Trans. 1. 77 (8), 1831-1843 (1981).
- Mears, S. J., Deng, Y., Cosgrove, T., Pelton, R. Structure of sodium dodecyl sulfate bound to a poly (NIPAM) microgel particle. Langmuir. 13 (7), 1901-1906 (1997).
- Shah, R. K., Kim, J. W., Agresti, J. J., Weitz, D. A., Chu, L. Y. Fabrication of monodisperse thermosensitive microgels and gel capsules in microfluidic devices. Soft Matter. 4 (12), 2303-2309 (2008).
- Jack, C. R., Forbes, G., Dewanjee, M. K., Brown, M. L., Earnest, F. Polyvinyl alcohol sponge for embolotherapy: particle size and morphology. Am. J. Neuroradiol. 6 (4), 595-597 (1985).
- Derdeyn, C. P., Moran, C. J., Cross, D. T., Dietrich, H. H., Dacey, R. G. Polyvinyl alcohol particle size and suspension characteristics. Am. J. Neuroradiol. 16 (6), 1335-1343 (1995).
- Han, K., et al. Effect of flow rates on generation of monodisperse clay-poly(N-isopropylacrylamide) embolic microspheres using hydrodynamic focusing microfluidic device. Jpn. J. Appl. Phys. 50 (6), 06-12 (2011).
- Seo, K. D., Doh, J., Kim, D. S. One-step microfluidic synthesis of Janus microhydrogels with anisotropic thermo-responsive behavior and organophilic/hydrophilic loading capability. Langmuir. 29 (49), 15137-15141 (2013).
- Seo, K. D., Kim, D. S. Microfluidic synthesis of thermo-responsive poly(N-isopropylacrylamide)-poly(ethylene glycol) diacrylate microhydrogels as chemo-embolic microspheres. J. Micromech. Microeng. 24 (8), 085001 (2014).
- Seo, K. D., Kwak, B. K., Kim, D. S., Sánchez, S. Microfluidic-assisted fabrication of flexible and location traceable organo-motor. IEEE Trans. Nanobiosci. 14 (3), 298-304 (2015).
- Seo, K. D., Kim, D. S., Sánchez, S. Fabrication and application of complex-shaped microparticles via microfluidics. Lab Chip. , (2015).
- Shah, R. K., Kim, J. W., Weitz, D. A. Janus supraparticles by induced phase separation of nanoparticles in droplets. Adv. Mater. 21 (19), 1949-1953 (2009).
- Lone, S., et al. Microfluidic synthesis of Janus particles by UV-directed phase separation. Chem. Commun. 47 (9), 2634-2636 (2011).
- Hauber, K., Drier, T., Beebe, D. PDMS bonding by means of a portable, low-cost corona system. Lab chip. 6 (12), 1548-1549 (2006).
- Nisisako, T., Torii, T., Takahashi, T., Takizawa, Y. Synthesis of monodisperse bicolored Janus particles with electrical anisotropy using a microfluidic co-flow system. Adv. Mater. 18 (9), 1152-1156 (2006).
- Seiffert, S., Romanowsky, M. B., Weitz, D. A. Janus microgels produced from functional precursor polymers. Langmuir. 26 (18), 14842-14847 (2010).
- Peppas, N. A., Hilt, J. Z., Khademhosseini, A., Langer, R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 18 (11), 1345-1360 (2006).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneThis article has been published
Video Coming Soon