このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
共培養による線維形成性三次元膵臓がんスフェロイドの増殖
要約
膵臓がんは、依然として治療が最も困難ながんの1つです。したがって、治療効果を評価する前臨床モデルが再現性があり、臨床的に関連性があることが重要です。このプロトコールは、再現性があり、臨床的に関連性のある線維形成性スフェロイドを生成するための簡単な共培養手順を説明しています。
要約
膵管腺がん(PDAC)は、5年生存率が<12%の最も致命的ながんの1つです。治療の最大の障壁は、腫瘍を取り囲み、血管新生を減少させる高密度の線維形成性細胞外マトリックス(ECM)であり、一般にデスモプラスラと呼ばれます。がんの治療には、さまざまな薬剤の組み合わせや製剤が試験されており、その多くは前臨床で成功を収めていますが、臨床的には失敗しています。したがって、治療に対する腫瘍の反応を予測できる臨床的に関連性のあるモデルを利用できるようにすることが重要になります。このモデルは、切除された臨床腫瘍に対して以前に検証されています。ここでは、頑健なECMを自然に生成でき、その成長をサポートするための外部マトリックスソースや足場を必要としない、線維形成性三次元(3D)共培養スフェロイドを成長させるための簡単なプロトコルについて説明します。
簡単に説明すると、ヒト膵臓星状細胞(HPaSteC)とPANC-1細胞を使用して、それぞれ1:2の比率で細胞を含む懸濁液を調製します。細胞は、ポリHEMAコーティングされた96ウェル低接着Uウェルプレートに播種されています。プレートを遠心分離して、細胞が最初のペレットを形成するようにします。プレートは5%CO2で37°Cのインキュベーターに保存され、培地は3日ごとに交換されます。プレートを指定された間隔でイメージングして、スフェロイドの体積を測定できます。14日間の培養後、成熟した線維形成性スフェロイドが形成され(すなわち、平均体積0.048 + 0.012 mm3(451 μm x 462.84 μm))、実験的治療評価に利用できます。成熟したECM成分には、コラーゲンI、ヒアルロン酸、フィブロネクチン、ラミニンが含まれます。
概要
膵臓がんの予後不良にはさまざまな理由が関連していますが、その中には、検出が遅れる原因となる簡単に検出できるバイオマーカーがないことが挙げられます。もう一つの大きな理由は、組織を取り巻く間質が厚いため、血液供給が減少することです。大量の細胞外マトリックス(ECM)、細胞間相互作用、内皮細胞、さまざまな免疫細胞、周皮細胞、増殖性筋線維芽細胞、線維芽細胞集団、および非腫瘍細胞の存在(一緒にして線維形成反応を構成する)1が、PDACの化学療法および放射線治療耐性2の原因となる厚い間質を構成します.がん細胞と間質細胞は、複雑でダイナミックな双方向の相互作用を持っています。一部の要素は疾患の進行を弱めたり加速させたりしますが、ほとんどのプロセスは腫瘍の発生中に適応します1。これにより、成長因子、血管新生促進因子、プロテアーゼ、接着分子が豊富な環境が提供されます。これらの因子は、血管新生、細胞増殖、転移、および浸潤を促進します3,4。これらが一緒になって、腫瘍に対する免疫と薬物特権の聖域となり、薬剤耐性をもたらします。
デスモプラスラは、免疫細胞および膵臓星細胞(PSC)とともに、さまざまなECMタンパク質からなる複雑な混合物です。これらが一緒になって、細胞が成長するための足場を形成する傾向があります。PSCは、間質コンパートメント5の最大のコンポーネントの1つです。マトリックスメタロプロテアーゼ(MMP)、マトリックスメタロプロテアーゼの組織阻害剤(TIMP)、がん関連線維芽細胞(CAF)6などの酵素を産生する能力は、それらが線維形成反応の進行に重要な役割を果たす可能性が高いことを示唆しています。ECM、がん関連線維芽細胞(CAF)、および血管系は、PDACの主要な側面です。CAFの中で、筋線維芽細胞と炎症性CAFは、腫瘍誘発性の特性に関与する活性クロストークに関与していると推測されています7。腫瘍上の線維芽細胞形成が広範囲に及ぶほど、予後は不良になります8,9,10。
確立された細胞株による単層細胞培養は、薬物毒性を解析するための有用なツールであり続けており、概念実証や創薬研究の出発点として適しています。しかし、確立された細胞培養株には、生殖細胞DNAと臨床的関連性が欠けています11。それらは平らな表面で成長するため、腫瘍の一部である場合とは異なる in vitro 選択基準を受け、異常に分裂し、分化した表現型を失います12。全体として、シングルセル培養は腫瘍の不均一性を制限するため、臨床的関連性を失います。腫瘍の微小環境(ECMなど)の複雑さを正確に表現することはできません。3D培養は、複雑な腫瘍微小環境をより忠実に再現することができます。
3D培養は、1970年代に健康な細胞とその腫瘍性細胞に導入されました13。スフェロイド14を通じて悪性組織の形態と構造を研究するために、いくつかの技術が使用されてきた。間質細胞との共培養は、TMEシグナルをモデル化できます。EMTマーカーのアップレギュレーションは、細胞を星状細胞と共培養したときに見られた15。PDACスフェロイドと間質との相互作用は、ECMコンポーネントとの共培養によってモデル化できます。PSCと特異的に共培養すると、臨床的に関連性のある薬物細胞毒性データが得られることが報告されています16,17,18。PSCはまた、アポトーシスを回避し、さまざまなパラクリン因子19を介してがん細胞の増殖を刺激し、EMT移行を誘導することにより、薬剤耐性を助けます。したがって、薬物または薬物送達システムの成功を評価するために使用される基準に、早い段階からPSCを含めることが重要になります。膵臓癌細胞単独と比較して、PSCが増殖を促進し、組み合わせてより速い増殖をサポートする能力は、免疫不全マウスで2つの細胞株の皮下側面注射を評価したときにもin vivoで見られました20。
細胞タイプがECMコンポーネントと相互作用する能力も、共培養スフェロイドを成長させる際に考慮すべきことが重要です。BxPC-3とPANC-1は、コラーゲンへの結合において同等の親和性を有することが報告されています。2つの細胞株はラミニンにも同等に結合しますが、BxPC-3の方がよりよく結合するという報告があります21,22,23,24,25。遊走に関しては、Stahle et al.26は、BxPC-3と比較してPANC-1細胞の運動性が5倍速いことを示しました。PANC-1細胞は主に単一細胞として遊走することも報告されていますが、BxPC-3細胞は密集したシートとして遊走します。細胞の選択は、腫瘍25のサイズにも影響する。BxPC-3腫瘍は、PANC-1から得られた腫瘍よりも27.28大きいことが示されましたが、1つの研究では反対の29のケースが示されました。サイズと運動性の違いにもかかわらず、どちらの細胞もマウスで腫瘍を形成するために長期間の潜伏が必要であることが報告されています。この期間は、BxPC-3 では特に長く、4 週間から 4 か月の範囲です25。しかし、BxPC-328またはBxPC-3がん幹細胞30がより早く目に見える腫瘍を形成したという文献もあり、腫瘍の成長期間にばらつきが見られる可能性があることを示唆しています。したがって、ここに記載されている期間は、腫瘍増殖率の最初のガイドラインとしてのみ役立つはずです。
BxPC-3細胞は、表面に緩い細胞と密集したコアを持つスフェロイドを形成しますが、PANC-1細胞は、多孔質で堅牢なスフェロイド31 とコンパクトなスフェロイドの両方を形成することが報告されています。PANC-1細胞はまた、分化が少なく、より攻撃的であることが報告されています32。攻撃的な性質32 を最前線に保ち、PANC-1細胞の高い運動性、コンパクトなスフェロイドを形成する能力、およびECM成分と相互作用する能力と組み合わせて、PANC-1細胞がスフェロイド研究に選ばれました。
ここ数年、スフェロイド培養は、二次元(2D)培養と比較して、その臨床的関連性において優位性を実証することに成功しています。その関連性は、動物研究の代替としてこの技術を使用し、腫瘍の生物学をよりよく理解するために活用されています。スフェロイドの臨床的関連性は、特にPSCと共培養された場合、剛性33、TGF-βの発現34,35,36,37,38、E-カドヘリン、F-アクチン18,34,36,37、α-SMA 34,35,37などのスフェロイドのさまざまな機能を研究するためにそれらを使用することを可能にしました。38、乳酸デヒドロゲナーゼ(LDHA)32、HIF-1α35,39、薬剤耐性16,37,40、細胞遊走41、細胞浸潤37、線維化35、放射線耐性42、表現型変化18、不均一性36、相互作用の細胞レベル39およびECM成分の実証37,38、39。記載されているデータを取得するために使用されたプロトコルの多くは、Matrigel、吊り下げ法、プリントされた型、またはその他の足場に依存して、スフェロイドとECMの増殖をサポートします。また、この研究では通常、非ヒト線維芽細胞または患者から新たに単離された星状細胞を使用します。腫瘍を生体内疾患に類似させるためには、星状細胞を使用することが重要であるが、新たな抽出物に関連する患者間のばらつきが、これらの研究の再現を困難にしている。
このプロトコルは、開発が容易で、再現性があり、臨床的に関連性があり、足場のないモデルを実証することを目的としており、ECMを自然に生成する共培養の能力のみに依存しています。そのために、PANC-1細胞(単一細胞として移動する自然な傾向があるため)とヒト膵臓星細胞(HPaSteC)を混合したシンプルな共培養法が選択されました。これは、幹細胞のように振る舞い、薬剤耐性が高いためです。Durymanov et al.38による研究をベースラインとして使用して、以下に詳述するプロトコルは、細胞比や培地交換間の期間などのパラメータをさらに最適化した後に確立されました。このプロトコールから得られたスフェロイドは、新薬候補評価のモデルシステムとして使用することができます40。
さらに、回転楕円体の文化に詳しくないユーザーにとっては、MISpheroIDナレッジベースの開発について論じたPeirsman et al.43の著作が参考になるかもしれない。これは、ラボプロトコル間の不均一性に対処するのに役立つ可能性のあるいくつかの最小限の情報ガイドラインを確立します。いくつかの制限はありますが、この研究は、培地、細胞株、スフェロイド形成方法、および最終的なスフェロイドサイズの選択が、スフェロイドの表現型特性を決定する上で重要であることを示しました。
プロトコル
1. 2D細胞培養
- 無菌条件下で10%ウシ胎児血清(FBS)を添加したダルベッコのModified Eagle Medium(DMEM)でPANC-1細胞を増殖させます。パッセージの前に70%〜80%のコンフルエントまで成長し、20番目の パッセージを超えて使用しないでください。ステップ 4.2.1 で説明されているプロセスを参照してください。
注:参考として、20mLの培地中の1 x 106細胞は、70〜80%の密度に達するまでに約2〜3日かかります。 - 2% FBS、1% PenStrep、および1% Stellate Cell Growth Supplement(SteCGS)を添加したステラ細胞培地で、メーカーが提供するキットを使用して無菌条件下でHPaSteC細胞を増殖させます。製造元の指示に従って(一部変更を加えて)、ステップ4.2.2に示すように、Poly-L-Lysineコーティングフラスコで成長させます。
- トリプシン中和溶液(TNS)が使用されるため、トリプシン化中のFBS中和ステップをメーカーのプロトコルからスキップします。ステップ4.2.2.2で説明したように、全量10 mLのTNSで細胞懸濁液を中和します。
注:参考値として、20 mLの培地中の0.5 x 106細胞および1 x 106細胞は、90%のコンフルエンシーを達成するために、それぞれ3日および2日を要します。 - 90%のコンフルエント度で細胞を採取します。Passage 7を超えるセルは使用しないでください。
- トリプシン中和溶液(TNS)が使用されるため、トリプシン化中のFBS中和ステップをメーカーのプロトコルからスキップします。ステップ4.2.2.2で説明したように、全量10 mLのTNSで細胞懸濁液を中和します。
- 細胞を5%CO2を含む37°Cに維持し、相対湿度が90%〜95%の滅菌インキュベーターで保持します。すべての細胞培養研究をT-75フラスコで実施します。
2. ポリ(2 -ヒドロキシエチルメタクリレート(ポリHEMA)溶液コーティング、96ウェルプレート用
- 95%エタノールを使用して5 mg/mLのポリHEMA溶液を調製し、37°Cの加熱攪拌機を用いて一晩混合します(例:500 mLの溶液には2.5 gのポリHEMAが必要です)。
- スターラーを追加する前に、開始ボリュームの位置をマークします。95%エタノールを使用して、翌日の溶液の損失を補います。
- 0.22μmフィルターを使用して滅菌フードで最終溶液をろ過し、4°Cの冷蔵庫で保存します。
注:Poly-HEMAは、表面の疎水性を高めるために使用され、細胞が付着するのを抑止する役割を果たします。この研究では、細胞がウェルに付着せず、単層として成長する必要があるため、ポリHEMAは、プレートの自然な低付着特性に対する追加の抑止力として使用され、それによってそれらを超低接着プレートにします。 - 細胞培養フード内の96ウェル丸型ポリスチレン底部マイクロウェルプレートの各ウェルに50 μLの冷溶液を加えて、プレートをコーティングします。
- 蓋をしたままウェルを37°Cの熱風オーブンに3日間放置し、プレートが完全に乾いていることを確認します。
- 細胞播種前に、細胞培養フード内でプレートを30分間UV滅菌します。長期保存のためにしっかりと閉じたジップロックに保管し、使用前に滅菌してください。
3. 2D細胞培養計画
- HPaSteC細胞が90%に達するのと同じ日に、PANC-1細胞が70%〜80%のコンフルエント度で利用可能になるように、実験のタイミングを計ります。
注:PANC-1またはHPaSteCのいずれかの細胞を約100万個含むバイアルは、同じ日に液体窒素から増殖すると、多少のばらつきはありますが、両方の細胞株を同じ日に準備するには、8日間のPANC-1培養と6〜7日間のHPaSteC培養が必要です。
4. 3D文化の成長
- プロセスを開始する前に、 図 1 のプロセス全体の概要を参照してください。
注意:PANC-1とHPaSteCはどちらも生物学的起源です。PANC-1(がん細胞株)の取り扱いには注意してください。 - 細胞のトリプシンジングとカウント
- PANC-1細胞をトリプシンゼしてカウントします。
- 上清を捨て、PANC-1細胞を9 mLのHBSS(一度に3 mL)で洗浄します。使用済みの試薬をフード内の20〜25%V / V漂白剤溶液に廃棄して、生きている/生存可能な材料が完全に死ぬようにします。
- 2mLのトリプシンで細胞をトリプシン化します。約10分後(さらに時間がかかる場合があります)、すべての細胞が剥離したら、10 mLのDMEM + 10%FBSで懸濁液を中和します。3 mLから始めて、その後の洗浄ステップで1回の洗浄(残りの7回の洗浄)を使用して、トリプシン化細胞の最大収集を確保します。
- 各中和アリコートを同じ15 mLチューブにプールします。チューブ内の最終容量は 12 mL (10 mL 培地で中和されたトリプシンの~2 mL) に近くなります。
- 中和した細胞懸濁液を700 x g で2分間30秒間遠心分離します。上清を捨て、得られたペレットを1 mLのDMEM + 10%FBSに再懸濁します。.これから細胞計数用のアリコートを取得し、このセクションから得られた値を「カウントA」とラベル付けします。
- HPaSteC細胞をトリプシンゼしてカウントします。
- 上清を捨て、HPaSteC細胞を9 mLのHBSS(一度に3 mL)で洗浄します。
- 2mLのトリプシンで細胞をトリプシン化します。約10分後、すべての細胞が剥離したら、懸濁液を10mLのトリプシン中和溶液で中和します。3 mLから始めて、その後の洗浄ステップで1回の洗浄(残りの7回の洗浄)を使用して、トリプシン化細胞の最大収集を確保します。
- 各中和アリコートを同じ15 mLチューブにプールします。チューブ内の最終容量は 12 mL (10 mL の中和溶液で中和されたトリプシン ~2 mL) に近くなります。
- 中和した細胞懸濁液を700 x g で2分間30秒間遠心分離します。上清を捨て、得られたペレットを補充した星状細胞培地1 mLに再懸濁します。これから細胞計数用のアリコートを取得し、このセクションから得られた値を「カウントB」とラベル付けします。
- PANC-1細胞をトリプシンゼしてカウントします。
- 初期細胞懸濁液の希釈
- 必要に応じて(0.5時間を超えないで)、AとBの両方のカウントを取得した後、またはPANC-1とHPaSteCの両方をトリプシン化して中和した後、しばらくの間、このステップで一時停止します。未使用の培地は37°Cに、細胞懸濁液は室温(RT;25°C±2°C)で保存してください。
- 以前に取得したカウントAとカウントBを使用して、アリコートを採取し、それぞれの希釈液を(別々のチューブで)して、最終容量が1 mL(希釈剤:DMEM + 両方のバイアルの10%FBS)で、最終カウントが各細胞タイプで110,000〜140,000細胞になるようにします。この希釈が行われたら、各希釈の細胞数を確認して、カウントC(PANC-1)とカウントD(HPaSteC)を求めます。
- カウントCとカウントDを取得した後、必要に応じて(15〜30分以内に)このステップで一時停止します。ステップ4.3.3.2で最終希釈を行った後は停止しないでください。細胞の沈降を避けるための実験。未使用の培地は37°Cで、細胞懸濁液はRT(25°C±2°C)で保存してください。
- HPaSteC: PANC-1: 60:120でウェルごとに必要な細胞の最終数を維持します。計算には 110 ウェルを使用して、過剰を考慮します。ウェルあたり100 μLの細胞懸濁液の基本値で計算します(HPaSteC:PANC-1:60:120を使用した110ウェルの総懸濁液容量は11 mLです)。この懸濁液を滅菌済みの50 mL遠心分離チューブに入れて、混合を容易にします。
- カウントCおよびカウントDを使用し、十分な細胞懸濁液をDMEM + 10% FBS溶液にスパイクして、最終容量11 mL(11 mL中に6,600個のHPaSteC細胞および13,200個のPANC-1細胞)でHPaSteC: PANC-1: 60:120が得られます。1 mLピペットを使用して、攪拌しながらピペッティングで上下に溶液を十分に混合します。泡の形成を避けてください。
- シード
- 200 μLまたは100 μLのピペットを使用して、各ステップで最終懸濁液を混合し(ピペットの先端を動かして攪拌し、ピペッティングを上下させて攪拌)、ウェルごとに100 μLの懸濁液を添加します。100μLをウェルの角に沿って穏やかに加えます。
- 同じチップを複数のウェルに再利用し、脱落しない限り再利用してください。前後に混ぜて、プレートの前半(およそ最初の43ウェル)の各ウェルへの追加間で10または15(秒ではない)に数えます。後半(残りの43ウェル)では、懸濁液の量が少なく、必要な混合時間が少なくて済むため、各ウェル間の混合数を5〜10に減らします。
- 疎水性のポリHEMAコーティングにより、懸濁液がプレートから跳ね返り、分布が不均一になるため、リピートピペッターを使用しないでください。
- すべてのウェルが満たされた後、余分な懸濁液を廃棄します。
- 200 μLまたは100 μLのピペットを使用して、各ステップで最終懸濁液を混合し(ピペットの先端を動かして攪拌し、ピペッティングを上下させて攪拌)、ウェルごとに100 μLの懸濁液を添加します。100μLをウェルの角に沿って穏やかに加えます。
- 遠心分離
- 細胞懸濁液を含む最終プレートを遠心分離して、すべての細胞をペレットとしてまとめます。このステップでの汚染を防ぐために、プレートの境界の周りにプレートをパラフィルムで慎重に包みます。ラッピング後、カウンターバランスで1000 x g のプレートを2分間遠心分離します。
- プレートを細胞培養フードに戻し(エタノール溶射手袋を使用して取り扱います)、パラフィルムをはがし、プレートを37°C、5%CO2のインキュベーターに保管します。エタノールと水の混合物をプレートにスプレーしないでください。
- 文化の維持
- 3日目までに、細胞が集まってスフェロイドを形成する様子を、光学顕微鏡で4倍から5倍の倍率で観察します。以下に説明する培養維持プロトコルに従ってください。
- 3日目に、5〜6 mLのDMEM + 10% FBSを測定し、ウェルの側面に沿って各ウェルに50 μLを加えます。
- 添加中にメディアを混合したり攪拌したりすると、スフェロイドが損傷したり、誤ってスフェロイドが除去されたりするため、メディアを混合したり攪拌したりしないでください。ウェルあたりの最終容量は~150 μLです。
- 3日目以降は、 図2 を参照して、ウェルの構造と、ハローと呼ばれる媒体に見られる光の反射(ウェルの円筒形部分と湾曲した部分の間)の位置について理解を深めてください。ハローの近くで操作を行い、スフェロイドが損傷を受けないように注意して行ってください。
- 6日目に、10〜11mLのDMEM + 10%FBSを測定します。1 mLピペットを使用して、ハローまで培地を引き出し、複数のウェルから培地をバルクで引き出します。これをプレート全体に対して行い、その後、メディアを交換します。上澄み液をまとめて捨てるか、引き出した状態で捨ててください。
- 各ウェルの側面に沿って100 μLの培地を添加して、廃棄した培地を新しい培地と交換します。ウェル内の最終容量は200μL近くになります。
- ハローの外側に媒体を引き抜くと、誤って回転楕円体を引き抜くリスクが高まるため、引っ張らないでください。
- 10/11日目に、10〜11 mLのDMEM + 10%FBSを測定します。1 mLピペットを使用して、ハローまで培地を引き出し、複数のウェルから培地をバルクで引き出します。これをプレート全体に対して行い、その後、メディアを交換します。上澄み液をまとめて捨てるか、引き出した状態で捨ててください。
- 各ウェルの側面に沿って100 μLの培地を添加して、廃棄した培地を新しい培地と交換します。ウェル内の最終容量は200μL近くになります。
- 3日目に、5〜6 mLのDMEM + 10% FBSを測定し、ウェルの側面に沿って各ウェルに50 μLを加えます。
- 3日目までに、細胞が集まってスフェロイドを形成する様子を、光学顕微鏡で4倍から5倍の倍率で観察します。以下に説明する培養維持プロトコルに従ってください。
- 最終日の評価
- 14日目にスフェロイドで薬物治療を行います。使用前に明視野倒立顕微鏡を4倍対物レンズで撮影し、スフェロイドの開始体積を決定します。次の式を使用して体積(V)を計算します。
V = 0.5 × L × W2 - 上記の式に従って回転楕円体体積を計算します。ここで、L は長軸の長さ、W は幅 (長軸に垂直な最長の線) です。すべての元の単位をマイクロメートルからミリメートルに変換して、立方ミリメートル単位の体積を取得します。
- 14日目の各ウェルの開始容量は、蒸発により150〜200μLです。薬物治療の場合は、各ウェルからハローに達するまで培地を取り除き、2倍の強度の100μLの薬液と交換し、穏やかな混合(各ウェル間で2まで数えながら培地を前後に穏やかに混合します)を加えます。
- 2倍の強度の溶液を使用して、最終的な薬物溶液を1倍にし、ウェルから古い培地を完全に除去してスフェロイドが乱されるのを防ぎます。この時間枠から3日以内にスフェロイドを使用してください。
- 14日目にスフェロイドで薬物治療を行います。使用前に明視野倒立顕微鏡を4倍対物レンズで撮影し、スフェロイドの開始体積を決定します。次の式を使用して体積(V)を計算します。
- スフェロイドコレクション
- ステップ4.7.2で説明されているように、収集前にスフェロイドの体積を測定します。
- 実験のニーズに応じて、14日目または17日目に、ハローに達するまで各ウェルから培地を除去して、スフェロイドを採取します。
- 1 mLピペットを使用して培地を除去し、複数のウェルから15 mLの円錐形遠心チューブに上清を回収します。
- すべてのウェルにハローレベルの培地が揃ったら、1 mLピペットの設定を200〜300 μLに下げます。
- プレートをフードの背景または暗い背景(チューブラックなど)に合わせ、スフェロイドが下部に沈殿することを確認します。
- 先端をスフェロイドの近くにそっと挿入し(ハローの下へ)、少量の培地(例えば、目視で推定される50μL近く)を前後にピペットで動かし、スフェロイドを乱します。回転楕円体の真上に先端を置かないでください、それはその構造を損傷する可能性があるためです。
- この手順では、すべてのメディアをウェルから引き出さないでください。親指の動きをピペットのピストンでしっかりと制御します。
- 回転楕円体が中心に残らなくなり、背景に対して移動しているのが見えるまで、メディアを移動します。スフェロイドを吸い出すのに十分な培地を引き上げ、目的の場所に移動します(たとえば、同じ処理セットの一部であるウェルに他のスフェロイドとプールします)。
- 廃棄物の廃棄
- 漂白剤で中和されたすべての液体サンプルは、それぞれの研究所の環境および健康安全部門のプロトコルに従って廃棄します。バイオハザード廃棄物を含むすべての使用済みピペットとフラスコをオートクレーブして廃棄します。
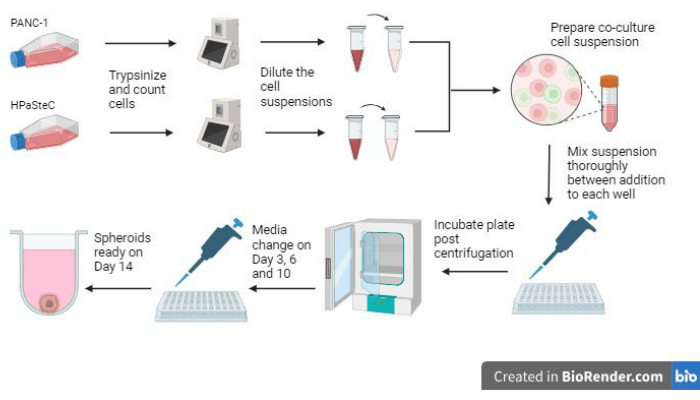
図1:3D線維形成性膵臓がんスフェロイドの増殖プロセスの概要 (BioRenderを使用して生成)。この図は、関連する基本的なプロセスの概要を示しています。すなわち、細胞をトリプシンし、初期細胞数を使用して希薄な細胞懸濁液を作製し、希釈した細胞懸濁液を使用して共培養物を調製し、各ウェルに細胞懸濁液を添加し、培養物をインキュベートし、培地の維持と最終的なスフェロイド形成を14日目に期待どおりに行います。 この図の拡大版を表示するには、ここをクリックしてください。

図2:U底ウェルの構造。 右の画像は、井戸の「ハロー」部分を示すために誇張された形状です。この図は、ハローの上で作業することがスフェロイドの成長と偶発的な損失を避けるために重要であるため、井戸の「ハロー」部分がどこにあるかを定義することを目的としています。 この図の拡大版を表示するには、ここをクリックしてください。
5. ECM成分の評価と共焦点顕微鏡
- 免疫染色を使用して、Durymanov et al.38 によって記述されたプロトコルに従って、薬物療法に対する ECM の反応を評価します。
- スフェロイドをプールし、100 μLのPBSで2回洗浄します。最適な切断温度(OCT)組織包埋培地に埋め込み、凍結して-80°Cに維持します。
- 凍結した腫瘍ブロックを10 μmの切片に切断し、切片をアセトン-メタノール(1:1)混合物に15分間固定し、RTで風乾させます。
- ECM成分を決定するには、ウサギモノクローナル抗フィブロネクチン一次抗体、ウサギポリクローナル抗I型コラーゲン抗体、ウサギポリクローナル抗ラミニン抗体、およびヒツジポリクローナル抗ヒアルロン酸抗体で凍結切片を免疫染色します。
- Alexa Fluor 488で標識したヤギ抗ウサギIgG、またはAlexa Fluor 568で標識したロバ抗ヒツジを二次抗体として使用します。
注:一次抗体と二次抗体は、それぞれ1:200と1:300で希釈しました。 - 20倍/0.45対物レンズを装備した共焦点レーザー走査型顕微鏡を使用して、すべての回転楕円体の切片の画像を取得します。
結果
スフェロイドの増殖に関与する最も重要な3つのステップは、初期細胞数、スフェロイドの播種中の混合ステップ、およびスフェロイドを成長させるためのタイムリーな培地交換の実施です(図1)。さらに、3日目以降のFigure 2 onのメディア変更に精通することは、ウェルあたりのメディア量の増加による効果的?...
ディスカッション
スフェロイドを増殖させるために選択された期間および細胞比は、以前に報告された研究に基づいていた38。これらの研究を最適化しようと、NIH3T3細胞をHPaSteC細胞に置き換えようとしたところ、スフェロイドの体積とアポトーシスパターンは、PANC-1:HPaSteC比が120:60のときに報告された最適化パラメータ(PANC-1:NIH3T3:: 120:12)と非常によく似ていることが?...
開示事項
著者は何も開示していません。
謝辞
記載されている研究は、サウスダコタ州知事経済開発局、サウスダコタ州評議委員会競争的研究助成プログラム(SD-BOR-CRGP)、およびサウスダコタ州立大学の薬学部の支援を受けて行われました。
資料
| Name | Company | Catalog Number | Comments |
| Axio Observer inverted microscope | Carl Zeiss | 0450-354 | |
| Cellometer Auto T4 | Nexcelom Bioscience LLC | Auto-T4 | |
| DMEM, powder, high glucose | Gibco | 12100046 | |
| Donkey anti-sheep conjugated with Alexa Fluor 568 | Abcam | ab175712 | |
| Fetal Bovine Serum | Cytiva | SH3091003HI | |
| Goat antirabbit IgG labeled with Alexa Fluor 488 | Abcam | ab150077 | |
| Hanks Balanced Salt Solution (HBSS) | Gibco | 14175145 | |
| Human Pancreatic Stellate Cells (HPaSteC) | ScienCell | 3830 | |
| Microscope Nikon | Nikon | Eclipse Ts 100 | |
| Nunc 96-Well Polystyrene Round Bottom Microwell Plates | Thermo Scientific | 12-565-331 | |
| Olympus Fluoview FV1200 confocal laser | Olympus | N/A | Discontinued product |
| PANC-1 | ATCC | CRL-1469 | |
| Poly-HEMA | Sigma | P3932 | |
| Rabbit polyclonal anti-laminin antibodies | Abcam | ab11575 | |
| Rabbit polyclonal anti-type I collagen antibodies | Abcam | ab34710 | |
| Sheep polyclonal anti-hyaluronic acid antibodies | Abcam | ab53842 | |
| Stellate cell media complete kit | ScienCell | 5301 | |
| Trypsin | MP Biomedicals, LLC | 153571 | Trypsin solution prepared according to manufacturers protocol and used at 0.25%w/v |
| Trypsin Neutralization Solution (TNS) | ScienCell | 103 |
参考文献
- Hingorani, S. R. Epithelial and stromal co-evolution and complicity in pancreatic cancer. Nat Rev Cancer. 23 (2), 57-77 (2023).
- Laklai, H., et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 22 (5), 497-505 (2016).
- Binker, M. G., Binker-Cosen, M. J., Binker-Cosen, A. A., Cosen-Binker, L. I. Microenvironmental factors and extracellular matrix degradation in pancreatic cancer. J Pancreas. 15 (4), 280-285 (2014).
- Spano, D., Zollo, M. Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis. 29 (4), 381-395 (2012).
- Ware, M. J., et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials. 108, 129-142 (2016).
- Sunami, Y., Häußler, J., Kleeff, J. Cellular heterogeneity of pancreatic stellate cells, mesenchymal stem cells, and cancer-associated fibroblasts in pancreatic cancer. Cancers. 12 (12), 3770 (2020).
- Öhlund, D., et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 214 (3), 579-596 (2017).
- Pandol, S., Edderkaoui, M., Gukovsky, I., Lugea, A., Gukovskaya, A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 7 (11), S44-S47 (2009).
- Watanabe, I., et al. Advanced pancreatic ductal cancer: fibrotic focus and β-catenin expression correlate with outcome. Pancreas. 26 (4), 326-333 (2003).
- Hu, G., et al. Tumor-infiltrating podoplanin+ fibroblasts predict worse outcome in solid tumors. Cell Physiol Biochem. 51 (3), 1041-1050 (2018).
- Kapałczyńska, M., et al. 2D and 3D cell cultures-a comparison of different types of cancer cell cultures. Arch Med Sci. 14 (4), 910-919 (2018).
- Monberg, M. E., et al. Occult polyclonality of preclinical pancreatic cancer models drives in vitro evolution. Nat Commun. 13 (1), 3652 (2022).
- Emerman, J. T., Pitelka, D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 13 (5), 316-328 (1977).
- Kelm, J. M., Timmins, N. E., Brown, C. J., Fussenegger, M., Nielsen, L. K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 83 (2), 173-180 (2003).
- Kikuta, K., et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 403 (3-4), 380-384 (2010).
- Lee, J. -. H., et al. Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance. J Exp Clin Cancer Res. 37 (1), 4 (2018).
- Wong, C. -. W., Han, H. -. W., Tien, Y. -. W., Hsu, S. -. H. Biomaterial substrate-derived compact cellular spheroids mimicking the behavior of pancreatic cancer and microenvironment. Biomaterials. 213, 119202 (2019).
- Liu, X., et al. 3D heterospecies spheroids of pancreatic stroma and cancer cells demonstrate key phenotypes of pancreatic ductal adenocarcinoma. Transl Oncol. 14 (7), 101107 (2021).
- Vonlaufen, A., et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 68 (7), 2085-2093 (2008).
- Bachem, M. G., et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 128 (4), 907-921 (2005).
- Greco, E., et al. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1β not by transforming growth factor-β1. Int J Biol Markers. 20 (4), 235-241 (2005).
- Arao, S., Masumoto, A., Otsuki, M. β1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells. Pancreas. 20 (2), 129-137 (2000).
- Sawai, H., Yamamoto, M., Okada, Y., Sato, M., Akamo, Y., Takeyama, H., Manabe, T. Alteration of integrins by Interleukin-1alpha in human pancreatic cancer cells. Pancreas. 23 (4), 399-405 (2001).
- Chen, J., et al. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol Rev. 96 (3), 1025-1069 (2016).
- Deer, E. L., et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 39 (4), 425 (2010).
- Stahle, M., et al. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 116, 3835-3846 (2003).
- Aubert, M., et al. Decrease of human pancreatic cancer cell tumorigenicity by α1, 3galactosyltransferase gene transfer. Int J Cancer. 107 (6), 910-918 (2003).
- Miknyoczki, S. J., Chang, H., Klein-Szanto, A., Dionne, C. A., Ruggeri, B. A. The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft modelsof human pancreatic ductal adenocarcinoma. Clin Cancer Res. 5 (8), 2205-2212 (1999).
- Fukasawa, M., Korc, M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 10 (10), 3327-3332 (2004).
- Hernández-Camarero, P., et al. Pancreatic (pro) enzymes treatment suppresses BXPC-3 pancreatic Cancer Stem Cell subpopulation and impairs tumour engrafting. Sci Rep. 9 (1), 11359 (2019).
- Ware, M. J., et al. Generation of homogenous three-dimensional pancreatic cancer cell spheroids using an improved hanging drop technique. Tissue Eng Part C Methods. 22 (4), 312-321 (2016).
- Longati, P., et al. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer. 13, 95 (2013).
- Kpeglo, D., et al. Modeling the mechanical stiffness of pancreatic ductal adenocarcinoma. Matrix Biol Plus. 14, 100109 (2022).
- Jang, S. -. D., et al. Anti-cancer activity profiling of chemotherapeutic agents in 3D co-cultures of pancreatic tumor spheroids with cancer-associated fibroblasts and macrophages. Cancers. 13 (23), 5955 (2021).
- Pednekar, K. P., Heinrich, M. A., van Baarlen, J., Prakash, J. Novel 3D µtissues mimicking the fibrotic stroma in pancreatic cancer to study cellular interactions and stroma-modulating therapeutics. Cancers. 13 (19), 5006 (2021).
- Kim, S. -. K., et al. Phenotypic heterogeneity and plasticity of cancer cell migration in a pancreatic tumor three-dimensional culture model. Cancers. 12 (5), 1305 (2020).
- Hwang, H. J., Oh, M. -. S., Lee, D. W., Kuh, H. -. J. Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. J Exp Clin Cancer Res. 38 (1), 258 (2019).
- Durymanov, M., et al. Subcutaneous inoculation of 3D pancreatic cancer spheroids results in development of reproducible stroma-rich tumors. Transl Oncol. 12 (1), 180-189 (2019).
- Giustarini, G., Teng, G., Pavesi, A., Adriani, G. Characterization of 3D heterocellular spheroids of pancreatic ductal adenocarcinoma for the study of cell interactions in the tumor immune microenvironment. Front Oncol. 13, 1156769 (2023).
- Alseud, K., et al. Synthesis and biological activity of 11-oxygenated and heterocyclic estrone analogs in pancreatic cancer monolayers and 3D spheroids. Bioorg Med Chem. , (2024).
- Fitzgerald, A. A., et al. Fibroblast activation protein regulates natural killer cell migration, extravasation and tumor infiltration. bioRxiv. , 429622 (2021).
- Wishart, G., Gupta, P., Schettino, G., Nisbet, A., Velliou, E. 3D tissue models as tools for radiotherapy screening for pancreatic cancer. Br J Radiol. 94 (1120), 20201397 (2021).
- Peirsman, A., et al. MISpheroID: a knowledgebase and transparency tool for minimum information in spheroid identity. Nat Methods. 18 (11), 1294-1303 (2021).
- Durymanov, M., Kroll, C., Permyakova, A., Reineke, J. Role of endocytosis in nanoparticle penetration of 3D pancreatic cancer spheroids. Mol Pharm. 16 (3), 1074-1082 (2019).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved