화학적 변형을 통한 살리실산의 결정화
Overview
출처: 케리 M. 둘리와 마이클 G. 벤턴, 화학 공학부, 루이지애나 주립 대학, 배턴 루지, LA
생화학의 처리는 결정화, 초원심분리기, 막 여과 및 예비 염색체와 같은 단위 작동을 포함하며, 이 모든 것은 공통적으로 작은 분자에서 큰 것을 분리하거나 액체로부터 고체를 분리할 필요가 있습니다. 이들 중 결정화는 톤수 관점에서 가장 중요합니다. 이러한 이유로, 그것은 일반적으로 제약, 화학 및 식품 가공 산업에 사용. 중요한 생화학적 예로는 키랄 분리, 항생제 정제1개, 전구체로부터 아미노산2개 분리,3개 및 기타 많은 의약품,4-5가지 식품 첨가물,6-7 및 농약 정화등이 있습니다. 8 이러한 요인이 건조, 여과 및 고체 전달과 같은 다운스트림 처리 작업의 비용에 영향을 미치기 때문에 결정 형태 및 크기 분포의 제어는 경제성을 처리하는 데 매우 중요합니다. 결정화에 대한 자세한 내용은 전문 교과서 또는 단위 운영 교과서를 참조하십시오. 9
결정화기 단위(그림 1)는 (a) 고체 함량, 형태 및 결정 크기 분포에 대한 과포화 및 냉각/가열 속도와 같은 주요 파라미터의 효과를 연구할 수 있습니다. (b) 및 결정화 공정의 온라인 제어. 동요 속도 및 온도와 같은 조건을 변경하여 과포화를 제어할 수 있습니다. 결정화의 다른 분류는 냉각을 포함, 증발, pH 스윙과 화학 적 수정. 이 실험에서 오프라인 현미경은 생물학적 생물의 전형적인 크기 범위인 10-1000 μm의 크기에서 측정됩니다.

그림 1: P&ID 회로도(왼쪽)와 크리스탈라이저의 사진(오른쪽). 이 그림의 더 큰 버전을 보려면 여기를 클릭하십시오.
본 실험에서는 기본인 염리실산(NaSAL)의 수성 용액의 신속한 반응으로부터 살리실산(SAL) (아스피린의 선구자)를 생성하고, 40~80°C에서 황산(H2SO4)을생성하여"화학적 변형"또는 "pH-swing" 결정화를 시연할것이다.
Na+SAL + 0.5 H2SO4 SAL (ppt) + Na+ + 0.5 SO42-
산제품 황산나트륨은 여전히 용해성입니다. 이 장치는 2개의 공급 탱크, 3개의 가변 속도(연동) 펌프, 결정화기(균일한 온도 및 농도근근상에 교반된 탱크, ~5L), 온도 제어를 위한 순환 용탕, 파워 컨트롤러, 제품 탱크 및 NaOH 용액을 사용하여 사료 재생을 위한 메이크업 탱크(원하는 경우)로 구성됩니다. 샘플은 잔류 용용 성살리산 이온에 대한 UV-Vis 분광계에 의해 분석될 것이며, 살리실산 결정 생성물은 건조및 계량될 것이다. pH 프로브를 사용하여 반응 조건이 변경될 때 정상 상태를 확인할 수 있습니다.
Procedure
유기 (살리실산, NaSAL) 및 산 (황산, 0.25 M = 0.50 N) 용액은 결정화기에게 공급됩니다. NaSAL, 살리실산 또는 용액 및 0.25 M 황산을 취급할 때 라텍스 장갑을 착용하십시오.
전체 시스템은 도 1의인터페이스와 유사한 인터페이스를 가진 상용 분산 컨트롤러를 사용하여 PC에서 제어됩니다. 모든 온-오프 또는 3방향 솔레노이드 밸브 및 컨트롤러 세트 포인트는 이 인터페이스를 사용하여 작동하고 변경할 수 있습니다. 회로도는 장치와 관련된 아날로그 값(유량, 온도)의 추세를 보여줍니다.
1. 결정화기 시작
실행이 시작될 때 모든 연속 컨트롤러는 수동 모드여야 하며 모든 솔레노이드 밸브는 닫히거나(온-오프) 또는 재활용(3way) 모드여야 합니다.
- 결정화기가 물과 살리실산 슬러리와 함께 교반
Results
Application and Summary
이 실험은 원시 농도, 흐름 및 온도 측정을 취하고 MSMPR 이론을 사용하여 크고 복잡한 결정화기 시스템을 설계하는 데 필요한 주요 매개 변수를 추정하는 방법을 보여 주어. 높은 결정 수율을 얻고 결정의 평균 크기를 제어하는 데 거주 시간이 수행하는 중요한 역할이 탐구되었습니다. 종종 매우 큰 결정이 바람직하지 하기 때문에 최적의 체류 시간이 있다. 혼합도 마찬가지입니다 - 혼합은 고체 결...
References
- C. Wibowo, L. O’Young and K.M. Ng, Chem. Eng. Prog., Jan. 2004, pp. 30-39.
- W.J. Genck, Chem. Eng. Prog., Oct. 2004, pp. 26-32.
- S. Takamatsu and D.D.Y. Ryu, Biotechnol. Bioeng., 32, 184-191 (1988).
- F. Wang and K.A. Berglund, Ind. Eng. Chem. Res., 39, 2101-2104 (2000).
- Y. Kim, S. Haam, Y.G. Shul, W.-S. Kim, J.K. Jung, H.-C. Eun and K.-K. Koo, Ind. Eng. Chem. Res., 42, 883-889 (2003).
- K. Hussain, G. Thorsen and D. Malthe-Sorenssen, Chem. Eng. Sci., 56, 2295-2304 (2001).
- H. Gron, A. Borissova and K.J. Roberts, Ind. Eng. Chem. Res., 42, 198-206 (2003).
- F. Lewiner, G. Fevotte, J.P. Klein and F. Puel, Ind. Eng. Chem. Res., 41, 1321-1328 (2002).
- For example: W.L. McCabe, J.C. Smith, and P. Harriott, “Unit Operations of Chemical Engineering”, 7th Ed., McGraw-Hill, New York, 2005, Ch. 27, or C.J. Geankoplis, “Transport Processes and Unit Operations”, 3rd Ed., 1993, Ch. 12.
- P. Barrett, Chem. Eng. Prog., Aug. 2003, pp. 26-32.
- R. Franck, R. David, J. Villermaux and J.P. Klein, Chem. Eng. Sci., 43, 69-77 (1988).
- J. Garside, Chem. Eng. Sci., 40, 3-26 (1985).
- H. Zhao, J.-X. Wang, Qi-An Wang, J.-F. Chen and J. Yun, Ind. Eng. Chem. Res. 46, 8229-8235 (2007).
- J.S. Kwon, M. Nayhouse, G. Orkoulas and P.D. Christofides, Ind. Eng. Chem. Res., 53, 15538-15548 (2014).
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
화학적 변형을 통한 살리실산의 결정화
Chemical Engineering
24.3K Views

핀 튜브 열교환기의 열전달 효율 테스트
Chemical Engineering
17.9K Views

트레이 건조기를 사용한 대류 및 전도열 전달 조사
Chemical Engineering
44.0K Views

프로필렌 글리콜 용액의 점도
Chemical Engineering
32.9K Views

실리카 알루미나 분말의 다공성 측정
Chemical Engineering
9.6K Views

압출을 통한 멱 법칙 모델 시연
Chemical Engineering
10.2K Views

가스 흡착 장치
Chemical Engineering
36.8K Views

증기-액체 평형
Chemical Engineering
89.3K Views

환류비가 트레이 증류 효율에 미치는 영향
Chemical Engineering
77.8K Views

액체-액체 추출의 효율성
Chemical Engineering
48.5K Views

액상 반응기: 자당 반전
Chemical Engineering
9.7K Views

충전 층 반응기의 단상 및 2상 흐름
Chemical Engineering
19.0K Views

폴리디메틸실록산에 대한 부가 중합의 반응 속도
Chemical Engineering
16.1K Views

촉매 반응기 에틸렌의 수소화
Chemical Engineering
30.4K Views

스핀 앤 칠의 열전달 평가
Chemical Engineering
7.4K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유

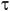 체적 유량을 변화시킴으로써(거주 시간)를 제어하지만, 스토이치오메트릭 동등성을 유지한다.
체적 유량을 변화시킴으로써(거주 시간)를 제어하지만, 스토이치오메트릭 동등성을 유지한다.