Для просмотра этого контента требуется подписка на Jove Войдите в систему или начните бесплатную пробную версию.
Method Article
Синтез поли (
В этой статье
Резюме
We present a protocol to synthesize Janus microhydrogels composed entirely of the same base material, poly(N-isopropylacrylamide) (PNIPAAm), with a clearly compartmentalized structure base on the phase separation of a supersaturated NIPAAm monomer solution. The synthesized Janus microhydrogels show unique properties such as anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability.
Аннотация
Janus microparticles are compartmentalized particles with differing molecular structures and/or functionality on each of their two sides. Because of this unique property, Janus microparticles have been recognized as a new class of materials, thereby attracting a great deal of attention from various research fields. The versatility of these microparticles has been exemplified through their uses as building blocks for self-assembly, electrically responsive actuators, emulsifiers for painting and cosmetics, and carriers for drug delivery. This study introduces a detailed protocol that explicitly describes a synthetic method for designing novel Janus microhydrogels composed of a single base material, poly(N-isopropylacrylamide) (PNIPAAm). Janus microdroplets are firstly generated via a hydrodynamic focusing microfluidic device (HFMD) based on the separation of a supersaturated aqueous NIPAAm monomer solution and subsequently polymerized through exposure to UV irradiation. The resulting Janus microhydrogels were found to be entirely composed of the same base material, featured an easily identifiable compartmentalized morphology, and exhibited anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability. We believe that the proposed method introduces a novel hydrogel platform with the potential for advanced synthesis of multi-functional Janus microhydrogels.
Введение
Hydrogels are a network of hydrophilic polymer chains.1 An increasing amount of research in the field of hydrogels has promoted significant advances and revealed their similarity to biological tissues; the properties of hydrogels allow the uptake of large amounts of water while maintaining their structure. Environmentally responsive hydrogels have also been studied extensively because of their ability to swell or shrink reversibly in response to external stimuli.2 Several triggers, including temperature,3-5 pH,6,7 light,8,9 electric fields,10,11 and specific molecules, such as glucose,12,13 have been suggested to control the geometric shape of hydrogels. Among the many environmentally responsive hydrogels currently available, poly(N-isopropylacrylamide) (PNIPAAm), a well-known thermo-responsive hydrogel, exhibits volume shrinkage above a low critical solution temperature (LCST) of 32 °C.14 A recent study by Sasaki et al.15 reported the intriguing liquid-liquid phase separation of supersaturated NIPAAm, which is the monomer of PNIPAAm. According to this report, supersaturated NIPAAm was dissolved with a 10-fold molar excess of H2O, and soon after, the solution separated into two liquid phases when allows to stand at a temperature above 25 °C; by contrast, dilute NIPAAm was dissolved homogeneously under the same conditions.
Microparticles made of environmentally responsive hydrogels are fascinating candidates for application in drug delivery,16,17 catalysis,18 sensing,19,20 and photonics.21 Traditional synthetic methods including emulsion polymerization, are used to produce hydrogel microparticles with polydispersity.22,23 However, certain applications require microparticles with a narrow size distribution, for example, to stabilize the pharmacokinetics of drug delivery.24 Irregularly shaped or polydisperse embolic microparticles aggregate proximally into clusters, leading to chronic inflammatory responses in embolic particles for cancer therapeutic treatment.25,26
The microfluidic approach is at the forefront of research as a means of fabricating micro-sized particles with narrow size distributions and complex shapes.27-31 The advantages of fabricating microparticles in the microfluidic device are predicated by the small characteristic length of the microfluidic device, which results in a low Reynolds number. In contrast to traditional bulk emulsification where drops are formed in parallel, microdroplets produced in microfluidic devices are generated in series and subsequently polymerized into microparticles upon exposure to UV irradiation. The fundamental principle of droplet formation using a microfluidic device is balance between the interfacial tension and the shear force of the sheath fluid acting on the core fluid.
Despite the obvious advantages of microfluidic fabrication of droplets/particles, Janus droplets/particles consisting of the same base material are rarely reported because the internal morphology of these droplets/particles is generally disturbed by the diffusion and perturbation of the core fluids. To circumvent this intrinsic limitation, two groups recently reported the preparation of the Janus microparticles by employing heat-induced phase separation of colloidal nanoparticles and UV-directed phase separation.32,33
To this end, we report a microfluidic approach to synthesize Janus microhydrogels entirely composed of a single base material and obtain a product with clearly compartmentalized morphology. Our approach is based on the primary concept of liquid-liquid phase separation of supersaturated NIPAAm monomer. The resulting Janus microhydrogels were found to possess unique properties including anisotropic thermo-responsiveness and organophilic/hydrophilic loading capability.
Access restricted. Please log in or start a trial to view this content.
протокол
1. Изготовление мастер-формы для гидродинамического Сосредоточение микрожидкостных устройств (ФГДМ) через фотолитографии
- Дизайн фотошаблона для ФГМД (Рисунок 1a) с помощью разработки программного обеспечения помощью компьютера (CAD) в соответствии с протоколом производителя.
- Промыть 4 'кремниевую пластину с ацетоном, изопропиловый спирт (IPA) и деионизированную (ДИ) воды для удаления органических и неорганических пыли из пластины.
- Очистите кремниевой пластины с O 2 плазмы при 100 Вт мощности в течение 5 мин , чтобы увеличить прочность сцепления между пластиной и SU-8.
- Спин-пальто 4 мл негативного фоторезиста, СУ-8 2150, на пластину со скоростью 3000 оборотов в минуту в течение 30 секунд , чтобы достичь толщины 150 мкм (Б1 на рисунке 1b).
- Поместите пластину, покрытую СУ-8 на плитке в течение 5 мин при 65 ° C, установите температуру до 95 ° С, а затем оставить пластину на плитке в течение 30 минут до мягкого выпекать.
- Поместитеразработан фотошаблона над пластиной и подвергать воздействию УФ - излучения (260 мДж см -2, 26 сек для 10 мВт см -2) в маске выравнивателя (b2 на рис 1b).
- Выполнение действий после воздействия выпекания на плитке (65 ° С в течение 5 мин и затем 95 ° С в течение 12 мин).
- Разработка облатку путем погружения в ванну для проявителя SU-8 в течение 10 мин, а затем перенести его в свежую проявителя в течение 5 секунд, чтобы получить чистую поверхность.
- Промыть пластины в течение 20 секунд с дистиллированной водой и высушить его в течение 10 секунд с N 2 газа (b3 на рисунке 1b). Используйте изготовленную пластину в качестве мастер-формы для полидиметилсилоксана (PDMS) литья в разделе 2.
2. Изготовление ФГМД через PDMS Литейно
- Используйте узорной пластину, полученную в разделе 1 в качестве основной формы для литья PDMS.
- Смешайте PDMS предварительно полимера и отвердителя гомогенно в массовом соотношении от 10: 1; например, используют 1 г отвердителя для 10 г PDMS предварительно Polymэ.
- Налейте PDMS форполимера в основную форму и дегазировать ее в течение 1 ч в вакуумной камере (b4 на рисунке 1b).
- Поместите мастер-формы с PDMS форполимера в сушильном шкафу при температуре 65 ° С в течение 3 часов.
- Нарезать отвержденных PDMS в размер одного чипа с использованием острым скальпелем. Аккуратно снимите отвержденного PDMS реплики от мастер формы вручную.
- Повторите шаги с 2,2 до 2,5, чтобы получить идентичный PDMS реплики.
- Обомните входе и на выходе отверстия в одну из реплик с использованием дырочного перфоратор с немного меньшим диаметром, чем наружный диаметр соединительной трубки.
- Применение плазменной обработки воздуха в зоне скрепления каждой реплики с помощью коронного протравливатель. 34
Внимание: Используйте коронный протравливателя в районе с хорошей вентиляцией , чтобы избежать озона отложений. - Отбросьте 5 мкл метанола на воздушно-плазменной обработке областей. Мелко совместите два идентичных PDMS реплики для изготовления ФГМД вручную manipu таже, и проверьте выравнивание с помощью микроскопа (b5 на рисунке 1b).
Примечание: воздушно-плазменной обработке PDMS реплики довольно липким и трудно манипулировать. Таким образом, 5 мкл метанола добавляют к воздушной плазменной обработки поверхности, чтобы функционировать в качестве смазочного материала. - Поместите ФГДМ в сушильном шкафу до 65 ° С в течение ночи , чтобы укрепить связь между двумя PDMS репликами (В6 на рисунке 1b). Бонд два одинаковых PDMS реплики, чтобы увеличить высоту микроканала части ФГМД и во избежание образования комков микрокапель в микрожидком канале во время работы.
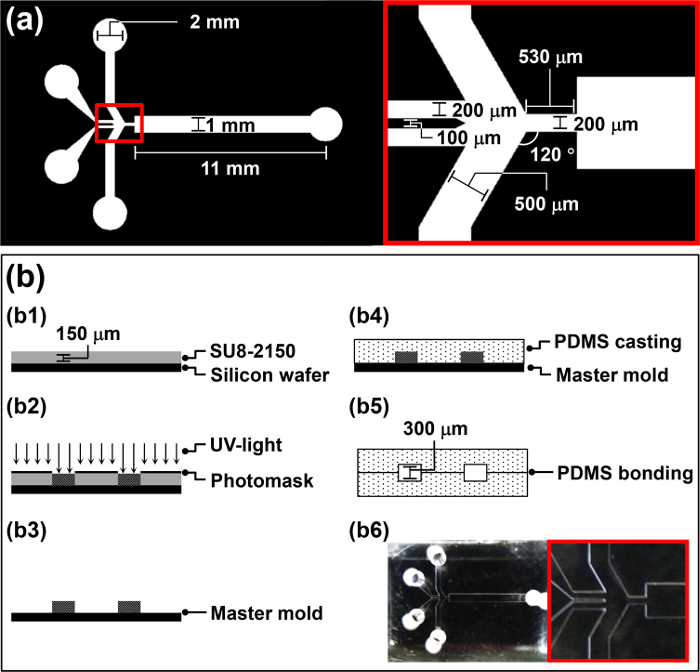
Рисунок 1: Обзор процедуры ФГМД Fabrication (а) Конструктивные параметры фотошаблона для ФГМД.. (Б) Иллюстрация процедуры изготовления для ФГМД.ftp_upload / 52813 / 52813fig1large.jpg "целевых =" _blank "> Пожалуйста, нажмите здесь, чтобы посмотреть увеличенную версию этой фигуры.
3. Получение NIPAAm богатых (N-богатых) и NIPAAm бедных (N-бедных) фаз путем фазового разделения перенасыщенного NIPAAm
- Растворите NIPAAm мономера в деионизированной воде при Aw / д соотношении 1: 1 с использованием вихревой смеситель; например, растворить 10 г NIPAAm в 10 мл деионизированной воды (первое изображение рис 2а).
Примечание: После того , как мономер NIPAAm полностью растворяли при комнатной температуре, раствор появляется мутный (второе изображение рис 2а). Это явление является первым кий, что растворимость индуцированное разделение фаз пересыщенного NIPAAm мономера успешно произошло. - Дайте раствору мономера отдыхать в вертикальном положении при комнатной температуре в течение не менее 15 мин. Верхняя фаза представляет собой N-фаза , обогащенная, а более плотный нижний фаза является N-бедных фаза (третий образ рис 2а). Плотность йФазы е N-богатых и N-бедные 0,93 ± 0,01 и 0,99 ± 0,01 г см -3, соответственно. 15
- Когда интерфейс разделения двух фаз становится ясно, осторожно извлечь 2 мл раствора мономера из N-богатых и N-бедных фаз, не нарушая этот интерфейс, с помощью пипетки.
- Добавить 4 мг N, N 'метиленбисакриламида (MBAAm) в качестве сшивающего агента и 4 мг 4- (2-гидроксиэтокси) фенил- (2-гидрокси-2-пропил) кетона в качестве фотоинициатора к извлеченным N богатых и N мономерные растворы Бедные подготовить основные жидкости 1 и 2 для низкой концентрации сшивателя (2 мг мл -1) образца (b1 и b2 на рис 2b).
- Повторите предыдущий шаг 3.3 и добавьте 80 мг MBAAm и 4 мг 4- (2-гидроксиэтокси) фенил- (2-гидрокси-2-пропил) кетона в каждом из добытой N-богатых и N-бедного раствора мономера для приготовления основных растворов 1 и 2 для высокой концентрации сшивателя (40 мг мл -1) образца (В3 и В4 на рисЮр 2b).
- Растворить 10% вес нефти поверхностно -активного вещества в минеральное масло , чтобы приготовить жидкость оболочки (В5 на рисунке 2b).
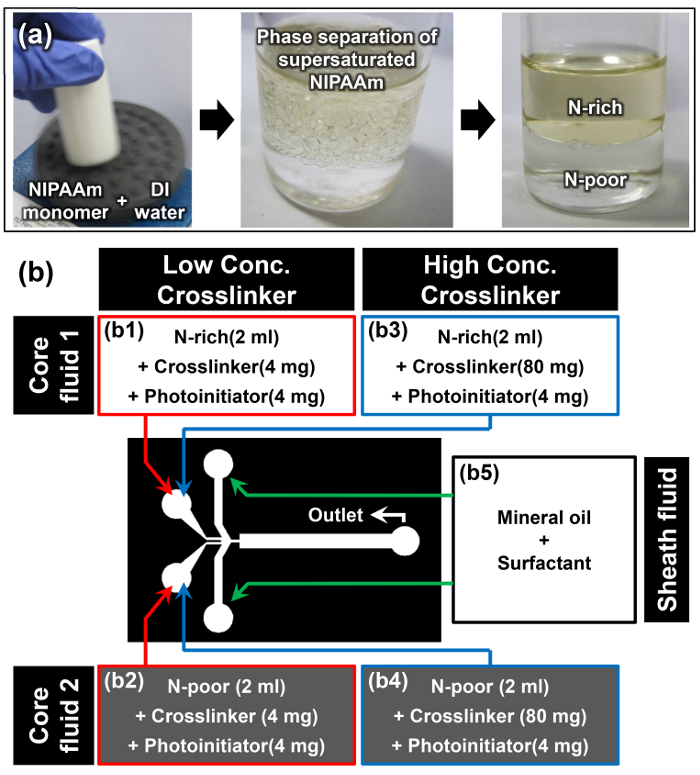
Рис . 2: Подготовка материала для Janus Microhydrogel синтеза (а) Получение N-богатых и N-бедных растворов мономеров путем разделения фаз перенасыщенной NIPAAm. (Б) Подробная информация о материалах и экспериментальной установки , используемой в протоколе. Пожалуйста , нажмите здесь , чтобы посмотреть увеличенную версию этой фигуры.
4. Синтез Janus Microhydrogels Использование ФГМД
- Нагрузка 2 мл основных растворов 1 и 2 (b1, b2 или В3, В4 в рисунке 2b) и жидкость оболочка (b5 на рисунке 2b) на три отдельных 3 мл шприцы.
- Установите шприцы в шприц насосов и соединить каждый шприц к соответствующему входному отверстию жидкости ФГМД с помощью трубки (рис (б). С помощью трубки для соединения выпускного отверстия для текучей среды ФГДМ к коллекторного резервуара.
- Установить шприцевые насосы и настоять основных жидкостей 1 и 2 и оболочки жидкости при скорости потока 2, 2, и 10 мкл мин -1, соответственно.
- (Необязательно) Настройте скорость потока основных жидкостей 1 и 2 для регулировки относительного соотношения объема каждой стороны микрокапли Janus.
- Расположите источник света УФ перпендикулярно около 1 см от коллекторного резервуара. Включить источник ультрафиолетового света и визуально контролировать непрерывное производство Януса microhydrogels.
Внимание: Использование УФ-защитные очки при мониторинге производства microhydrogel. - Соберите сфабрикованные microhydrogels Janus в коническую пробирку и промойте их с помощью IPA. Затем, центрифугировать коническую трубку (780 г в течение 5 мин), чтобы решитьmicrohydrogels.
- Повторите шаг 4,6 несколько раз, чтобы удалить минеральное масло, окружающую microhydrogels Janus полностью.
- Повторите шаг 4,6, но использование деионизированной воды с поверхностно-активным веществом воды 0,005% (об / об) вместо IPA, чтобы удалить остатки IPA вокруг microhydrogels Janus.
- Хранить полностью размыты microhydrogels Януса в 10 мл флакон, содержащий ДИ воды в.
5. Анализ анизотропного Thermo-отзывчивости Janus Microhydrogels
- С помощью пипетки поместить Janus microhydrogels синтезирован из раздела 4 в 24-луночный планшет. Дайте microhydrogels урегулировать в течение 15 секунд до тех пор, пока монослой формируется на нижней поверхности скважины.
- Получить изображение microhydrogel Janus при 24 ° C, используя вертикальный оптический микроскоп с объективом 5X.
- Установить термоэлектрический модуль под луночного планшета и управления напряжением этого модуля для повышения температуры раствора, содержащего Janus микрогидрогели до 32 ° С.
- Получить изображение microhydrogel Janus при 32 ° C еще раз, используя вертикальный оптический микроскоп с объективом 5X.
- Повторите шаги 5.2-5.4 24-х раз, следя за тем, чтобы выбрать другой Janus microhydrogel для статистического анализа.
- Из 25 изображений различных microhydrogels Janus при 24 и 32 ° С, измерить радиус PN-богатых и бедных PN-частей microhydrogels Janus с использованием программного обеспечения для анализа изображений в соответствии с инструкциями изготовителя.
Access restricted. Please log in or start a trial to view this content.
Результаты
На рисунке 3а представлена схема экспериментальной установки для синтеза Janus microhydrogels через ФГДМ. N-богатых и N-бедные фазы точно впрыскивается в ФГМД в качестве основных жидкостей 1 и 2, а затем объединяются и разбивается на Janus микрокапель в отверстие с ...
Access restricted. Please log in or start a trial to view this content.
Обсуждение
Две несмешивающихся базовые материалы, как правило, используются для синтеза microhydrogels Janus. До недавнего времени Януса microhydrogels , состоящие из того же основного материала были редко сообщается и сообщили Janus microhydrogels не имеют четкой внутренней морфологии из - за возмущения , вызванного смеш...
Access restricted. Please log in or start a trial to view this content.
Раскрытие информации
The authors declare that they have no competing financial interests.
Благодарности
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP (Nos. 2014R1A2A1A01006527 and 2011-0030075).
Access restricted. Please log in or start a trial to view this content.
Материалы
| Name | Company | Catalog Number | Comments |
| Silicon wafer | LG Siltron | 4", Test grade | Wafer for master mold fabrication |
| Acetone | Samchun Pure Chemical | A0097 | Cleaning silicon wafer |
| Isopropyl alcohol (IPA) | Daejung Chemicals & Metals | 5035-4404 | Cleaning silicon wafer |
| Water purification system | Merck Millipore | EMD Millipore RIOs Essential 5 | Prepering deionized water |
| O2 plasma machine | Femto Science | VITA-A | Cleaning silicon wafer |
| SU-8 2150 negative photoresist | MicroChem | Y111077 0500L1GL | Photoresist for master mold fabrication |
| Hot plate | Misung Scientific | HP330D, HP150D | Baking SU-8 |
| SU-8 developer | Microchem | Y020100 4000L1PE | Developing SU-8 |
| Mask aligner system for photolithograpy | Shinu Mst Co. | CA-6M | Photolithography |
| Sylgard 184 silicone elastomer kit | Dow Corning | 1064891 | PDMS casting |
| Laboratory Corona Treater | Electro-technic Products Inc. | Model BD-20AC | PDMS air plasma treatment |
| N-isopropylacrylamide (NIPAAm) | Sigma-Aldrich | 415324-50G | Monomer |
| N,N'-methylenebisacrylamide (MBAAm) | Sigma-Aldrich | 146072-100G | Crosslinker of NIPAAm |
| 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-propyl)ketone, Irgacure 2959 | BASF | 55047962 | Photoinitiator of NIPAAm |
| ABIL EM 90 | Evonik Industries | 201109 | Sufactant for oil |
| Vortex mixer | Scientific Industries Inc. | Vortex-Genie 2 | Mixing |
| Tygon tubing | Saint-Gobain | I.D. 1/32", O.D. 3/32", Wall 1/32" | Connecting tube between syringes and HFMD |
| UV light source | Hamamatsu | Spot light source LC8 | Polymerization from NIPAAm to PNIPAAm |
| Syringes, NORM-JECT (3 ml) | Henke-Sass Wolf GmbH | 22767 | Loading of materials |
| Syringe pump | KD Scientific | KDS model 200 | Perfusion of materials |
| Tegitol Type NP-10 | Sigma-Aldrich | NP10-500ML | Surfactant for water |
| Oil red O | Sigma-Aldrich | O0625-25G | Dye for N-rich phase |
| Oil Blue N | Sigma-Aldrich | 391557-5G | Dye for N-rich phase |
| Yellow food dye | Edentown F&B | NA | Dye for N-poor phase |
| Green food dye | Edentown F&B | NA | Dye for N-poor phase |
| Power supply | Agilent | E3649A | Power source for thermoelectric module |
| Thermoelectric module | Peltier | FALC1-12710T125 | Temparature control |
| Centrifuge machine | Labogene | 1248R | Settling down microhydrogels |
| 24-well plate | SPL Life Sciences | 32024 | Reservoir for observation |
| Optical microscope | Nikon | ECLIPSE 80i | Optical observation |
| Image analysis software | IMT i-Solution Inc. | iSolutions DT | Measurement of radius |
Ссылки
- Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Delivery Rev. 54 (1), 3-12 (2002).
- Qiu, Y., Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev. 53 (3), 321-339 (2001).
- Hirokawa, Y., Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 81 (12), 6379-6380 (1984).
- Bae, Y. H., Okano, T., Hsu, R., Kim, S. W. Thermo-sensitive polymers as on-off switches for drug release. Macromol. Rapid Commun. 8 (10), 481-485 (1987).
- Yoshida, R., et al. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 374 (6519), 240-242 (1995).
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 40 (12), 820-823 (1978).
- Tanaka, T., et al. Phase transitions in ionic gels. Phys. Rev. Lett. 45 (20), 1636-1639 (1980).
- Zhao, Y. L., Stoddart, J. F. Azobenzene-based light-responsive hydrogel system. Langmuir. 25 (15), 8442-8446 (2009).
- Alvarez-Lorenzo, C., Bromberg, L., Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 85 (4), 848-860 (2009).
- Tanaka, T., Nishio, I., Sun, S. T., Ueno-Nishio, S. Collapse of gels in an electric field. Science. 218 (4571), 467-469 (1982).
- Kwon, I. C., Bae, Y. H., Kim, S. W. Electrically credible polymer gel for controlled release of drugs. Nature. 354 (6351), 291-293 (1991).
- Obaidat, A. A., Park, K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials. 18 (11), 801-806 (1997).
- Kataoka, K., Miyazaki, H., Bunya, M., Okano, T., Sakurai, Y. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off regulation of insulin release. J. Am. Chem. Soc. 120 (48), 12694-12695 (1998).
- Heskins, M., Guillet, J. E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Part A Pure Appl. Chem. 2 (8), 1441-1455 (1968).
- Sasaki, S., Okabe, S., Miyahara, Y. Thermodynamic properties of N-isopropylacrylamide in water: solubility transition, phase separation of supersaturated solution, and glass formation. J. Phys. Chem. B. 114 (46), 14995-15002 (2010).
- Bromberg, L., Alakhov, V. Effects of polyether-modified poly(acrylic acid) microgels on doxorubicin transport in human intestinal epithelial Caco-2 cell layers. J. Controlled Release. 88 (1), 11-22 (2003).
- Coughlan, D. C., Quilty, F. P., Corrigan, O. I. Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopropylacrylamide) hydrogels. J. Controll. Release. 98 (1), 97-114 (2004).
- Bergbreiter, D. E., Case, B. L., Liu, Y. S., Caraway, J. W. Poly(N-isopropylacrylamide) soluble polymer supports in catalysis and synthesis. Macromolecules. 31 (18), 6053-6062 (1998).
- Lapeyre, V., Gosse, I., Chevreux, S., Ravaine, V. Monodispersed glucose-responsive microgels operating at physiological salinity. Biomacromolecules. 7 (12), 3356-3363 (2006).
- Hoare, T., Pelton, R. Engineering glucose swelling responses in poly(N-isopropylacrylamide)-based microgels. Macromolecules. 40 (3), 670-678 (2007).
- Xu, S., Zhang, J., Paquet, C., Lin, Y., Kumacheva, E. From hybrid microgels to photonic crystals. Adv. Funct. Mater. 13 (6), 468-472 (2003).
- Clarke, J., Vincent, B. Stability of non-aqueous microgel dispersions in the presence of free polymer. J. Chem. Soc., Faraday Trans. 1. 77 (8), 1831-1843 (1981).
- Mears, S. J., Deng, Y., Cosgrove, T., Pelton, R. Structure of sodium dodecyl sulfate bound to a poly (NIPAM) microgel particle. Langmuir. 13 (7), 1901-1906 (1997).
- Shah, R. K., Kim, J. W., Agresti, J. J., Weitz, D. A., Chu, L. Y. Fabrication of monodisperse thermosensitive microgels and gel capsules in microfluidic devices. Soft Matter. 4 (12), 2303-2309 (2008).
- Jack, C. R., Forbes, G., Dewanjee, M. K., Brown, M. L., Earnest, F. Polyvinyl alcohol sponge for embolotherapy: particle size and morphology. Am. J. Neuroradiol. 6 (4), 595-597 (1985).
- Derdeyn, C. P., Moran, C. J., Cross, D. T., Dietrich, H. H., Dacey, R. G. Polyvinyl alcohol particle size and suspension characteristics. Am. J. Neuroradiol. 16 (6), 1335-1343 (1995).
- Han, K., et al. Effect of flow rates on generation of monodisperse clay-poly(N-isopropylacrylamide) embolic microspheres using hydrodynamic focusing microfluidic device. Jpn. J. Appl. Phys. 50 (6), 06-12 (2011).
- Seo, K. D., Doh, J., Kim, D. S. One-step microfluidic synthesis of Janus microhydrogels with anisotropic thermo-responsive behavior and organophilic/hydrophilic loading capability. Langmuir. 29 (49), 15137-15141 (2013).
- Seo, K. D., Kim, D. S. Microfluidic synthesis of thermo-responsive poly(N-isopropylacrylamide)-poly(ethylene glycol) diacrylate microhydrogels as chemo-embolic microspheres. J. Micromech. Microeng. 24 (8), 085001(2014).
- Seo, K. D., Kwak, B. K., Kim, D. S., Sánchez, S. Microfluidic-assisted fabrication of flexible and location traceable organo-motor. IEEE Trans. Nanobiosci. 14 (3), 298-304 (2015).
- Seo, K. D., Kim, D. S., Sánchez, S. Fabrication and application of complex-shaped microparticles via microfluidics. Lab Chip. , (2015).
- Shah, R. K., Kim, J. W., Weitz, D. A. Janus supraparticles by induced phase separation of nanoparticles in droplets. Adv. Mater. 21 (19), 1949-1953 (2009).
- Lone, S., et al. Microfluidic synthesis of Janus particles by UV-directed phase separation. Chem. Commun. 47 (9), 2634-2636 (2011).
- Hauber, K., Drier, T., Beebe, D. PDMS bonding by means of a portable, low-cost corona system. Lab chip. 6 (12), 1548-1549 (2006).
- Nisisako, T., Torii, T., Takahashi, T., Takizawa, Y. Synthesis of monodisperse bicolored Janus particles with electrical anisotropy using a microfluidic co-flow system. Adv. Mater. 18 (9), 1152-1156 (2006).
- Seiffert, S., Romanowsky, M. B., Weitz, D. A. Janus microgels produced from functional precursor polymers. Langmuir. 26 (18), 14842-14847 (2010).
- Peppas, N. A., Hilt, J. Z., Khademhosseini, A., Langer, R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 18 (11), 1345-1360 (2006).
Access restricted. Please log in or start a trial to view this content.
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеСмотреть дополнительные статьи
This article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены